Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals and Reagents
2.2. Preparation of Chicory Root Extracts
2.3. Purification of Chicory Extracts
2.4. Analysis of Sesquiterpene Lactones Concentration
2.5. Isothermal Titration Calorimetry (ITC)
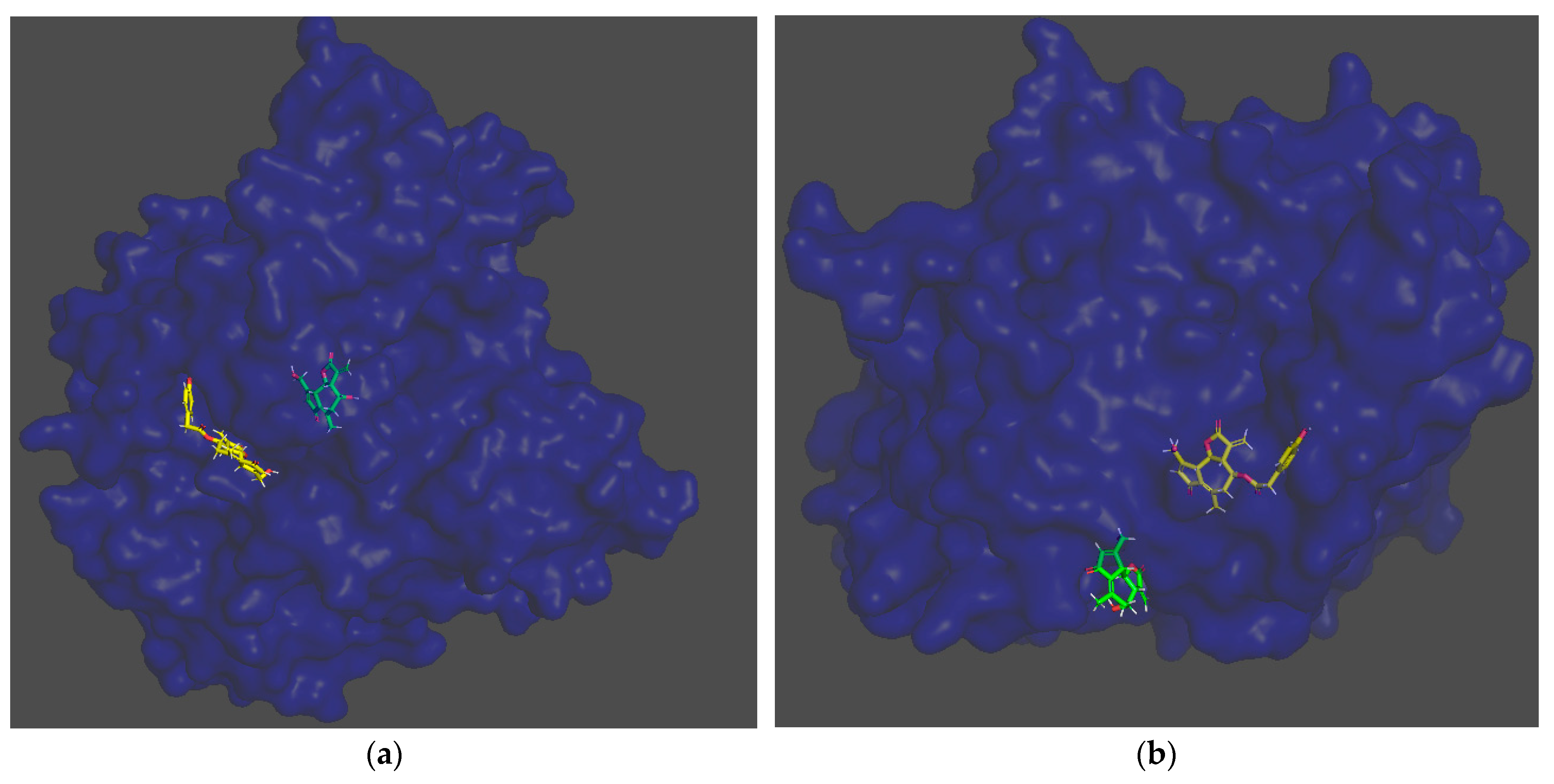
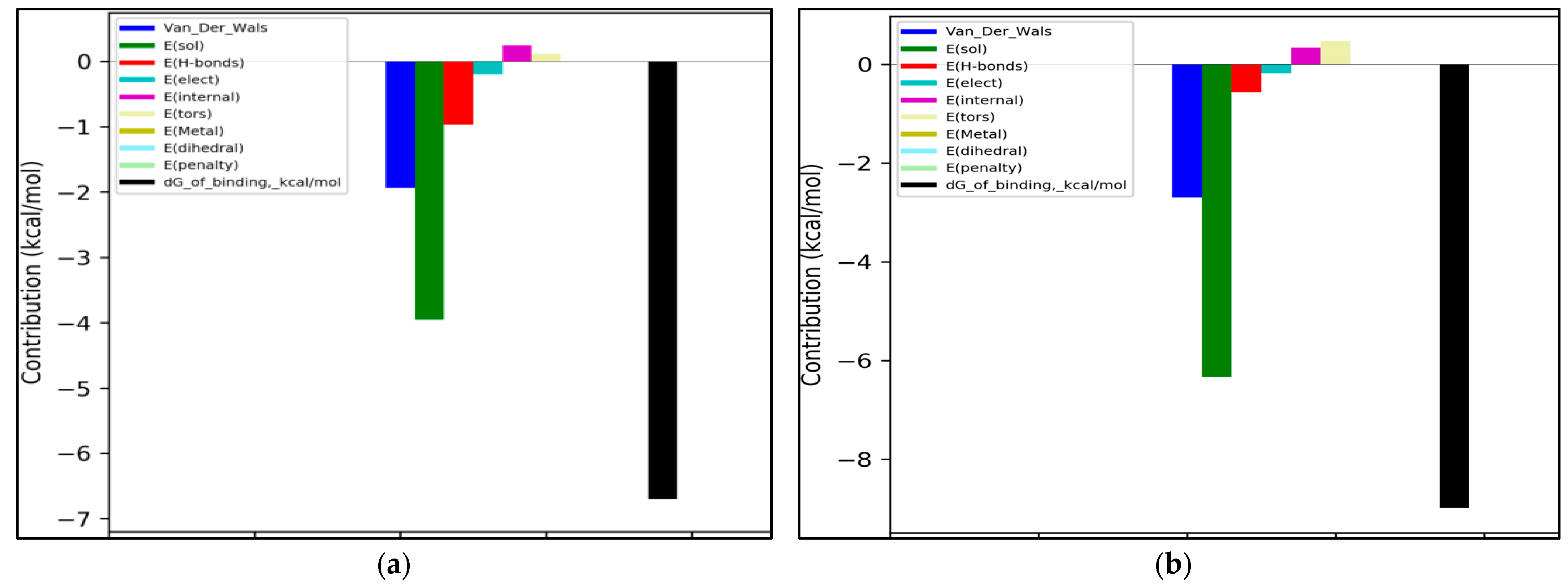
2.6. Molecular Modeling
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Chicory Root Extracts
3.2. Evaluation of Sesquiterpene Lactones Preparations as AChE Inhibitors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enzyme Nomenclature. Available online: https://iubmb.qmul.ac.uk/enzyme/ (accessed on 25 June 2022).
