Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin
Abstract
1. Introduction
2. Extraction, Purification, and Characterization of Phloretin
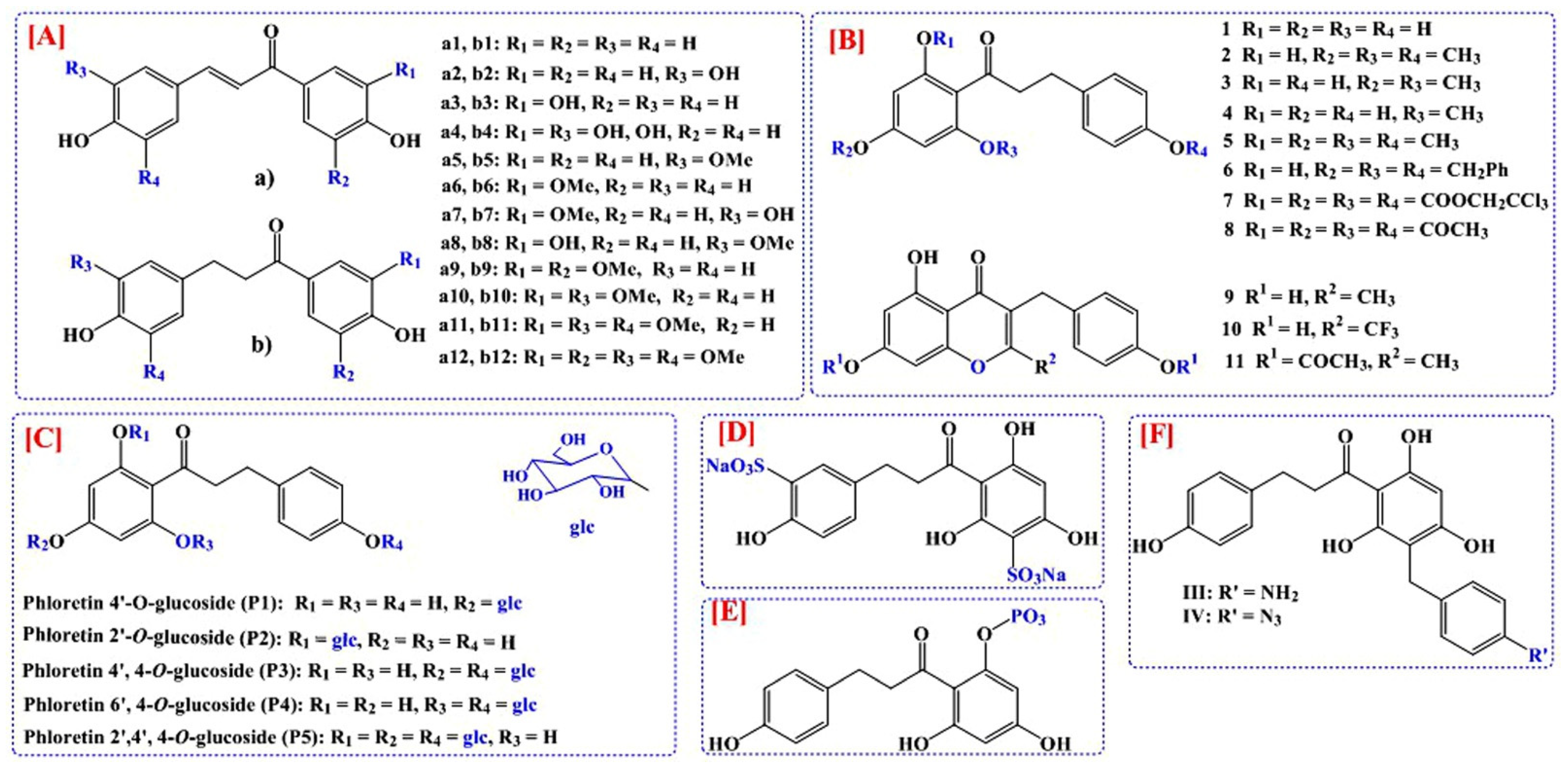
3. Development of Analogs of Phloretin for the Improvement of Bioavailability
4. Pharmacokinetics of Phloretin
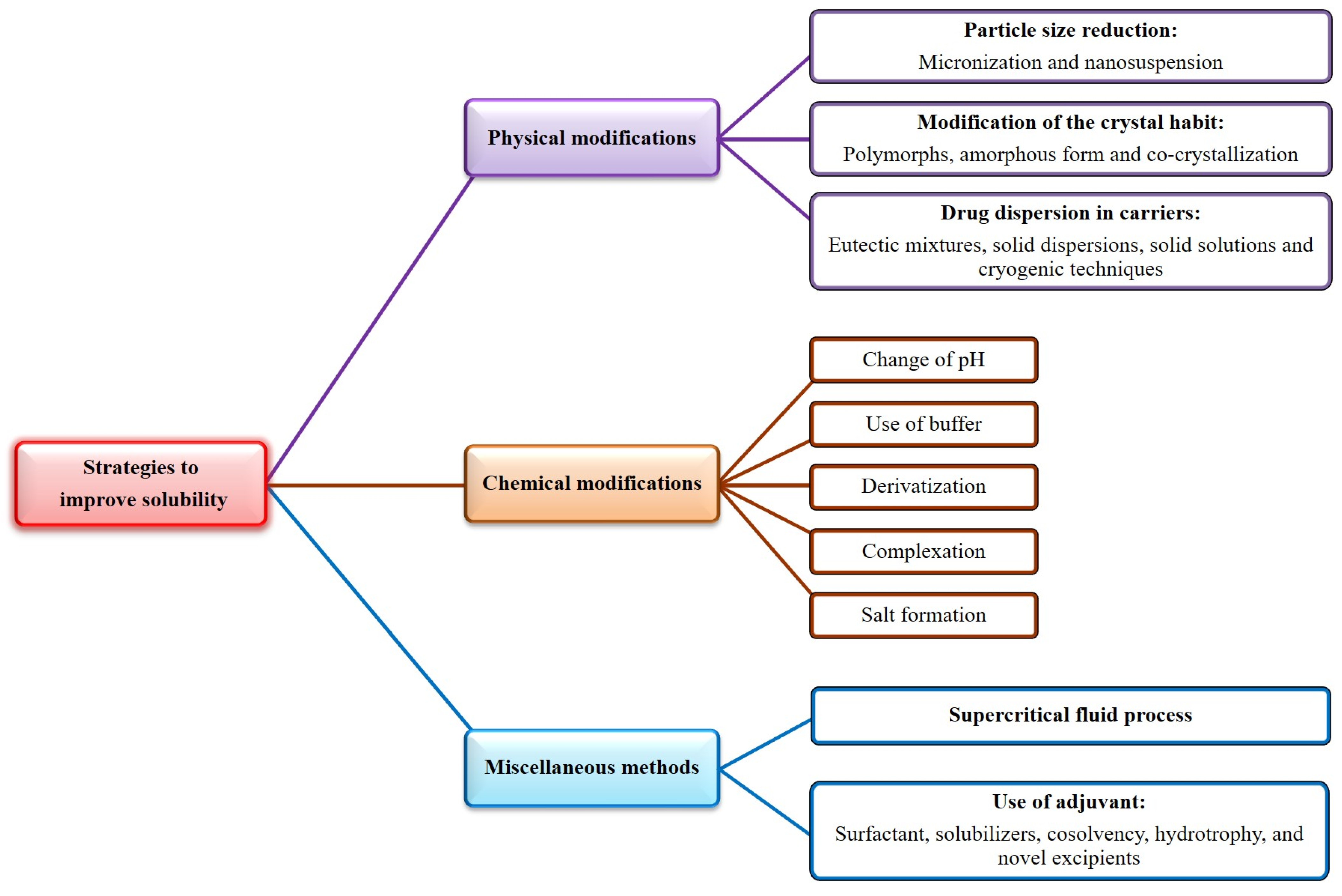
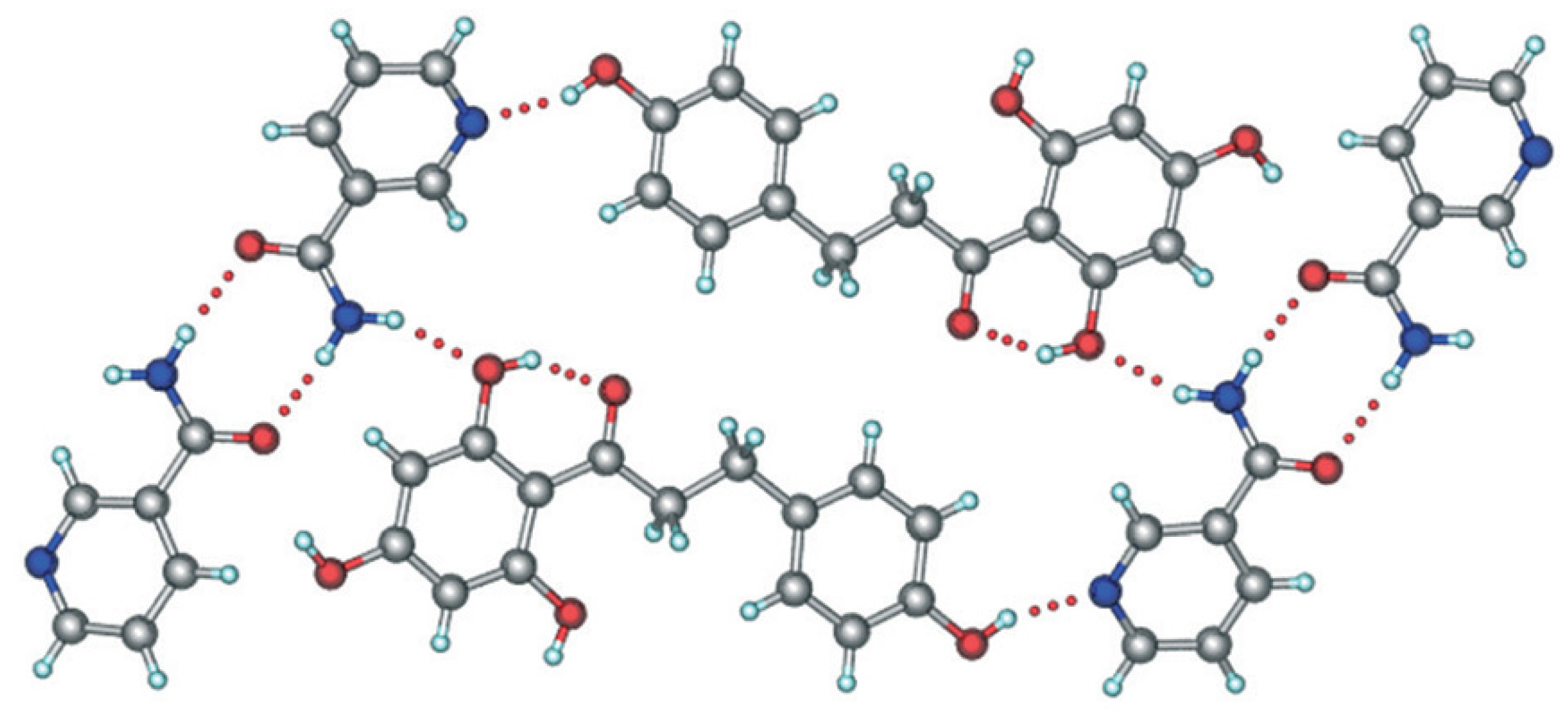
5. Physicochemical and Pharmaceutical Characteristics of Phloretin
6. Pharmacological Potentials and Molecular Mechanisms of Phloretin
6.1. Anticancer Activity
6.2. Antidiabetic Activity
6.3. Antiobesity Activity
6.4. Cardiovascular Protective Activity
6.5. Hepatoprotective Activity
6.6. Anti-Inflammatory and Antioxidant Activities
6.7. Neuroprotective Activity
6.8. Immunosuppressant Activity
6.9. Antimicrobial Activity
| SN | Activity | Mechanism | Reference |
|---|---|---|---|
| 1. | Anticancer | ||
| Reduced LC3B-II expression in low-glucose and glucose-free media; Reversed doxorubicin- and tamoxifen-induced cytoprotective autophagy; Downregulated mTOR/ULK1 signaling; Facilitated apoptosis through Bcl2 and Bax; Downregulated PI3K/Akt/mTOR signaling | [3,10] | |
| ROS mediated cell death; Arrested cell cycle in G0-G1 phase; Reduced expression of cyclin D1, CDK4, and CDK6 | [4] | |
| Arrested cell cycle in G2/M phase; Reduced p-JNK and p38 expression | [5] | |
| Arrested cell cycle in G0-G1 phase; Increased p27 expression; Decreased cdk2, cdk4, cdk6, cyclin D, and cyclin E expressions; Inhibited PI3K/AKT/mTOR signaling; Induced mitochondrial apoptosis pathways; Increased ROS production; Down-regulated Bcl-2; Up-regulated Bax, Bak, and c-PARP | [8] | |
| Increased activity of p53; Increased levels of Bax; Decreased levels of Bcl-2 | [6,125] | |
| Decreased Bcl-2 expression; Increased cleaved-caspase-3 and -9 protein expression; Deregulated MMP-2 and -9 gene expression and protein levels | [6,7] | |
| Increased HSP70 penetration efficacy; Potentiated antitumor activity of HSP70 | [6,126] | |
| Potentiated anticancer effect of paclitaxel | [6,127] | |
| Marked anticancer activity | [6,9,64] | |
| 2. | Antidiabetic | Inhibited intestinal SGLT1 and GLUT2 to reduce glucose absorption | [11,12] |
| Inhibited renal SGLT2 to reduce renal tubular reabsorption of glucose, and thus increased urinary excretion of glucose | [13,14] | ||
| Activated PI3K/AKT signaling cascade by GLUT4 translocation and expression to improve glucose consumption and tolerance in type 2 diabetes | [15] | ||
| Inhibited production of AGEs and suppressed receptor expression for AGEs by Nrf2-dependant pathway and mitigated HFD-induced diabetes in C57BL/6 mice | [94] | ||
| Preserved nephrin and podocin contents to protect podocytes in diabetic nephropathy | [16] | ||
| 3. | Antiobesity | Inhibited adipogenicity by stimulating beta-catenin and adipocytes apoptosis; Stimulated phloretin OPG gene expression and OPG/RANKL ratio in adipocytes | [99] |
| Blocked weight gain induced by high-fat diet feeding; Reduced hepatic lipid accumulation; Reduced expression of macrophage markers and pro-inflammatory genes and increased adiponectin gene in white adipose tissue; Increased fatty acid oxidation genes expression and reduced expression of lipogenesis transcriptional factor | [96] | ||
| 4. | Cardioprotective | Reduced the activation of platelets and TNF-induced expression of endothelial adhesion molecules in HUVECs | [17] |
| Protected against hydrogen peroxide-induced apoptosis in primary culture by inhibiting the chloride ion channels; Inhibited uric acid-induced pro-inflammatory factors, p-NF-κB/p-ERK levels, and nuclear translocation of NF-κΒ p65, and improved endothelial tube formation in TNF-treated HUVECs | [18,19] | ||
| Protected myocardium against doxorubicin-triggered injury; Attenuated doxorubicin-produced oxidative stress and decreased nitric oxide contents in heart tissue; Prevented doxorubicin-triggered changes in hemodynamic parameters; Decreased pro-inflammatory cytokines, and plasma myocardial injury markers such as CK-MB, LDH, AST and ALT | [104] | ||
| Protected against hyperglycemia-triggered injury in diabetic cardiomyopathy by reducing fibrosis via restoring sirtuin 1 expression | [20] | ||
| Decreased hyperglycemia by inhibiting SGLT2 in the kidney and consequently prevented the development of hypertension | [14] | ||
| 5. | Hepatoprotective | Protected against acetaminophen, CCl4, and D-galactosamine-induced acute liver damage; Decreased levels of ALT, AST, GGT, ALP, and total bilirubin levels; Alleviated oxidative stress and lipid peroxidation in liver tissue | [21,22,23] |
| 6. | Anti-inflammatory | Activated Nrf2 signaling to decrease the release of IL-8 triggered by LPS and thereby produced anti-inflammatory effect in retinal pigment epithelium (ARPE-19 cells); Inhibited the glucose uptake in ARPE-19 cells | [28] |
