Mediation Effect of Platelet Traits on Associations of Central Obesity with Aging Biomarkers in Rural Adults of Henan, China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of Obesity Indices
2.4. Measurement of Platelet Traits
2.5. Measurement of Aging Biomarkers
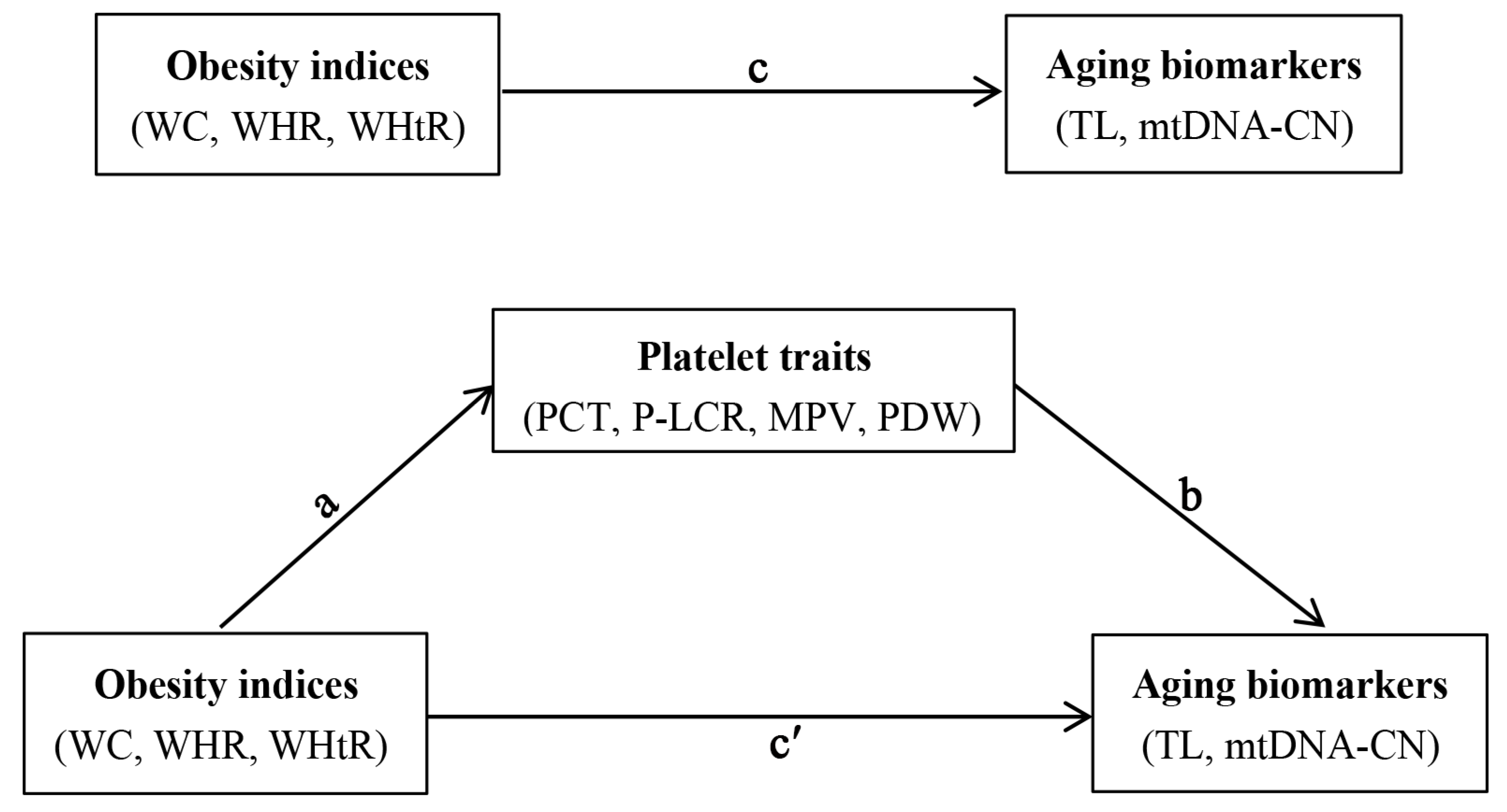
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
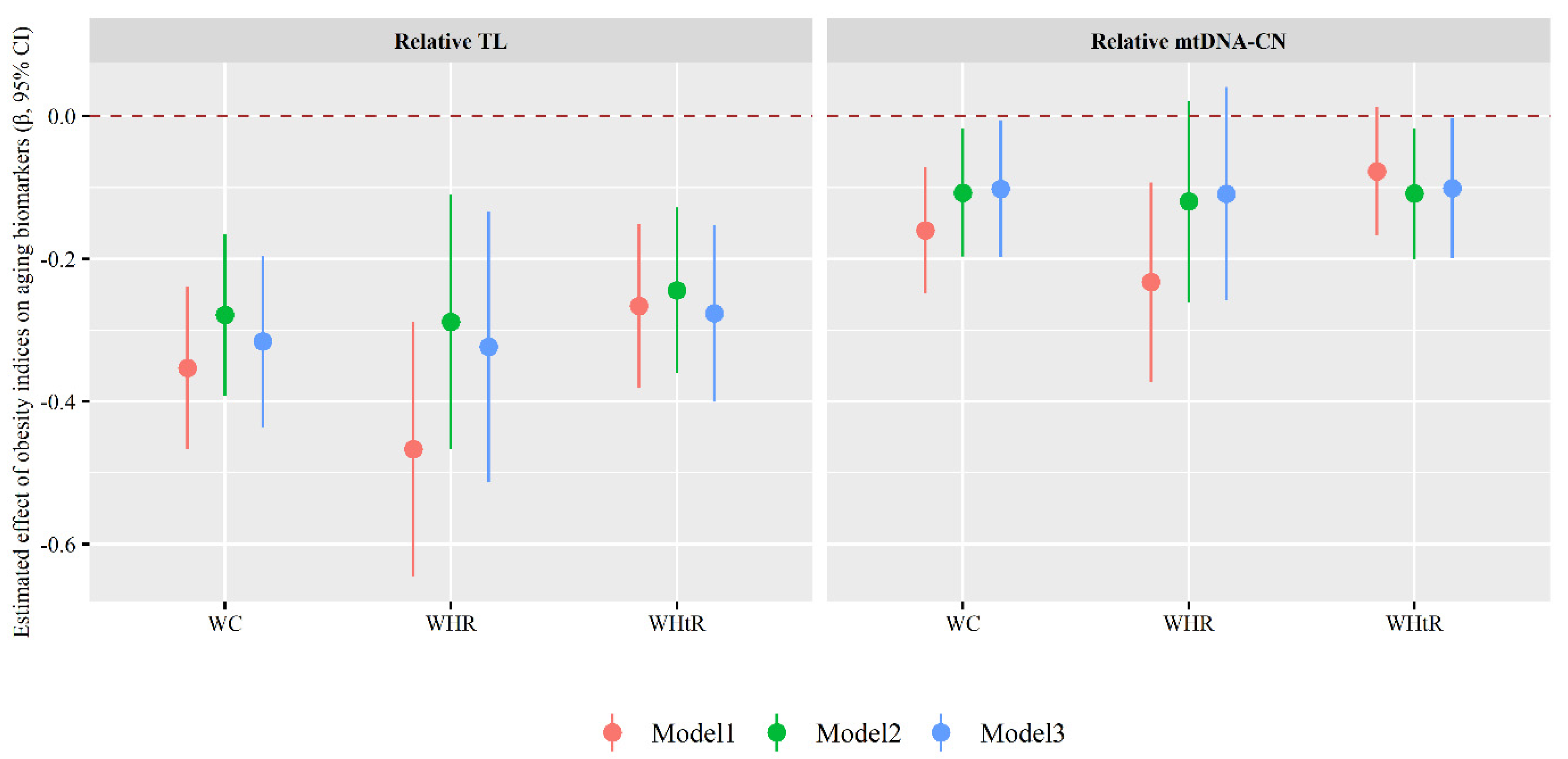
3.2. Association of Obesity Indices with Aging Biomarkers
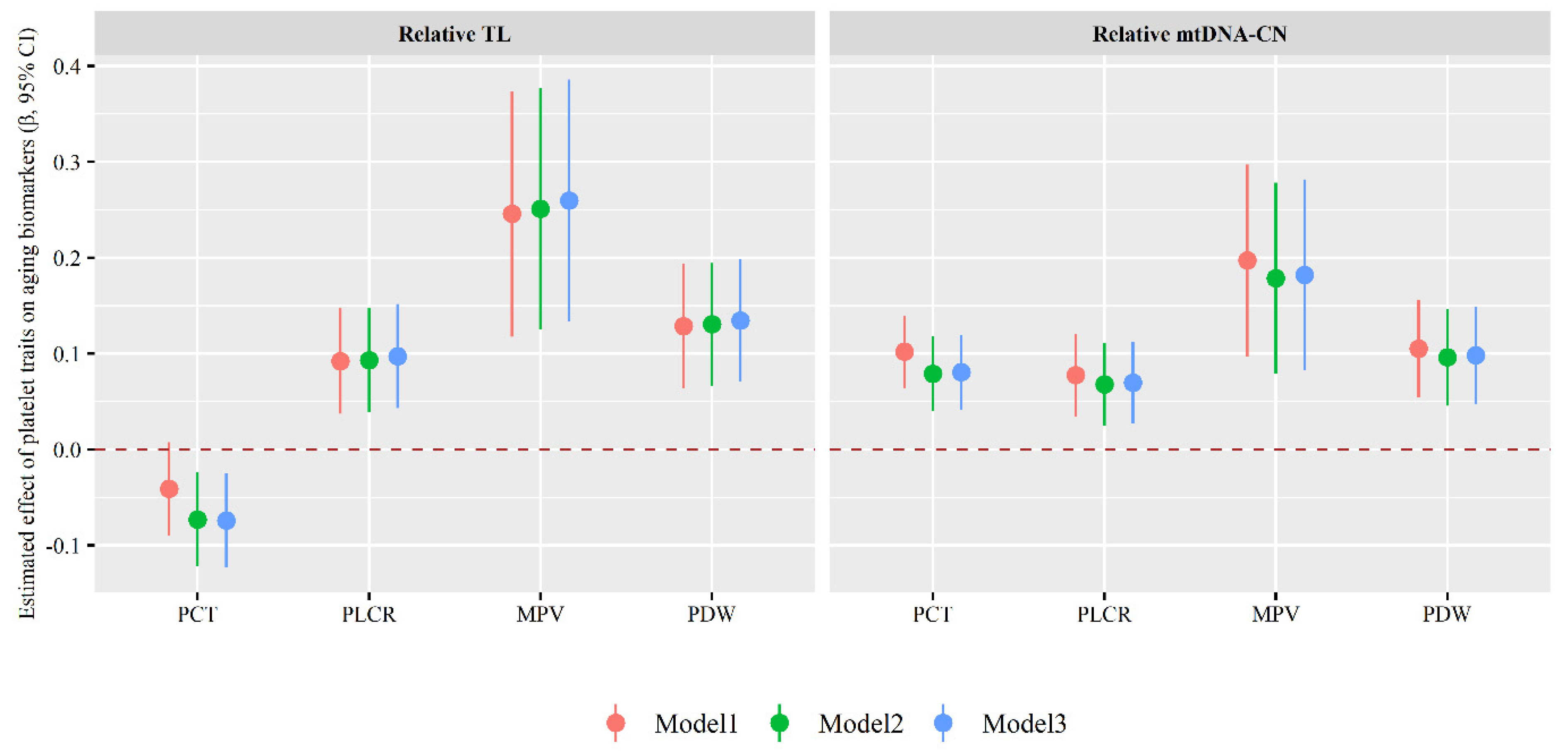
3.3. Association of Platelet Traits with Aging Biomarkers
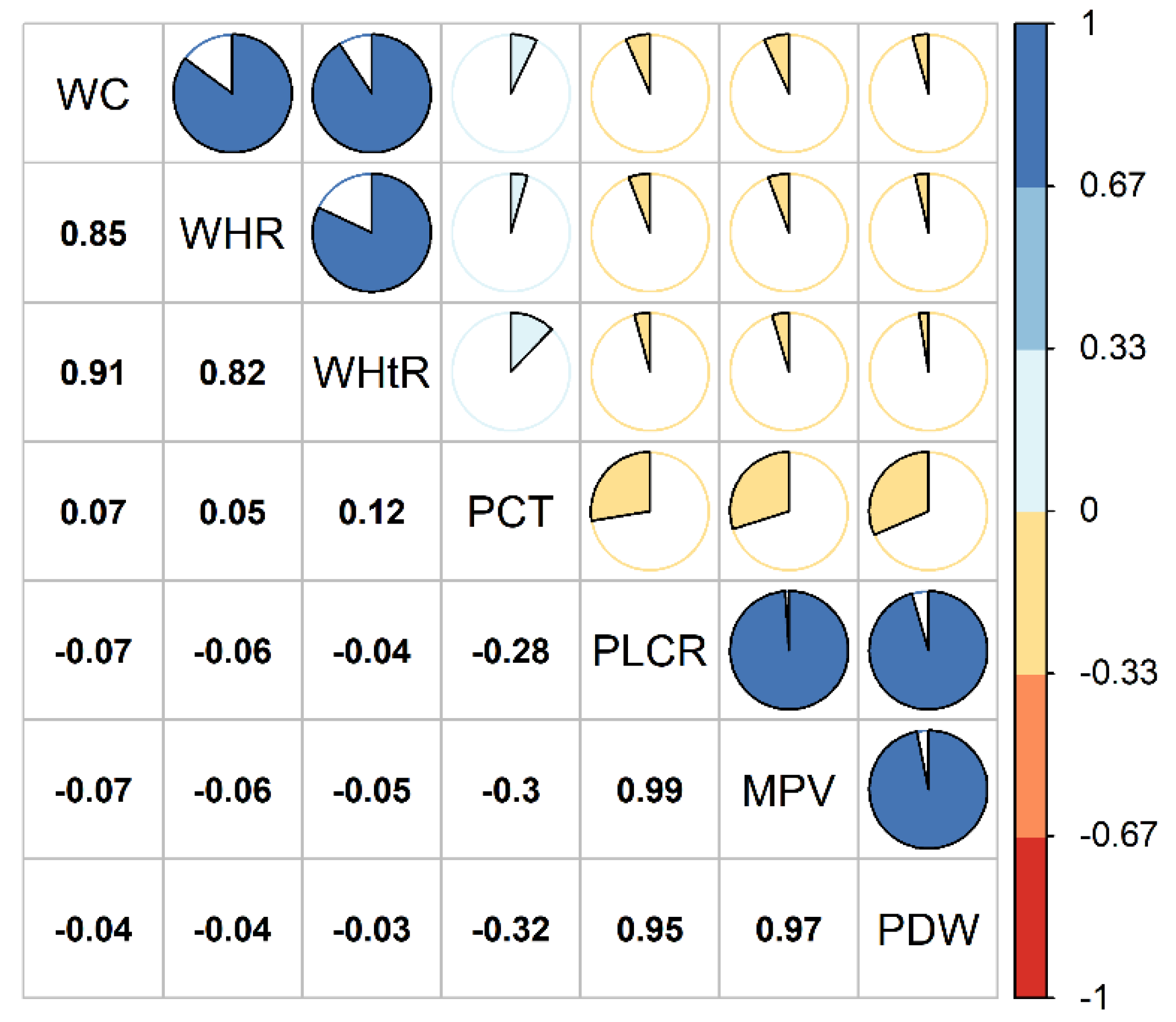
3.4. Association of Obesity Indices with Platelet Traits
3.5. Mediating Role of Platelet Traits in Associations of Obesity Indices with Aging Biomarkers
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Tyagi, T.; Patell, K.; Xie, Y.; Kadado, A.J.; Lee, S.H.; Yarovinsky, T.; Du, J.; Hwang, J.; Martin, K.A.; et al. Age associated non-linear regulation of redox homeostasis in the anucleate platelet: Implications for CVD risk patients. eBioMedicine 2019, 44, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.-C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef] [PubMed]
- Lejawa, M.; Osadnik, K.; Osadnik, T.; Pawlas, N. Association of Metabolically Healthy and Unhealthy Obesity Phenotypes with Oxidative Stress Parameters and Telomere Length in Healthy Young Adult Men. Analysis of the MAGNETIC Study. Antioxidants 2021, 10, 93. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal. 2019, 13, 303–318. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Zhelankin, A.V.; Sinyov, V.V.; Bobryshev, Y.V.; Orekhov, A.N. Mitochondrial Aging: Focus on Mitochondrial DNA Damage in Atherosclerosis—A Mini-Review. Gerontology 2015, 61, 343–349. [Google Scholar] [CrossRef]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- Cleal, K.; Norris, K.; Baird, D. Telomere Length Dynamics and the Evolution of Cancer Genome Architecture. Int. J. Mol. Sci. 2018, 19, 482. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andres, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.; Tedone, E.; Ludlow, A.; Huang, E.; Arosio, B.; Mari, D.; Shay, J.W. Quantitative mitochondrial DNA copy number determination using droplet digital PCR with single-cell resolution. Genome Res. 2019, 29, 1878–1888. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. Body mass index and leukocyte telomere length in adults: A systematic review and meta-analysis. Obes. Rev. 2013, 15, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Louzada, F.M.; Grun, L.K.; Jones, M.; Guma, F.T.C.R.; Mazzola, J.; Epifanio, M.; Stein, R.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef]
- Fazzini, F.; Lamina, C.; Raftopoulou, A.; Koller, A.; Fuchsberger, C.; Pattaro, C.; Del Greco, F.M.; Döttelmayer, P.; Fendt, L.; Fritz, J.; et al. Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14,176 individuals. J. Intern. Med. 2021, 290, 190–202. [Google Scholar] [CrossRef]
- Munusamy, S.; do Carmo, J.M.; Hosler, J.P.; Hall, J.E. Obesity-induced changes in kidney mitochondria and endoplasmic reticulum in the presence or absence of leptin. Am. J. Physiol. Renal Physiol. 2015, 309, F731–F743. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Litvinova, L.; Vulf, M.; Zatolokin, P.; Popadin, K.; Mazunin, I. From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index. Cells 2019, 8, 430. [Google Scholar] [CrossRef]
- Yilmaz, M.A.; Duran, C.; Basaran, M. The mean platelet volume and neutrophil to lymphocyte ratio in obese and lean patients with polycystic ovary syndrome. J. Endocrinol. Investig. 2015, 39, 45–53. [Google Scholar] [CrossRef]