- Cavalcante, S.F.D.A.; Simas, A.B.C.; Barcellos, M.C.; de Oliveira, V.G.M.; Sousa, R.B.; Cabral, P.A.d.M.; Kuèa, K.; França, T.C.C. Acetylcholinesterase: The “hub” for neurodegenerative diseases and chemical weapons convention. Biomolecules 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive compounds, antioxidant activity and inhibition of key enzymes relevant to Alzheimer’s disease from sweet pepper (Capsicum annuum) extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C.; Correia, B.L.; Stinghen, A.E.; Santos, C.A. Acetylcholinesterase inhibitory activity of uleine from Himatanthus lancifolius. Z. Naturforsch C J. Biosci. 2010, 65, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Grzelczyk, J.; Jaśkiewicz, A.; Żyżelewicz, D.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P. Evaluation of butyrylcholinesterase inhibitory activity by chlorogenic acids and coffee extracts assed in ITC and docking simulation models. Food Res. Int. 2018, 109, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for Alzheimer’s disease therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Puhlmann, M.L.; de Vos, W.M. Back to the roots: Revisiting the use of the fiber-rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient – nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Żary-Sikorska, E.; Król, B.; Milala, J.; Jurgoński, A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on cecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011, 105, 710–720. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Nunes Dos Santos, C. Sesquiterpene lactones: Promising natural compounds to fight inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef]