| Suppressed neuroinflammation in experimental autoimmune encephalomyelitis model via activation of Nrf2 signaling in macrophages, attributed to AMPK-dependent activation of autophagy and consequent degradation of Keap1 | [24] | ||
| Reduced levels of BUN, UACR, tubular necrosis, ECM deposition, and interstitial fibroblasts in mice with hyperuricemia-induced renal dysfunction; Reduced renal inflammatory cells infiltration, cytokines (NLRP3 and IL-1β), mitochondrial ROS, and morphological lesions; Inhibited renal GLUT9 and promoted urinary uric acid excretion | [111] | ||
| Inhibited Propionibacterium acnes-induced TLR2-mediated inflammatory signaling in human keratinocytes | [25] | ||
| Improved histopathological changes in the colon of mice with dextran sulfate sodium-induced ulcerative colitis; Inhibited TNF-α, IL-1β, IL-12 IL-17A and IFN-γ levels; Activation of NF-κB pathway, increased TLR4 expression, and reduced PPARγ expression in ulcerative colitis were restored; Escherichia coli and Lactobacillus levels were re-balanced | [2] | ||
| Reduced inflammation, eosinophil infiltration, Th2 cytokine production, and oxidative stress in ovalbumin-induced asthmatic mice | [26] | ||
| Reduced formation of inflammatory cytokines (TNF, IL-6, IL-1, and IL-17) in collagen-induced arthritic mice | [27] | ||
| 7. | Antioxidant | Antioxidant action in DPPH and ABTS assay | [29] |
| Reduced the matrix MMP-1, tyrosinase, and elastase activity due to its dihydrochalcone structure | [30,31] | ||
| Augmented antioxidant defense mechanisms by phlorizin (glycone of phloretin) to ameliorate LPS-induced cognitive deficit, and diabetes-induced depression, memory impairment, delayed wound healing, and peripheral neuropathy | [32,33,34,35] | ||
| 8. | Neuroprotective | ||
| a. Alzheimer’s disease | Improved learning and memory performance of animals with Alzheimer’s disease-like condition induced by scopolamine and amyloid β; Inhibited activity of acetylcholinesterase; Increased BDNF levels;Decreased oxidative stress by enhancing activities of GSH, SOD and CAT, and decreasing levels of MDA; Decreased TNF-α-triggered neuroinflammation; Reduced accumulation of amyloid-β in the CA1 hippocampal region and number of pyknotic nuclei in the hippocampal DG;Exhibited protective effect on the synaptophysin; Increased count of Ki67- and doublecortin in the DG; No change in PSD-95 levels | [120,121,122] | |
| b. Parkinson’s disease | Improved motor performance of animals with Parkinson’s disease-like condition induced by MPTP; Increased dopamine levels and tyrosine hydroxylase enzyme expression; Suppressed neuroinflammation: reduced expression of GFAP, iba1, iNOS and COX2; Reduced levels of proinflammatory cytokines (IL-β, IL-6, and TNF-α) | [123] | |
| 9. | Immunosuppressant | Suppressed proliferation of T lymphocytes and expression of CD69 and CD25, and arrested cell cycle in G0/G1 phase | [36] |
| 10. | Antimicrobial | Suppressed production of Escherichia coli O157:H7 biofilm and reduced colon inflammation without causing harm to the beneficial commensal Escherichia coli biofilms | [37] |
| Inhibited Mycobacterium tuberculosis by reducing the expression of inflammatory molecules such as ILs and TNF-α | [38] | ||
| Inhibited Staphylococcus aureus, Listeria monocytogenes, methicillin-resistant Staphylococcus aureus clinical strains, and Salmonella typhimurium | [39] | ||
| Inhibited Listeria monocytogenes | [40,41] | ||
| Decreased Salmonella typhimurium bacterial load of infected mice | [42] | ||
| Inhibited Candida albicans without inducing any tissue necrosis in mouse model of oral candidiasis | [124] | ||
| Inhibited plant pathogenic fungi Phytophthora capsici, Alternaria panax, Sclerotinia sclerotiorum, Rhizoctonia solani AG4, and Magnaporthe grisea on rice and tomato seedlings | [62] |
7. Safety and Adverse Effects of Phloretin
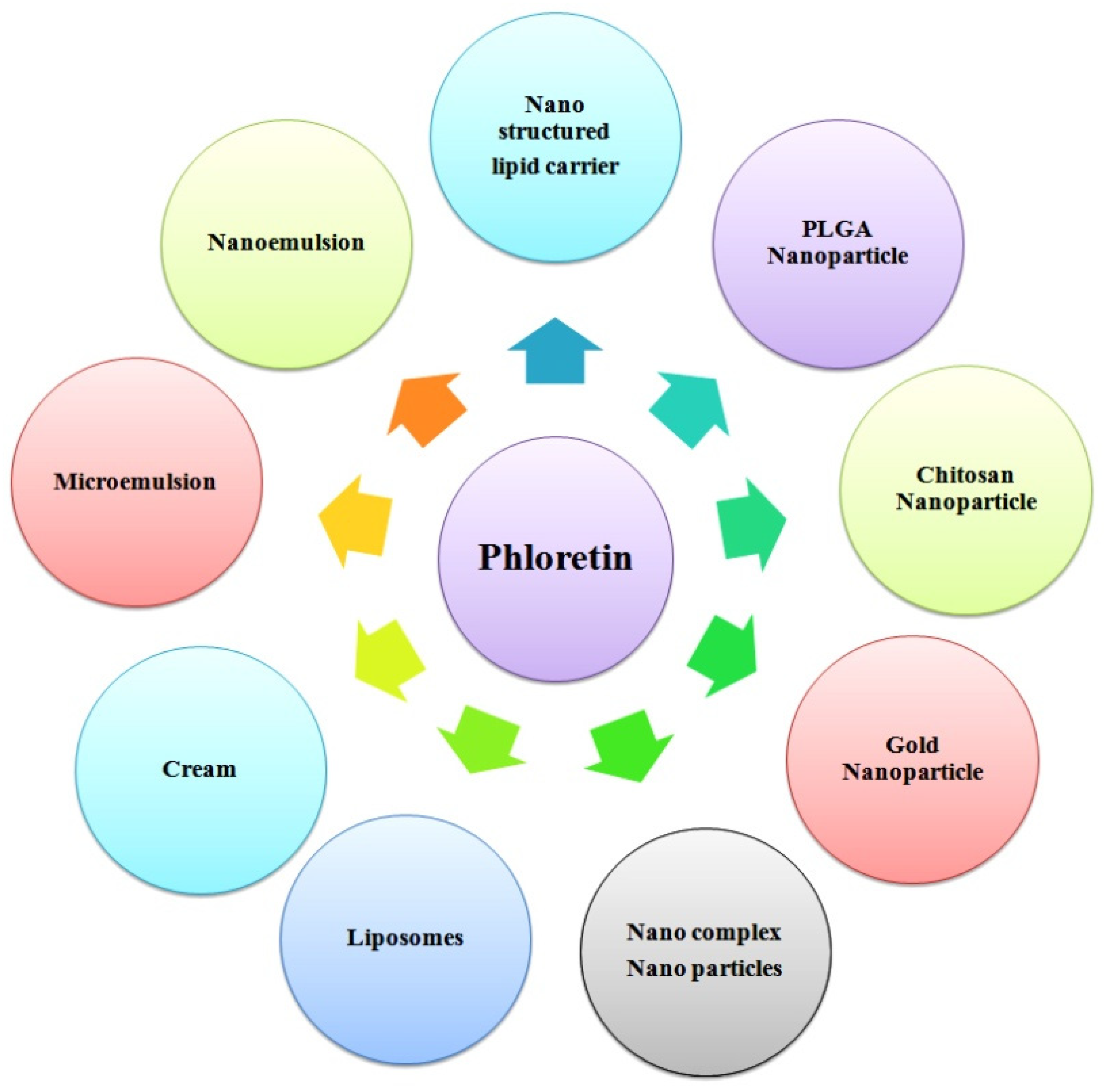
8. Pharmaceutical Development of Phloretin
9. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.-W. The apple dihydrochalcone phloretin suppresses growth and improves chemosensitivity of breast cancer cells via inhibition of cytoprotective autophagy. Food Funct. 2021, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yin, X.; Ma, D.; Su, Z. Anticancer activity of Phloretin against the human oral cancer cells is due to G0/G1 cell cycle arrest and ROS mediated cell death. J. BUON 2020, 25, 344–349. [Google Scholar] [PubMed]