- Muscari, A.; De Pascalis, S.; Ludovico, C.; Castaldini, N.; Antonelli, S.; Bianchi, G.; Magalotti, D.; Zoli, M.; Cenni, A. Determinants of mean platelet volume (MPV) in an elderly population: Relevance of body fat, blood glucose and ischaemic electrocardiographic changes. Thromb. Haemost. 2008, 99, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, C.; Yao, P.; Chen, W.; He, M.; Wang, Y.; Liang, Y.; Miao, X.; Wei, S.; Xu, T.; et al. Association of Adiposity Indices with Platelet Distribution Width and Mean Platelet Volume in Chinese Adults. PLoS ONE 2015, 10, e0129677. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davì, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2011, 13, 27–42. [Google Scholar] [CrossRef]
- Erdal, E.; Inanir, M. Platelet-to-lymphocyte ratio (PLR) and Plateletcrit (PCT) in young patients with morbid obesity. Rev. Assoc. Med. Bras. 2019, 65, 1182–1187. [Google Scholar] [CrossRef]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. RETRACTED: Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 72, 1218–1225. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Z.; Li, Y.; Wu, W.; Zhang, X.; Huo, W.; Yu, S.; Shen, L.; Li, L.; Tu, R.; et al. Cohort Profile: The Henan Rural Cohort: A prospective study of chronic non-communicable diseases. Int. J. Epidemiol. 2019, 48, 1756. [Google Scholar] [CrossRef]
- Li, R.; Li, S.; Pan, M.; Chen, H.; Liu, X.; Chen, G.; Chen, R.; Mao, Z.; Huo, W.; Wang, X.; et al. Physical activity attenuated the association of air pollutants with telomere length in rural Chinese adults. Sci. Total Environ. 2020, 759, 143491. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, S.; Pan, M.; Chen, H.; Liu, X.; Chen, G.; Chen, R.; Yin, S.; Mao, Z.; Huo, W.; et al. Physical activity counteracted associations of exposure to mixture of air pollutants with mitochondrial DNA copy number among rural Chinese adults. Chemosphere 2021, 272, 129907. [Google Scholar] [CrossRef]