- Chalatsa, I.; Arvanitis, D.A.; Mikropoulou, E.V.; Giagini, A.; Papadopoulou-Daifoti, Z.; Aligiannis, N.; Halabalaki, M.; Tsarbopoulos, A.; Skaltsounis, L.A.; Sanoudou, D. Beneficial effects of Sideritis scardica and Cichorium spinosum against amyloidogenic pathway and tau misprocessing in Alzheimer’s disease neuronal cell culture models. J. Alzheimer’s Dis. 2018, 64, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Gómez, A.; Ontiveros-Rodríguez, J.C.; Pablo-Pérez, S.S.; Vargas-Díaz, M.E.; Garduño-Siciliano, L. The potential role of sesquiterpene lactones isolated from medicinal plants in the treatment of the metabolic syndrome—A review. S. Afr. J. Bot. 2020, 135, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Giambanelli, E.; D’Antuono, L.F.; Ferioli, F.; Frenich, A.G.; Romero-González, R. Sesquiterpene lactones and inositol 4-hydroxyphenylacetic acid derivatives in wild edible leafy vegetables from Central Italy. J. Food Compos. Anal. 2018, 72, 1–6. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Budryn, G.; Nowak, A.; Efenberger-Szmechtyk, M. Novel biodegradable starch film for food packaging with antimicrobial chicory root extract and phytic acid as a cross-linking agent. Foods 2020, 9, 1696. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Destandau, E.; Boukhris, M.A.; Zubrzycki, S.; Akssira, M.; El Rhaffari, L.; Elfakir, C. Centrifugal partition chromatography elution gradient for isolation of sesquiterpene lactones and flavonoids from Anvillea radiata. J. Chromatogr. B 2015, 985, 29–37. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Yang, Y.; Ba, H.; Aisa, H.A. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss. et Huet. by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1176, 217–222. [Google Scholar] [CrossRef]