- Xu, M.; Gu, W.; Shen, Z.; Wang, F. Anticancer Activity of Phloretin Against Human Gastric Cancer Cell Lines Involves Apoptosis, Cell Cycle Arrest, and Inhibition of Cell Invasion and JNK Signalling Pathway. Med. Sci. Monit. 2018, 24, 6551–6558. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical basis of anti-cancer-effects of phloretin—A natural dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Ma, L.; Wang, R.; Nan, Y.; Li, W.; Wang, Q.; Jin, F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int. J. Oncol. 2016, 48, 843–853. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, C.; Pu, L.; Wei, C.; Jin, H.; Teng, Y.; Zhao, M.; Yu, A.C.H.; Jiang, F.; Shu, J.; et al. Phloretin induces cell cycle arrest and apoptosis of human glioblastoma cells through the generation of reactive oxygen species. J. Neurooncol. 2016, 128, 217–223. [Google Scholar] [CrossRef]
- Kim, J.L.; Lee, D.H.; Pan, C.H.; Park, S.J.; Oh, S.C.; Lee, S.Y. Role of phloretin as a sensitizer to TRAIL-induced apoptosis in colon cancer. Oncol. Lett. 2022, 24, 321. [Google Scholar] [CrossRef]
- Roy, S.; Mondru, A.K.; Chakraborty, T.; Das, A.; Dasgupta, S. Apple polyphenol phloretin complexed with ruthenium is capable of reprogramming the breast cancer microenvironment through modulation of PI3K/Akt/mTOR/VEGF pathways. Toxicol. Appl. Pharmacol. 2022, 434, 115822. [Google Scholar] [CrossRef]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Helliwell, P.A. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem. J. 2000, 350, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013, 1, 140–151. [Google Scholar] [CrossRef]
- Osorio, H.; Bautista, R.; Rios, A.; Franco, M.; Arellano, A.; Vargas-Robles, H.; Romo, E.; Escalante, B. Effect of phlorizin on SGLT2 expression in the kidney of diabetic rats. J. Nephrol. 2010, 23, 541–546. [Google Scholar] [PubMed]
- Shen, X.; Wang, L.; Zhou, N.; Gai, S.; Liu, X.; Zhang, S. Beneficial effects of combination therapy of phloretin and metformin in streptozotocin-induced diabetic rats and improved insulin sensitivity in vitro. Food Funct. 2020, 11, 392–403. [Google Scholar] [CrossRef]
- Liu, J.; Sun, M.; Xia, Y.; Cui, X.; Jiang, J. Phloretin ameliorates diabetic nephropathy by inhibiting nephrin and podocin reduction through a non-hypoglycemic effect. Food Funct. 2022, 13, 6613–6622. [Google Scholar] [CrossRef]
- Stangl, V.; Lorenz, M.; Ludwig, A.; Grimbo, N.; Guether, C.; Sanad, W.; Ziemer, S.; Martus, P.; Baumann, G.; Stangl, K. The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J. Nutr. 2005, 135, 172–178. [Google Scholar] [CrossRef]
- Malekova, L.; Tomaskova, J.; Novakova, M.; Stefanik, P.; Kopacek, J.; Lakatos, B.; Pastorekova, S.; Krizanova, O.; Breier, A.; Ondrias, K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch. 2007, 455, 349–357. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, Y.; Zhou, Y.; Zhao, M.; Chen, Y.; Cheng, J.; Lu, Y.; Liu, J. Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J. Cell. Mol. Med. 2017, 21, 2553–2562. [Google Scholar] [CrossRef]
- Ying, Y.; Jiang, C.; Zhang, M.; Jin, J.; Ge, S.; Wang, X. Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging 2019, 11, 2822–2835. [Google Scholar] [CrossRef]
- Ebadollahi Natanzi, A.R.; Mahmoudian, S.; Minaeie, B.; Sabzevari, O. Hepatoprotective activity of phloretin and hydroxychalcones against Acetaminophen Induced hepatotoxicity in mice. Iran. J. Pharm. Sci. 2011, 7, 89–97. [Google Scholar]
- Zuo, A.R.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Wang, X.M.; Peng, S.H.; Xie, Y.F.; Cao, S.W. Hepatoprotective effects and antioxidant, antityrosinase activities of phloretin and phloretin isonicotinyl hydrazone. J. Chin. Med. Assoc. 2014, 77, 290–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Y.; Chen, J.; Ren, D.; Yang, X.; Zhao, Y. Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food Agric. Immunol. 2017, 28, 211–222. [Google Scholar] [CrossRef]
- Dierckx, T.; Haidar, M.; Grajchen, E.; Wouters, E.; Vanherle, S.; Loix, M.; Boeykens, A.; Bylemans, D.; Hardonnière, K.; Kerdine-Römer, S. Phloretin suppresses neuroinflammation by autophagy-mediated Nrf2 activation in macrophages. J. Neuroinflamm. 2021, 18, 148. [Google Scholar] [CrossRef]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.-C.; Kim, Y. Target Proteins of Phloretin for Its Anti-Inflammatory and Antibacterial Activities Against Propionibacterium acnes-Induced Skin Infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Fang, L.W.; Liou, C.J. Phloretin Attenuates Allergic Airway Inflammation and Oxidative Stress in Asthmatic Mice. Front. Immunol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Wang, S.P.; Lin, S.C.; Li, S.; Chao, Y.H.; Hwang, G.Y.; Lin, C.C. Potent Antiarthritic Properties of Phloretin in Murine Collagen-Induced Arthritis. Evid. Based. Complement. Alternat. Med. 2016, 2016, 9831263. [Google Scholar] [CrossRef]
- Hytti, M.; Ruuth, J.; Kanerva, I.; Bhattarai, N.; Pedersen, M.L.; Nielsen, C.U.; Kauppinen, A. Phloretin inhibits glucose transport and reduces inflammation in human retinal pigment epithelial cells. Mol. Cell. Biochem. 2022, 1–13. [Google Scholar] [CrossRef]