- Pieters, N.; Janssen, B.G.; Dewitte, H.; Cox, B.; Cuypers, A.; Lefebvre, W.; Smeets, K.; Vanpoucke, C.; Plusquin, M.; Nawrot, T.S. Biomolecular Markers within the Core Axis of Aging and Particulate Air Pollution Exposure in the Elderly: A Cross-Sectional Study. Environ. Health Perspect. 2016, 124, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yin, W.; Li, P.; Hu, C.; Zhang, Y.; Wang, X.; Wang, G.; Gao, E.; Zhang, J.; Wang, L.; et al. Seasonal modification of the associations of exposure to polycyclic aromatic hydrocarbons or phthalates of cellular aging. Ecotoxicol. Environ. Saf. 2019, 182, 109384. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, N.K.; Sleiman, F.; Nasreddine, L.; Nasrallah, M.; Nakhoul, N.; Isma’eel, H.; Tamim, H. Short Telomere Length is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging Dis. 2018, 9, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Bethancourt, H.J.; Kratz, M.; Beresford, S.A.A.; Hayes, M.G.; Kuzawa, C.W.; Duazo, P.L.; Borja, J.B.; Eisenberg, D.T.A. No association between blood telomere length and longitudinally assessed diet or adiposity in a young adult Filipino population. Eur. J. Nutr. 2015, 56, 295–308. [Google Scholar] [CrossRef]
- Kozlitina, J.; Garcia, C.K. Red Blood Cell Size Is Inversely Associated with Leukocyte Telomere Length in a Large Multi-Ethnic Population. PLoS ONE 2012, 7, e51046. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Shin, Y.-A. How Does Obesity and Physical Activity Affect Aging? Focused on Telomere as a Biomarker of Aging. J. Obes. Metab. Syndr. 