- D’Antuono, F.; Ferioli, F.; Manco, M.A. The impact of sesquiterpene lactones and phenolics on sensory attributes: An investigation of a curly endive and escarole germplasm collection. Food Chem. 2016, 199, 238–245. [Google Scholar] [CrossRef]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.F.; Hilbert, J.L. A method for the simultaneous determination of chlorogenic acid and sesquiterpene lactone content in industrial chicory root foodstuffs. Sci. World J. 2014, 583180. [Google Scholar] [CrossRef] [Green Version]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite profiling of sesquiterpene lactones from Lactuca species. Major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; Den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT-Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Rachwał-Rosiak, D. Effect of green coffee polyphenols on properties of protein hydrolysates in model systems. J. Food Process. Preserv. 2017, 41, 1–10. [Google Scholar] [CrossRef]
- Paketurytė, V.; Linkuvienė, V.; Krainer, G.; Chen, W.Y.; Matulis, D. Repeatability, precision, and accuracy of the enthalpies and Gibbs energies of a protein–ligand binding reaction measured by isothermal titration calorimetry. Eur. Biophys. J. 2019, 48, 139–152. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK(a) prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending force field coverage for drug-like small molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead finder: An approach to improve accuracy of protein-ligand docking, binding energy estimation, and virtual screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef]