- Leu, S.J.; Lin, Y.P.; Lin, R.D.; Wen, C.L.; Cheng, K.T.; Hsu, F.L.; Lee, M.H. Phenolic constituents of Malus doumeri var. formosana in the field of skin care. Biol. Pharm. Bull. 2006, 29, 740–745. [Google Scholar] [CrossRef]
- Auner, B.G.; O’Neill, M.A.A.; Valenta, C.; Hadgraft, J. Interaction of phloretin and 6-ketocholestanol with DPPC-liposomes as phospholipid model membranes. Int. J. Pharm. 2005, 294, 149–155. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as Novel Radical Scavenging Antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin ameliorates type 2 diabetes-induced depression in mice by mitigating oxidative stress and modulating brain-derived neurotrophic factor. J. Diabetes Metab. Disord. 2021, 20, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Effect of apple peel extract on diabetes-induced peripheral neuropathy and wound injury. J. Diabetes Metab. Disord. 2021, 20, 119–130. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Badwaik, H.R.; Raval, A.; Ajazuddin; Nakhate, K.T. Ameliorative potential of phloridzin in type 2 diabetes-induced memory deficits in rats. Eur. J. Pharmacol. 2021, 913, 174645. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Ye, Y.; Zhou, Y.; Mu, J.; Zhao, X. Anti-inflammatory and immunosuppressive effect of phloretin. Yao Xue Xue Bao 2009, 44, 480–485. [Google Scholar]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157: H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Jeon, D.; Jeong, M.-C.; Jnawali, H.N.; Kwak, C.; Ryoo, S.; Jung, I.D.; Kim, Y. Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation. Molecules 2017, 22, 183. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Teng, Z.; Zhou, X.; Wang, X.; Zhang, B.; Lu, G.; Niu, X.; Yang, Y.; Deng, X. Phloretin Attenuates Listeria monocytogenes Virulence Both In vitro and In vivo by Simultaneously Targeting Listeriolysin O and Sortase A. Front. Cell. Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef][Green Version]
- Zhao, P.; Zhang, Y.; Deng, H.; Meng, Y. Antibacterial mechanism of apple phloretin on physiological and morphological properties of Listeria monocytogenes. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Shuai-Cheng, W.; Ben-Dong, F.; Xiu-Ling, C.; Jian-Qing, S.; Yun-Xing, F.; Zhen-Qiang, C.; Dao-Xiu, X.; Zong-Mei, W. Subinhibitory concentrations of phloretin repress the virulence of Salmonella typhimurium and protect against Salmonella typhimurium infection. Antonie Van Leeuwenhoek 2016, 109, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Geohagen, B.C.; Korsharskyy, B.; Vydyanatha, A.; Nordstroem, L.; LoPachin, R.M. Phloretin cytoprotection and toxicity. Chem. Biol. Interact. 2018, 296, 117–123. [Google Scholar] [CrossRef]
- Londzin, P.; Siudak, S.; Cegieła, U.; Pytlik, M.; Janas, A.; Waligóra, A.; Folwarczna, J. Phloridzin, an Apple Polyphenol, Exerted Unfavorable Effects on Bone and Muscle in an Experimental Model of Type 2 Diabetes in Rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.X.; Zou, J.B.; Zhang, X.F.; Shi, Y.J.; Guo, D.Y. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Guo, D.; Liu, J.; Fan, Y.; Cheng, J.; Shi, Y.; Zou, J.; Zhang, X. Optimization, characterization and evaluation of liposomes from Malus hupehensis (Pamp.) Rehd. extracts. J. Liposome Res. 2020, 30, 366–376. [Google Scholar] [CrossRef]
- Sharifi-Rad, A.; Mehrzad, J.; Darroudi, M.; Saberi, M.R.; Chamani, J. Oil-in-water nanoemulsions comprising Berberine in olive oil: Biological activities, binding mechanisms to human serum albumin or holo-transferrin and QMMD simulations. J. Biomol. Struct. Dyn. 2021, 39, 1029–1043. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Vega-Villa, K.; Miranda, N.D.; Andrews, P.K. HPLC-UV Analysis of Phloretin in Biological Fluids and Application to Pre-Clinical Pharmacokinetic Studies. J. Chromatogr. Sep. Technol. 2010, 1, 101. [Google Scholar] [CrossRef]
- Zhang, T.; Lian, J.; Wang, P.; Xu, Y.; Wang, Y.; Wei, X.; Fan, M. Purification and characterization of a novel phloretin-2′-O-glycosyltransferase favoring phloridzin biosynthesis. Sci. Rep. 2016, 6, 35274. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Lin, H.; Jiang, S.; Han, L.; Hou, S.; Lin, S.; Cheng, Z.; Bian, W.; Zhang, X.; et al. Enhanced oral bioavailability and bioefficacy of phloretin using mixed polymeric modified self-nanoemulsions. Food Sci. Nutr. 2020, 8, 3545–3558. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Toprak, M. Biophysical study of phloretin with human serum albumin in liposomes using spectroscopic methods. Eur. Biophys. J. 2020, 49, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Abu-Azzam, O.; Nasr, M. In vitro anti-inflammatory potential of phloretin microemulsion as a new formulation for prospective treatment of vaginitis. Pharm. Dev. Technol. 2020, 25, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective Effects of a New Phloretin Derivative against UVB-Induced Damage in Skin Cell Model and Human Volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef]