2019, 28, 92–104. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Lanaspa, M.A.; Kuwabara, M.; Orlicky, D.J.; Cicerchi, C.; Bales, E.; Garcia, G.E.; Roncal-Jimenez, C.A.; Sato, Y.; Johnson, R.J. Obesity causes renal mitochondrial dysfunction and energy imbalance and accelerates chronic kidney disease in mice. Am. J. Physiol. Physiol. 2019, 317, F941–F948. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Braud, L.; Palacios-Ramirez, R.; Karlsson, S.; Boujardine, N.; Motterlini, R.; Jaisser, F.; Cat, A.N.D. MR (Mineralocorticoid Receptor) Induces Adipose Tissue Senescence and Mitochondrial Dysfunction Leading to Vascular Dysfunction in Obesity. Hypertension 2019, 73, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cui, N.-H.; Zhang, S.; Wang, X.-B.; Ming, L. Leukocyte Mitochondrial DNA Copy Number and Risk of Thyroid Cancer: A Two-Stage Case-Control Study. Front. Endocrinol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Meyer, A.; Salewsky, B.; Buchmann, N.; Steinhagen-Thiessen, E.; Demuth, I. Relative Leukocyte Telomere Length, Hematological Parameters and Anemia—Data from the Berlin Aging Study II (BASE-II). Gerontology 2016, 62, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Storey, R. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Quan, Y.; Xin, Y.; Tian, G.; Zhou, J.; Liu, X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxidative Med. Cell. Longev. 2020, 2020, 9423593. [Google Scholar] [CrossRef]
- Qi, A.; Zhou, H.; Zhou, Z.; Huang, X.; Ma, L.; Wang, H.; Yang, Y.; Zhang, D.; Li, H.; Ren, R.; et al. Telomerase activity increased and telomere length shortened in peripheral blood cells from patients with immune thrombocytopenia. J. Clin. Immunol. 2013, 33, 577–585. [Google Scholar] [CrossRef]