- Stierand, K.; Rarey, M. From modeling to medicinal chemistry: Automatic generation of two-dimensional complex diagrams. Chem. Med. Chem. 2007, 2, 853–860. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Torres, M.D.; Camilo, C.; Mariotti-Celis, M.S.; Domínguez, H.; Pérez-Correa, H.R. Multi-response optimal hot pressurized liquid recovery of extractable polyphenols from leaves of maqui (Aristotelia chilensis [Mol.] Stuntz). Food Chem. 2021, 357, 129729. [Google Scholar] [CrossRef]
- Zaragoza-Huesca, D.; Martínez-Cortés, C.; Banegas-Luna, A.J.; Pérez-Garrido, A.; Vegara-Meseguer, J.M.; Peñas-Martínez, J.; Rodenas, M.C.; Espín, S.; Perez-Sánchez, H.; Martínez-Martínez, I. Identification of kukoamine A, zeaxanthin, and clexane as new furin inhibitors. Int. J. Mol. Sci. 2022, 23, 2796. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Scozzafava, A.; Supuran, C.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.-H.; Qi, L.-W.; Li, P. Structural relationship and binding mechanisms of five flavonoids with bovine serum albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, S.; Wei, Y.; Zhou, J.; Zhang, L.; Wang, C.; Guo, Y. Monitoring enzyme reaction and screening of inhibitors of acetylcholinesterase by quantitative matrix-assisted laser desorption/ionization Fourier transform mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 1849–1855. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Zheng, J.; Li, Q.; Liu, X.; Wang, D. Low Oral bioavailability and partial gut microbiotic and phase II metabolism of brussels/witloof chicory sesquiterpene lactones in healthy humans. Nutrients 2020, 12, 3675. [Google Scholar] [CrossRef]
- Bajda, M.; Wiȩckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- McHardy, S.F.; Wang, H.L.; McCowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase inhibitors and reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef]