- Pandey, R.P.; Li, T.F.; Kim, E.-H.; Yamaguchi, T.; Park, Y.I.; Kim, J.S.; Sohng, J.K. Enzymatic Synthesis of Novel Phloretin Glucosides. Appl. Environ. Microbiol. 2013, 79, 3516–3521. [Google Scholar] [CrossRef]
- Minsat, L.; Peyrot, C.; Brunissen, F.; Renault, J.-H.; Allais, F. Synthesis of Biobased Phloretin Analogues: An Access to Antioxidant and Anti-Tyrosinase Compounds for Cosmetic Applications. Antioxidants 2021, 10, 512. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.W.; Zhang, W.; Xu, R.; Gao, F.; Liu, Y.F.; Li, Y.J. Synthesis, Crystal Structure, and Biological Evaluation of a Series of Phloretin Derivatives. Molecules 2014, 19, 16447–16457. [Google Scholar] [CrossRef]
- Shimoda, K.; Otsuka, T.; Morimoto, Y.; Hamada, H.; Hamada, H. Glycosylation and malonylation of quercetin, epicatechin, and catechin by cultured plant cells. Chem. Lett. 2007, 36, 1292–1293. [Google Scholar] [CrossRef]
- Peerce, B.E.; Clarke, R. A phosphorylated phloretin derivative. Synthesis and effect on intestinal Na ϩ-dependent phosphate absorption. Am. J. Physiol. Liver Physiol. 2022, 0641, 848–855. [Google Scholar]
- Difdrich, D.F. Photoafflnity-Labeling Analogs of Phlorizin and Phloretin: Synthesis and Effects on Cell Membranes. Methods Enzymol. 1990, 191, 755–780. [Google Scholar]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Jo, S.; Kim, J.; Choi, G.J. Control Efficacy of Phloretin Isolated from Apple Fruits Against Several Plant Diseases. Plant Pathol. J. 2010, 26, 280–285. [Google Scholar] [CrossRef]
- Kindt, M.; Orsini, M.C.; Costantini, B. Improved High-Performance Liquid Chromatography—Diode Array Detection Method for the Determination of Phenolic Compounds in Leaves and Peels from Different Apple Varieties. J. Chromatogr. Sci. 2007, 45, 507–514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, X.; Xing, Y.; Zhou, Z.; Yao, Y. Dihydrochalcone Compounds Isolated from Crabapple Leaves Showed Anticancer Effects on Human Cancer Cell Lines. Molecules 2015, 20, 21193–21203. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of ultrasound-assisted extraction of phloretin and other phenolic compounds from apple tree leaves (Malus domestica Borkh.) and comparison of different cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef]
- Adamcová, A.; Horna, A.; Šatínský, D. Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity. Pharmaceuticals 2022, 15, 244. [Google Scholar] [CrossRef]
- Petkovska, A.; Gjamovski, V.; Stanoeva, J.P.; Stefova, M. Characterization of the Polyphenolic Profiles of Peel, Flesh and Leaves of Malus domestica Cultivars Using UHPLC-DAD-HESI-MS. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gulati, A.; Bhushan, S. RP-HPLC-DAD Determination of Phenolics in Industrial Apple Pomace. Food Anal. Methods 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Lommen, A.; Godejohann, M.; Venema, D.P.; Hollman, P.C.H.; Spraul, M. Application of Directly Coupled HPLC-NMR-MS to the Identification and Confirmation of Quercetin Glycosides and Phloretin Glycosides in Apple Peel. Anal. Chem. 2000, 72, 4431–4435. [Google Scholar] [CrossRef]
- Preti, R.; Maria, A. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Xü, K.; Lü, H.; Qü, B.; Shan, H.; Song, J. High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep. Purif. Technol. 2010, 72, 406–409. [Google Scholar] [CrossRef]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of Phloridzin in Strawberries (Fragaria x ananassa Duch.) by HPLC − PDA − MS/MS and NMR Spectroscopy. J. Agric. Food Chem. 2003, 51, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Yadav, S.K. Impact of atmospheric non-thermal plasma and hydrothermal treatment on bioactive compounds and microbial inactivation of strawberry juice: A hurdle technology approach. Food Sci. Technol. Int. 2020, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Mattivi, F.; Vrhovsek, U. Overall dietary polyphenol intake in a bowl of strawberries: The influence of Fragaria spp. in nutritional studies. J. Funct. Foods 2014, 18, 1057–1069. [Google Scholar] [CrossRef]
- Jaroslawska, J.; Juskiewicz, J.; Wroblewska, M.; Jurgonski, A.; Krol, B. Polyphenol-Rich Strawberry Pomace Reduces Serum and Liver Lipids and Alters Gastrointestinal Metabolite Formation in Fructose-Fed Rats. J. Nutr. Nutr. Physiol. Metab. Nutr. Interact. 2011, 141, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Pubchem. PubChem Compd. Summ. CID 4788, Phloretin; 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phloretin (accessed on 1 August 2022).