- Mollica, L.; Fleury, I.; Belisle, C.; Provost, S.; Roy, D.-C.; Busque, L. No Association Between Telomere Length and Blood Cell Counts in Elderly Individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 965–967. [Google Scholar] [CrossRef]
- Zribi, B.; Uziel, O.; Lahav, M.; Stahy, R.M.; Singer, P. Telomere Length Changes during Critical Illness: A Prospective, Observational Study. Genes 2019, 10, 761. [Google Scholar] [CrossRef]
- Trovati, M.; Anfossi, G. Mechanisms involved in platelet hyperactivation and platelet-endothelium interrelationships in diabetes mellitus. Curr. Diabetes Rep. 2002, 2, 316–322. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Trovati, M. Platelet dysfunction in central obesity. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.A.; Nesto, R.W. The platelet in diabetes: Focus on prevention of ischemic events. Diabetes Care 2003, 26, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.S.; Dayal, S. Modulators of platelet function in aging. Platelets 2019, 31, 474–482. [Google Scholar] [CrossRef]
- Elaib, Z.; Lopez, J.J.; Coupaye, M.; Zuber, K.; Becker, Y.; Kondratieff, A.; Repérant, C.; Pépin, M.; Salomon, L.; Teillet, F.; et al. Platelet Functions are Decreased in Obesity and Restored after Weight Loss: Evidence for a Role of the SERCA3-Dependent ADP Secretion Pathway. Thromb. Haemost. 2019, 119, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Longchamps, R.J.; Yang, S.Y.; Castellani, C.A.; Shi, W.; Lane, J.; Grove, M.L.; Bartz, T.M.; Sarnowski, C.; Liu, C.; Burrows, K.; et al. Genome-wide analysis of mitochondrial DNA copy number reveals loci implicated in nucleotide metabolism, platelet activation, and megakaryocyte proliferation. Hum. Genet. 2021, 141, 127–146. [Google Scholar] [CrossRef]