- Venkatesan, R.; Shim, W.S.; Yeo, E.J.; Kim, S.Y. Lactucopicrin potentiates neuritogenesis and neurotrophic effects by regulating Ca2+/CaMKII/ATF1 signaling pathway. J. Ethnopharmacol. 2017, 198, 174–183. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]

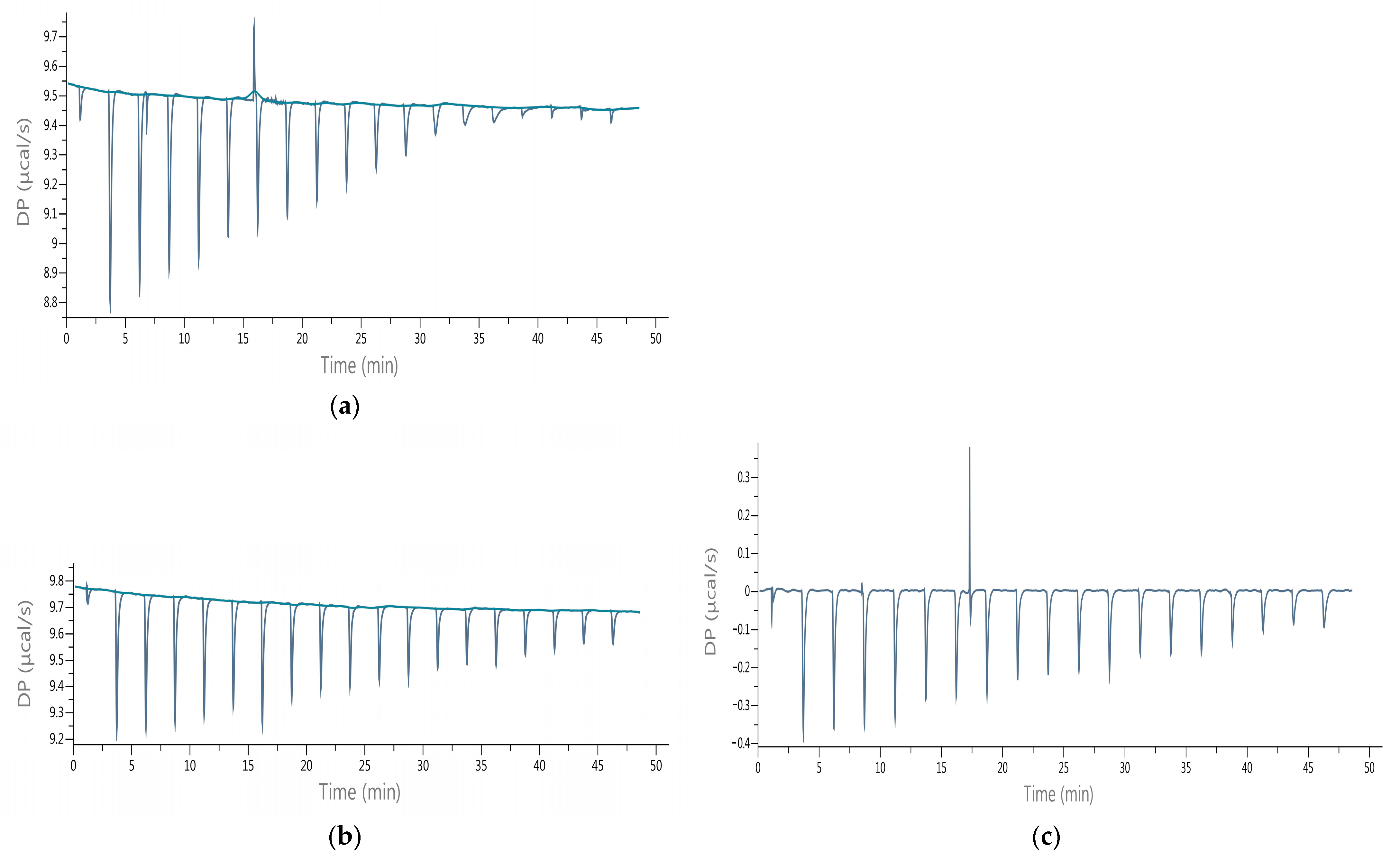
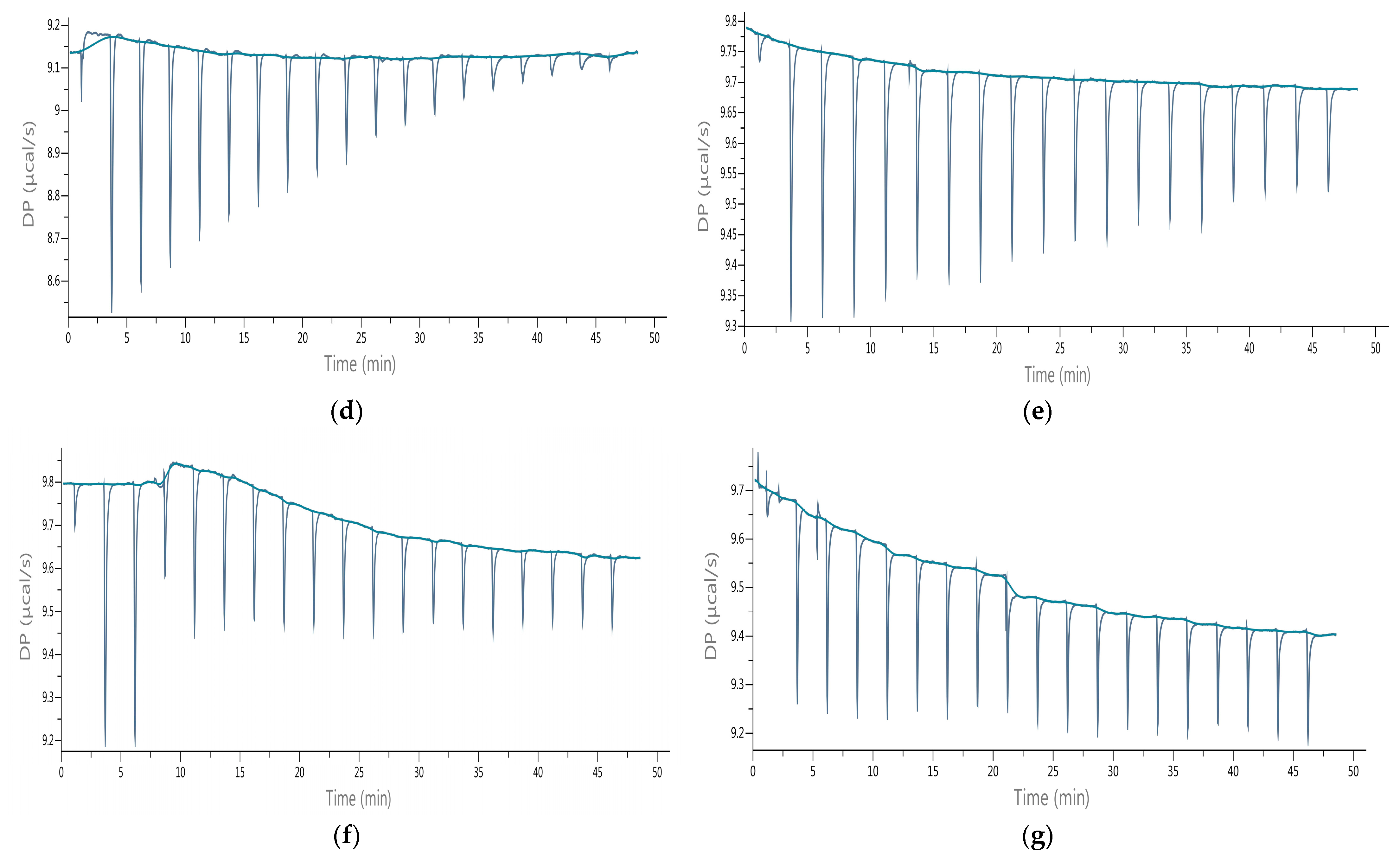
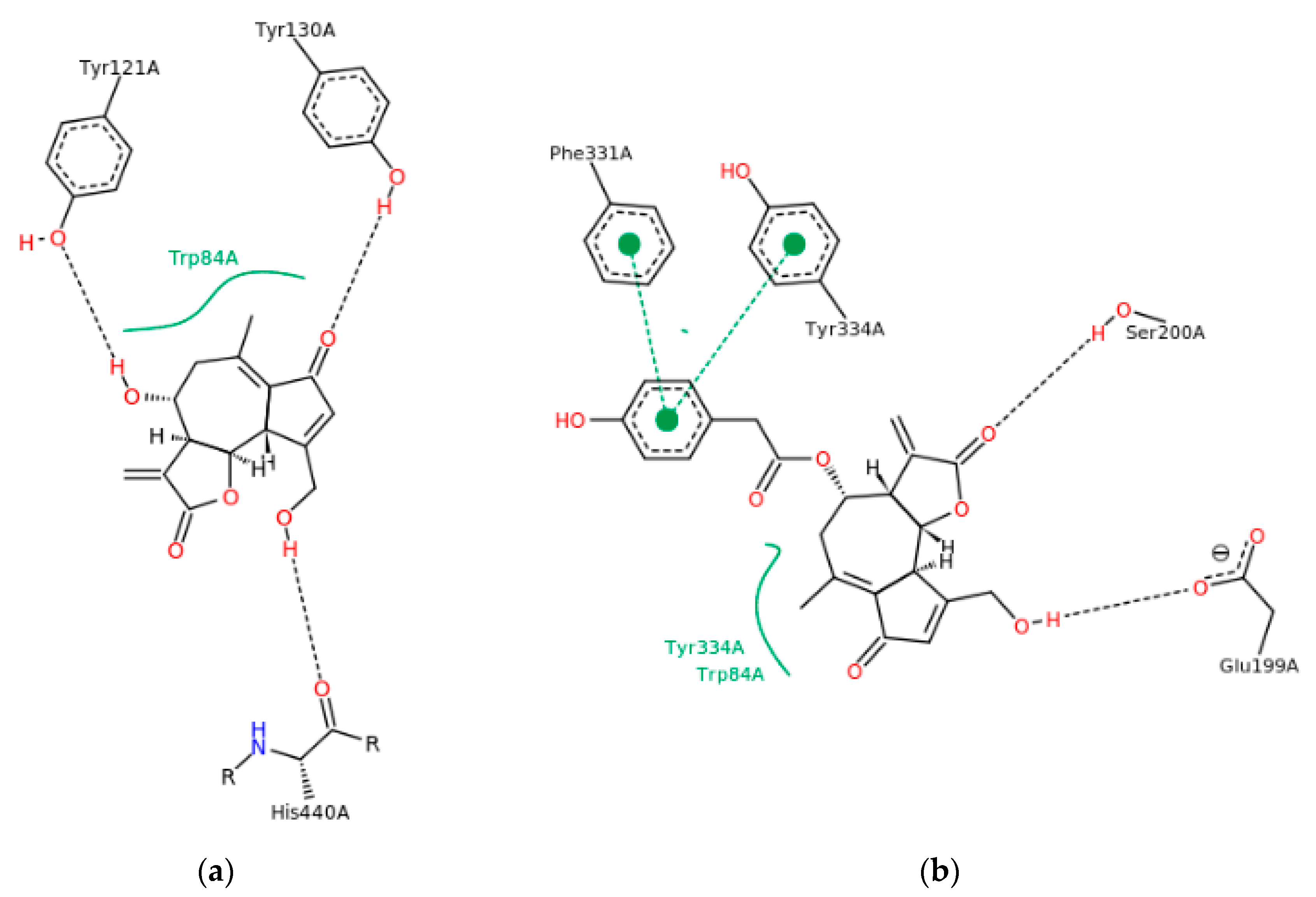
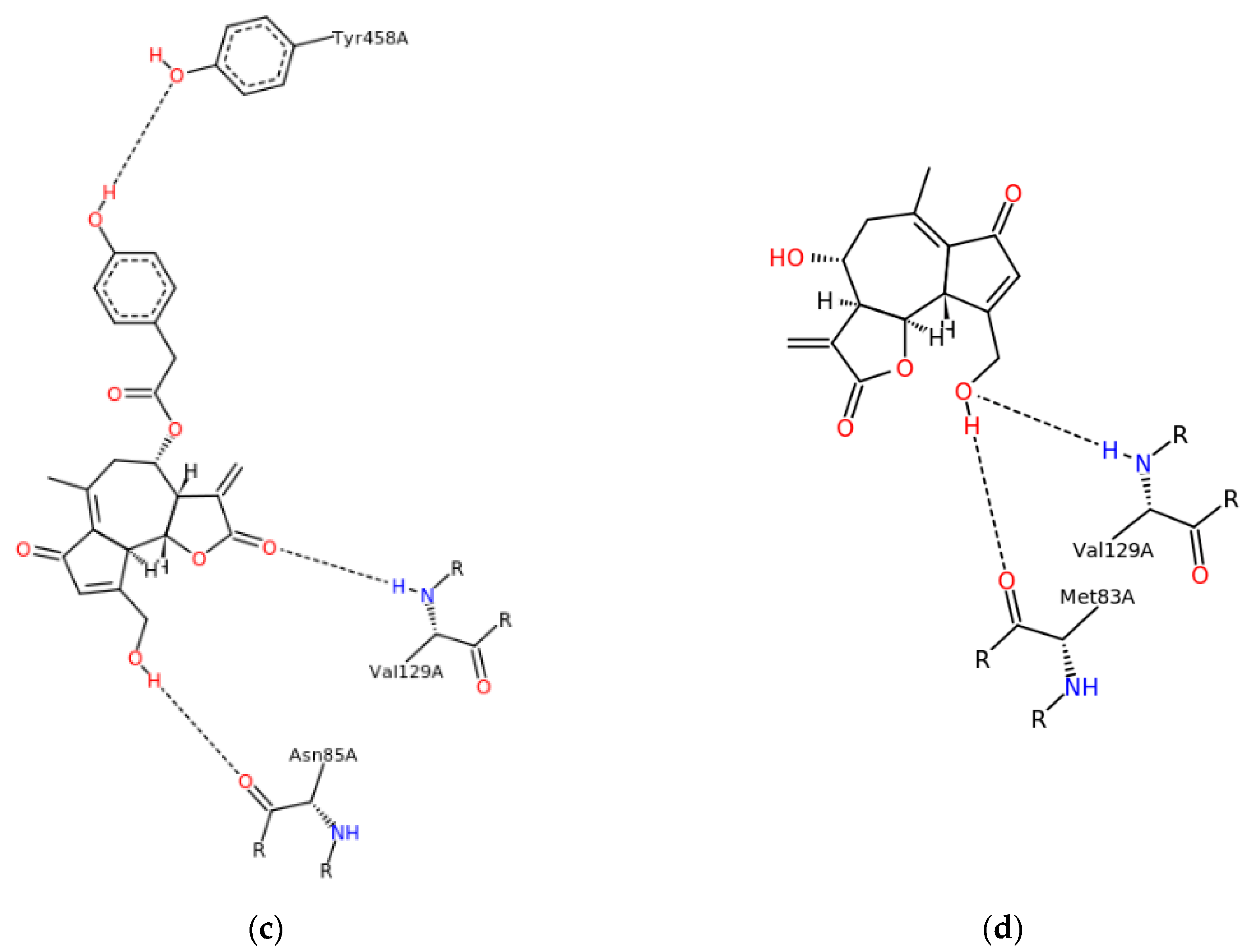
| Sesquiterpene Lactones (g/100 g db.) | Raw Extracts | Purified Extracts | ||||
|---|---|---|---|---|---|---|
| 70/30 | 50/50 | CPC 70/30 I | CPC 70/30 II | CPC 50/50 I | CPC 0/50 II | |
| 8-Deoxylactucin | 1.094 ± 0.084 a | 0.809 ± 0.068 b | 0.030 ± 0.004 d | 1.612 ± 0.034 c | 0.042 ± 0.009 d | 1.188 ± 0.044 a |
| Lactucin | 0.554 ± 0.038 c | 0.515 ± 0.041 c | 0.671 ± 0.023 a | 0.236 ± 0.021 b | 0.645 ± 0.016 a | 0.273 ± 0.014 d |
| 11(S),13-Dihydrolactucin | 0.328 ± 0.025 b | 0.285 ± 0.017 d | 0.323 ± 0.011 b | 0.175 ± 0.023 a | 0.311 ± 0.001 b | 0.125 ± 0.021 c |
| 8-Deoxylactucin oxalate | 1.129 ± 0.074 c | 0.946 ± 0.075 b | 0.100 ± 0.018 a | 1.830 ± 0.014 e | 0.105 ± 0.011 a | 1.359 ± 0.033 d |
| Lactucopicrin | 1.291 ± 0.054 a | 0.045 ± 0.003 d | 1.303 ± 0.001 e | 0.418 ± 0.012 c | 0.085 ± 0.028 b | 0.004 ± 0.001 f |
| 11(Z),13-Dihydrolactucopicrin | 0.083 ± 0.002 b | 0.057 ± 0.008 c | 0.120 ± 0.021 d | 0.007 ± 0.009 a | 0.088 ± 0.014 b | 0.006 ± 0.001 a |
| Total sesquiterpene lactones | 4.479 ± 0.297 a | 3.457 ± 0.212 d | 4.267 ± 0.013 f | 4.278 ± 0.021 c | 2.041 ± 0.005 e | 2.955 ± 0.014 b |
| Substance/ Extract | ∆H [kJ/mol] | ∆G [kJ/mol] | ΔGp [kJ/mol] | ∆S [J/mol × K] | KD [µmol/L] | KA × 103 [L/mol] | IA [%] | IC50 [μmol/L] | KI [µmol/L] |
|---|---|---|---|---|---|---|---|---|---|