- Li, B.; Li, R.; Yan, W. Solubilities of Phloretin in 12 Solvents at Different Temperatures. J. Chem. Eng. Data 2011, 56, 1459–1462. [Google Scholar] [CrossRef]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of phloretin and phloridzin in rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef]
- Bentes, A.L.A.; Borges, R.S.; Monteiro, W.R.; De Macedo, L.G.M.; Alves, C.N. Structure of dihydrochalcones and related derivatives and their scavenging and antioxidant activity against oxygen and nitrogen radical species. Molecules 2011, 16, 1749–1760. [Google Scholar] [CrossRef]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigne, C. Bioavailability of Phloretin and Phloridzin in Rats. Nutr. Metab. Commun. 2002, 131, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Behzad, S.; Sureda, A.; Barreca, D.; Fazel, S.; Rastrelli, L.; Mohammad, S. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm 2014, 16, 3451. [Google Scholar] [CrossRef]
- Aitipamula, S.; Shan, L.P.; Gupta, K.M. Polymorphism and distinct physicochemical properties of the phloretin–nicotinamide cocrystal. CrystEngComm 2022, 24, 560–570. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Kobori, M.; Shinmoto, H.; Tsushida, T.; Shinohara, K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997, 119, 207–212. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, J.; Bi, J.; Wu, X.; Lyu, J.; Gao, K. Synergistic inhibition of colon cancer cell growth by a combination of atorvastatin and phloretin. Oncol. Lett. 2018, 15, 1985–1992. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Agbeko, R.; Boison, D.; Ekloh, W.; Kuatsienu, L.E.; Biney, E.E.; Affum, O.O.; Kwarteng, J.; Nyarko, A.K. Phytomedicines Used for Diabetes Mellitus in Ghana: A Systematic Search and Review of Preclinical and Clinical Evidence. Evid. Based. Complement. Alternat. Med. 2019, 2019, 6021209. [Google Scholar] [CrossRef]
- Arora, S.K.; Verma, P.R.; Itankar, P.R.; Prasad, S.K.; Nakhate, K.T. Evaluation of pancreatic regeneration activity of Tephrosia purpurea leaves in rats with streptozotocin-induced diabetes. J. Tradit. Complement. Med. 2021, 11, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes. Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, N.; Mi, L.; Hu, Z.; Wang, L.; Liu, X.; Zhang, S. Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des. Devel. Ther. 2017, 11, 313–324. [Google Scholar] [CrossRef]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.D.; Lakhawat, S.S.; Yasmeen, N.; Pandey, A.; Singla, R.K. Biogenic Phytochemicals Modulating Obesity: From Molecular Mechanism to Preventive and Therapeutic Approaches. Evidence-Based Complement. Altern. Med. 2022, 2022, 6852276. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Dandekar, M.P.; Kokare, D.M.; Subhedar, N.K. Involvement of neuropeptide Y Y1 receptors in the acute, chronic and withdrawal effects of nicotine on feeding and body weight in rats. Eur. J. Pharmacol. 2009, 609, 78–87. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Rodríguez-Ramos, Á.; Torrecillas-Baena, B.; Dorado, G.; Quesada-Gómez, J.M.; Gálvez-Moreno, M.Á. Flavonoid Phloretin Inhibits Adipogenesis and Increases OPG Expression in Adipocytes Derived from Human Bone-Marrow Mesenchymal Stromal-Cells. Nutrients 2021, 13, 4185. [Google Scholar] [CrossRef]
- Thiriet, M. Cardiovascular Disease: An Introduction. Vasc. Behav. Chem. Environ. Genet. Factors 2019, 8, 1–90. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Pawar, H.D.; Mahajan, U.B.; Nakhate, K.T.; Agrawal, Y.O.; Patil, C.R.; Meeran, M.F.; Sharma, C.; Ojha, S.; Goyal, S.N. Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life 2022, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Agrawal, Y.; Sherikar, A.; Nakhate, K.T.; Patil, C.R.; Nagoor Meeran, M.F.; Ojha, S.; Goyal, S.N. Thymoquinone Produces Cardioprotective Effect in β-Receptor Stimulated Myocardial Infarcted Rats via Subsiding Oxidative Stress and Inflammation. Nutrients 2022, 14, 2742. [Google Scholar] [CrossRef] [PubMed]
- Wagh, S.S.; Patil, K.R.; Mahajan, U.B.; Bagal, P.D.; Wadkar, A.R.; Bommanhalli, B.; Patil, P.R.; Goyal, S.N.; Ojha, S.; Patil, C.R. Phloretin-induced suppression of oxidative and nitrosative stress attenuates doxorubicin-induced cardiotoxicity in rats. Asian Pac. J. Trop. Biomed. 2022, 12, 124. [Google Scholar]
- Sauri, I.; Uso, R.; Trillo, J.L.; Fernandez, A.; Holgado, J.L.; Lopez, C.; Vela, S.; Bea, C.; Ruiz, A.; Martinez, F. Impact of hypertension in the morbidity and mortality in diabetes mellitus: A real-world data. J. Hypertens. 2021, 39, e23. [Google Scholar] [CrossRef]
- Kaplan, N.M. Hypertension and diabetes. J. Hum. Hypertens. 2002, 16, S56–S60. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Khichariya, A.; Jeswani, G.; Choudhary, R.; Alexander, A.; Nakhate, K.T.; Badwaik, H.R. Formulation of Plumbagin Loaded Microemulsion: Evaluation of Anti-rheumatoid efficacy in Wistar Rat model. J. Mol. Liq. 2022, 363, 119851. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Memon, A.H.; Liang, H. Molecular model and in vitro antioxidant activity of a water-soluble and stable phloretin/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Liq. 2017, 236, 68–75. [Google Scholar] [CrossRef]
- Huang, W.C.; Dai, Y.W.; Peng, H.L.; Kang, C.W.; Kuo, C.Y.; Liou, C.J. Phloretin ameliorates chemokines and ICAM-1 expression via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human keratinocytes. Int. Immunopharmacol. 2015, 27, 32–37. [Google Scholar] [CrossRef]