| Characteristic | Median (Q25, Q75) or Number (%) |
|---|---|
| Age (years) a | 55.0 (49.0, 64.0) |
| Gender (Men) b | 2138 (42.0) |
| Average monthly income (yuan, Chinese RMB) b | |
| <500 | 1650 (32.4) |
| 500~999 | 1549 (30.4) |
| ≥1000 | 1892 (37.2) |
| Education level b | |
| Elementary school or below | 2420 (47.5) |
| Junior high school | 2151 (42.3) |
| High school or above | 520 (10.2) |
| Marital status b | |
| Married/Cohabitation | 4566 (89.7) |
| Unmarried/divorced/widowed | 525 (10.3) |
| Smoking status b | |
| Never smokers | 3639 (71.5) |
| Former smokers | 410 (8.1) |
| Current smokers | 1042 (20.5) |
| Alcohol consumption b | |
| Never drinkers | 3971 (78.0) |
| Former drinkers | 325 (6.4) |
| Current drinkers | 795 (15.6) |
| Physical activity b | |
| Low | 1126 (22.1) |
| Moderate | 2557 (50.2) |
| High | 1408 (27.7) |
| High fat diet b | 1382 (27.1) |
| More vegetable and fruit intake b | 3391 (66.6) |
| T2DM b | 269 (5.3) |
| Hypertension b | 1200 (23.6) |
| Hyperlipidemia b | 1682 (33.0) |
| Family history of T2DM b | 113 (2.2) |
| Family history of hypertension b | 948 (18.6) |
| Family history of hyperlipidemia b | 228 (4.5) |
| Variables | Mean | Q25 | Median | Q75 | IQR |
|---|---|---|---|---|---|
| Obesity indices | |||||
| WC (cm) | 82.81 | 75.90 | 82.80 | 89.60 | 13.70 |
| WHR (%) | 0.88 | 0.84 | 0.88 | 0.93 | 0.09 |
| WHtR (%) | 0.52 | 0.47 | 0.52 | 0.56 | 0.09 |
| Platelet traits | |||||
| PCT (%) | 0.23 | 0.19 | 0.23 | 0.27 | 0.08 |
| P-LCR (%) | 42.03 | 34.60 | 41.80 | 49.40 | 14.80 |
| MPV (fl) | 12.20 | 11.20 | 12.10 | 13.20 | 2.00 |
| PDW (fl) | 16.27 | 13.50 | 15.70 | 18.60 | 5.10 |
| Aging biomarkers | |||||
| Relative TL | 0.72 | 0.43 | 0.60 | 0.92 | 0.49 |
| Relative mtDNA-CN | 0.85 | 0.63 | 0.78 | 1.00 | 0.37 |