| Lactucin | −166.93 ± 0.22 e | −23.41 ± 1.82 a | −37.64 | −262.62 ± 6.21 b | 29.90 ± 0.11 f | 33.44 ± 0.21 a | 26.23 ± 0.12 e | 3.11 ± 0.21 a | 1.12 ± 0.06 c |
| Lactucopicrin | −74.45 ± 0.88 d | −22.41 ± 1.59 a | −47.90 | −262.27 ± 2.21 b | 169.00 ± 0.12 c | 5.92 ± 0.11 d | 79.07 ± 1.09 d | 1.74 ± 0.11 b | 1.32 ± 0.11 b |
| CPC 70/30 I | −69.93 ± 0.76 c | −20.94 ± 1.13 a | - | −212.14 ± 9.87 a | 147.00 ± 0.99 d | 6.81 ± 0.11 c | 91.19 ± 2.15 c | 1.65 ± 0.13 b | 1.16 ± 0.06 a |
| CPC 70/30 II | −58.74 ± 0.54 b | −22.24 ± 1.58 a | - | −263.68 ± 12.87 b | 299.02 ± 0.87 a | 3.34 ± 0.04 f | 98.41 ± 1.22 b | 1.55 ± 0.16 b | 1.18 ± 0.04 a |
| CPC 50/50 I | −58.49 ± 0.33 b | −22.99 ± 2.09 a | - | −289.61 ± 10.81 c | 180.01 ± 0.66 b | 5.56 ± 0.16 e | 98.61 ± 0.14 a | 1.51 ± 0.32 b | 1.43 ± 0.11 b |
| CPC 50/50 II | −57.72 ± 0.16 a | −23.43 ± 1.82 a | - | −261.92 ± 11.09 b | 134.02 ± 0.87 e | 7.46 ± 0.21 b | 98.26 ± 0.12 b | 1.53 ± 0.21 b | 1.12 ± 0.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkiewicz, A.; Budryn, G.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 3633. https://doi.org/10.3390/nu14173633
Jaśkiewicz A, Budryn G, Carmena-Bargueño M, Pérez-Sánchez H. Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies. Nutrients. 2022; 14(17):3633. https://doi.org/10.3390/nu14173633
Chicago/Turabian StyleJaśkiewicz, Andrzej, Grażyna Budryn, Miguel Carmena-Bargueño, and Horacio Pérez-Sánchez. 2022. "Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies" Nutrients 14, no. 17: 3633. https://doi.org/10.3390/nu14173633
APA StyleJaśkiewicz, A., Budryn, G., Carmena-Bargueño, M., & Pérez-Sánchez, H. (2022). Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies. Nutrients, 14(17), 3633. https://doi.org/10.3390/nu14173633