- Cui, D.; Liu, S.; Tang, M.; Lu, Y.; Zhao, M.; Mao, R.; Wang, C.; Yuan, Y.; Li, L.; Chen, Y. Phloretin ameliorates hyperuricemia-induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine 2020, 66, 153111. [Google Scholar] [CrossRef]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lai, C.L.; Liang, Y.T.; Hung, H.C.; Liu, H.C.; Liou, C.J. Phloretin attenuates LPS-induced acute lung injury in mice via modulation of the NF-κB and MAPK pathways. Int. Immunopharmacol. 2016, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, K.; Borkar, C.; Bharne, A. Chapter 2—Functional neuroanatomy and disorders of cognition. In Cognitive Informatics, Computer Modelling, and Cognitive Science; Sinha, G.R., Suri, J.S., Eds.; Academic Press: London, UK, 2020; pp. 21–47. ISBN 978-0-12-819445-4. [Google Scholar]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β-secretase. Biomed. Pharmacother. 2018, 101, 379–390. [Google Scholar] [CrossRef]
- Rangani, R.J.; Upadhya, M.A.; Nakhate, K.T.; Kokare, D.M.; Subhedar, N.K. Nicotine evoked improvement in learning and memory is mediated through NPY Y1 receptors in rat model of Alzheimer’s disease. Peptides 2012, 33, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, M.A.; Nakhate, K.T.; Kokare, D.M.; Singru, P.S.; Subhedar, N.K. Cocaine- and amphetamine-regulated transcript peptide increases spatial learning and memory in rats. Life Sci. 2011, 88, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Gothwal, A.; Nakhate, K.T.; Alexander, A.; Ajazuddin, A.; Gupta, U. Boosted Memory and Improved Brain Bioavailability of Rivastigmine: Targeting Effort to the Brain Using Covalently Tethered Lower Generation PAMAM Dendrimers with Lactoferrin. Mol. Pharm. 2018, 15, 4538–4549. [Google Scholar] [CrossRef]
- Ghumatkar, P.J.; Patil, S.P.; Jain, P.D.; Tambe, R.M.; Sathaye, S. Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol. Biochem. Behav. 2015, 135, 182–191. [Google Scholar] [CrossRef]
- Ghumatkar, P.J.; Patil, S.P.; Peshattiwar, V.; Vijaykumar, T.; Dighe, V.; Vanage, G.; Sathaye, S. The modulatory role of phloretin in Aβ25–35 induced sporadic Alzheimer’s disease in rat model. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 327–339. [Google Scholar] [CrossRef]
- Ghumatkar, P.; Peshattiwar, V.; Patil, S.; Muke, S.; Whitfield, D.; Howlett, D.; Francis, P.; Sathaye, S. The effect of phloretin on synaptic proteins and adult hippocampal neurogenesis in Aβ (1-42)-injected male Wistar rats. J. Pharm. Pharmacol. 2018, 70, 1022–1030. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Liu, J. Phloretin attenuates behavior deficits and neuroinflammatory response in MPTP induced Parkinson’s disease in mice. Life Sci. 2019, 232, 116600. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, N.; Zhang, S.; Zhang, L.; Liu, Q. Phloretin inhibited the pathogenicity and virulence factors against Candida albicans. Bioengineered 2021, 12, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, R.; Yan, X.; Liu, H.; Zhang, Y.; Mu, D.; Han, J.; Li, X. Phloretin induces apoptosis of human esophageal cancer via a mitochondria dependent pathway. Oncol. Lett. 2017, 14, 6763–6768. [Google Scholar] [CrossRef] [PubMed]
- Abkin, S.V.; Ostroumova, O.S.; Komarova, E.Y.; Meshalkina, D.A.; Shevtsov, M.A.; Margulis, B.A.; Guzhova, I. V Phloretin increases the anti-tumor efficacy of intratumorally delivered heat-shock protein 70 kDa (HSP70) in a murine model of melanoma. Cancer Immunol. Immunother. 2016, 65, 83–92. [Google Scholar] [CrossRef]
- Yang, K.; Tsai, C.; Wang, Y.; Wei, P.; Lee, C.; Chen, J.; Wu, C.; Ho, Y. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol. Carcinog. Publ. Coop. with Univ. Texas MD Anderson Cancer Cent. 2009, 48, 420–431. [Google Scholar] [CrossRef]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Kumari, L.; Nakhate, K.; Verma, V.S.; Sakure, K. Phytoconstituent plumbagin: Chemical, biotechnological and pharmaceutical aspects. Stud. Nat. Prod. Chem. 2019, 63, 415–460. [Google Scholar]
- Badwaik, H.; Giri, T.; Nakhate, K.; Kashyap, P.; Tripathi, D. Xanthan Gum and Its Derivatives as a Potential Bio-polymeric Carrier for Drug Delivery System. Curr. Drug Deliv. 2013, 10, 587–600. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Nakhate, K.; Kumari, L.; Sakure, K. Oral Delivery of Proteins and Polypeptides through Polysaccharide Nanocarriers. In Polysaccharide-Based Nano-Biocarrier in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–24. ISBN 0429449437. [Google Scholar]
- Khan, I.; Joshi, G.; Sarkar, B.; Nakhate, K.T.; Ajazuddin; Mantha, A.K.; Kumar, R.; Kaul, A.; Chaturvedi, S.; Mishra, A.K.; et al. Doxorubicin and Crocin Co-delivery by Polymeric Nanoparticles for Enhanced Anticancer Potential in Vitro and in Vivo. ACS Appl. Bio Mater. 2020, 3, 7789–7799. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Kumari, L.; Maiti, S.; Sakure, K.; Nakhate, K.T.; Tiwari, V.; Giri, T.K. A review on challenges and issues with carboxymethylation of natural gums: The widely used excipients for conventional and novel dosage forms. Int. J. Biol. Macromol. 2022, 209, 2197–2212. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, J.; Luo, Y. Encapsulation of Phloretin in a Ternary Nanocomplex Prepared with Phytoglycogen–Caseinate–Pectin via Electrostatic Interactions and Chemical Cross-Linking. J. Agric. Food Chem. 2020, 68, 13221–13230. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, R.; Wang, W.; Xia, Q. Nanostructured lipid carriers for the encapsulation of phloretin: Preparation and in vitro characterization studies. Chem. Phys. Lipids 2022, 242, 105150. [Google Scholar] [CrossRef] [PubMed]
- Casarini, T.P.A.; Frank, L.A.; Benin, T.; Onzi, G.; Pohlmann, A.R.; Guterres, S.S. Innovative hydrogel containing polymeric nanocapsules loaded with phloretin: Enhanced skin penetration and adhesion. Mater. Sci. Eng. C 2021, 120, 111681. [Google Scholar] [CrossRef]
- Nam, S.; Lee, S.Y.; Cho, H.-J. Phloretin-loaded fast dissolving nanofibers for the locoregional therapy of oral squamous cell carcinoma. J. Colloid Interface Sci. 2017, 508, 112–120. [Google Scholar] [CrossRef]
- Ranjanamala, T.; Vanmathiselvi, K.; Casimeer, S.C.; Ghidan, A.Y. Synthesis and Characterization of Dose-Dependent Antioxidants and Antimicrobial Activity of Phloretin Loaded PLGA Nanoparticles. Biointerface Res. Appl. Chem. 2022, 12, 3076–3089. [Google Scholar]
- Payne, J.N.; Badwaik, V.D.; Waghwani, H.K.; Moolani, H.V.; Tockstein, S.; Thompson, D.H.; Rajalingam, D. Development of dihydrochalcone-functionalized gold nanoparticles for augmented antineoplastic activity. Int. J. Nanomed. 2018, 13, 1917–1926. [Google Scholar] [CrossRef]
- Yang, J.Z.; Zhao, X.Y.; Zhou, K.; Zhang, Z.Q.; Xing-Ye, Z. Formulation optimization of phloretin nanostructured lipid carrier by Box-Behnken design-response surface method. Chinese J. Hosp. Pharm. 2021, 41, 2076–2081. [Google Scholar] [CrossRef]
- Kum, H.; Roh, K.-B.; Shin, S.; Jung, K.; Park, D.; Jung, E. Evaluation of anti-acne properties of phloretin in vitro and in vivo. Int. J. Cosmet. Sci. 2016, 38, 85–92. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Zielinski, J.; Hansenne, I. Anti-Aging Composition Containing Phloretin. Patent US9248082B2, 2 February 2016. [Google Scholar]
- Hu, X.; Zhou, Z.; Han, L.; Li, S.; Zhou, W. Preparation and characterization of phloretin by complexation with cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
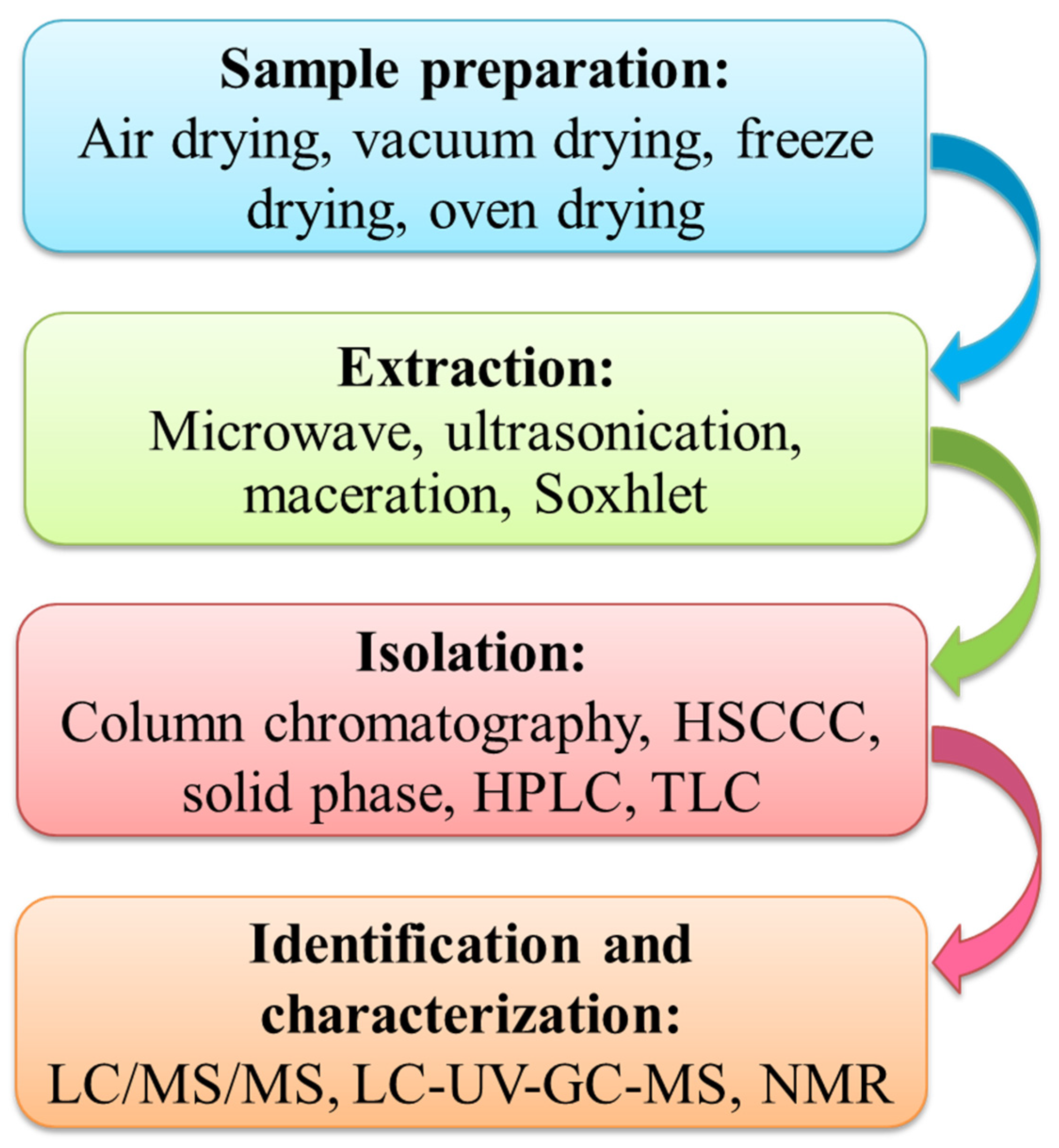
| Plant Part | Extraction Method | Extraction Solvent | Analytical Technique | Reference |
|---|---|---|---|---|
| Apple fruits | Solvent extraction | Methanol, n-hexane, CHCl3, ethyl acetate | HPLC-NMR | [62] |
| Apple leaves | Homogenization, centrifugation | Methanol, acetone | HPLC | [63] |
| Apple leaves | Solvent extraction | Ethanol, water | HPLC | [64] |
| Apple leaves | Ultrasound | Ethanol, water | LCMS | [65] |
| Apple leaves, bark, and buds | Centrifugation and sonication | Methanol, formic acid | HPLC-DAD | [66] |
| Apple peel, flesh, and leaves | Solvent extraction | Methanol, water | UPLC-DAD-HESI-MS | [67] |
| Apple pomace | Solvent extraction | Acetone, methanol, ethanol, ethyl acetate | RP-HPLC-DAD | [68] |
| Apple pomace | Solvent extraction | Acetone, methanol, ethanol, CHCl3, ethyl acetate | HPLC-DAD | [69] |
| Apple pulp and peel | Solvent extraction | Methanol | HPLC-NMR-MS | [70] |
| Apple pulp and peel | Solvent extraction and sonication | Methanol (1% HCl) | HPLC | [71] |
| Apple tree bark | Solvent extraction | Ethanol, ethyl acetate | HSCCC | [72] |
| Strawberry fruits | Homogenization, solvent extraction | Acetone, ethyl acetate, methanol | HPLC−PDA−MS/MS and NMR | [73] |
| Strawberry fruits | Centrifugation, solvent extraction | Methanol (HCl:Water, 50:50) | UPLC−PDA−MS/MS and NMR | [74] |
| Strawberry fruits | Solvent extraction | Acetone, water | UPLC−MS/MS | [75] |
| Strawberry pomace | Solvent extraction | Water, ethanol | HPLC-DAD | [76] |
| Particulars | Data | Reference |
|---|---|---|
| Molecular structure and other details | 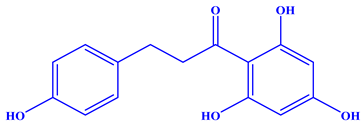 | [77] |
| Molecular formula: C15H14O5 | ||
| Synonyms: dihydronaringenin, phloretol | ||
| Color: pearl white powder | ||
| Melting point: 263.5 °C | ||
| Molecular weight: 274.27 | ||
| Solubility: slightly soluble in water, sparingly soluble in methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, butan-2-ol, pentan-1-ol, hexan-1-ol, ethyl acetate, butyl acetate, and 1,4-dioxane and DMSO | [78] | |
| Spectroscopic analysis | UV-Visible: λ = 225, 282.8, 369 | [67,68] |
| 1H NMR: δ: 2.86 (2H, t, J = 7.6 Hz, H-β), 3.20 (2H, t, J = 7.6 Hz, H-α), 5.80 (2H, s, H-3′, 5′), 6.65 (2H, d, J = 8.5 Hz, H-3, 5), 7.00 (2H, d, J = 8.5 Hz, H-2,6) | [62,64] | |
| 13C NMR: 132.6, 128.93, 114.7, 155.03, 114.7, 128.93, 103.91, 164.74, 94.34, 164.44, 94.34, 164.74, 45.93, 30.09, 205 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhate, K.T.; Badwaik, H.; Choudhary, R.; Sakure, K.; Agrawal, Y.O.; Sharma, C.; Ojha, S.; Goyal, S.N. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients 2022, 14, 3638. https://doi.org/10.3390/nu14173638
Nakhate KT, Badwaik H, Choudhary R, Sakure K, Agrawal YO, Sharma C, Ojha S, Goyal SN. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients. 2022; 14(17):3638. https://doi.org/10.3390/nu14173638
Chicago/Turabian StyleNakhate, Kartik T., Hemant Badwaik, Rajesh Choudhary, Kalyani Sakure, Yogeeta O. Agrawal, Charu Sharma, Shreesh Ojha, and Sameer N. Goyal. 2022. "Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin" Nutrients 14, no. 17: 3638. https://doi.org/10.3390/nu14173638
APA StyleNakhate, K. T., Badwaik, H., Choudhary, R., Sakure, K., Agrawal, Y. O., Sharma, C., Ojha, S., & Goyal, S. N. (2022). Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients, 14(17), 3638. https://doi.org/10.3390/nu14173638