| Relative TL | Relative mtDNA-CN | |||||
|---|---|---|---|---|---|---|
| Total Effect (95% CI) | Indirect Effect (95% CI) | Proportion of Mediation (%) | Total Effect (95% CI) | Indirect Effect (95% CI) | Proportion of Mediation (%) | |
| PCT | ||||||
| WC | −0.316 (−0.437, −0.195) | −0.011 (−0.022, −0.004) | 3.48 | −0.097 (−0.192, −0.001) | 0.013 (0.005, 0.024) | - a |
| WHR | −0.324 (−0.514, −0.134) | −0.018 (−0.035, −0.007) | 5.56 | −0.100 (−0.249, 0.050) | 0.020 (0.009, 0.037) | - a |
| WHtR | −0.280 (−0.404, −0.156) | −0.013 (−0.025, −0.005) | 4.64 | −0.099 (−0.197, −0.001) | 0.015 (0.007, 0.026) | - a |
| P-LCR | ||||||
| WC | −0.316 (−0.437, −0.195) | −0.010 (−0.021, −0.003) | 3.16 | −0.097 (−0.192, −0.001) | −0.008 (−0.016, −0.003) | 8.25 |
| WHR | −0.324 (−0.514, −0.134) | −0.016 (−0.033, −0.006) | 4.94 | −0.100 (−0.249, 0.050) | −0.012 (−0.025, −0.004) | - a |
| WHtR | −0.280 (−0.404, −0.156) | −0.012 (−0.023, −0.004) | 4.29 | −0.099 (−0.197, −0.001) | −0.009 (−0.018, −0.003) | 9.09 |
| MPV | ||||||
| WC | −0.316 (−0.437, −0.195) | −0.012 (−0.024, −0.005) | 3.80 | −0.097 (−0.192, −0.001) | −0.009 (−0.018, −0.003) | 9.28 |
| WHR | −0.324 (−0.514, −0.134) | −0.019 (−0.036, −0.007) | 5.86 | −0.100 (−0.249, 0.050) | −0.013 (−0.027, −0.005) | - a |
| WHtR | −0.280 (−0.404, −0.156) | −0.014 (−0.026, −0.006) | 5.00 | −0.099 (−0.197, −0.001) | −0.010 (−0.019, −0.004) | 10.10 |
| PDW | ||||||
| WC | −0.316 (−0.437, −0.195) | −0.009 (−0.019, −0.002) | 2.85 | −0.097 (−0.192, −0.001) | −0.006 (−0.014, −0.002) | 6.19 |
| WHR | −0.324 (−0.514, −0.134) | −0.013 (−0.029, −0.004) | 4.01 | −0.100 (−0.249, 0.050) | −0.010 (−0.022, −0.002) | - a |
| WHtR | −0.280 (−0.404, −0.156) | −0.010 (−0.021, −0.003) | 3.57 | −0.099 (−0.197, −0.001) | −0.008 (−0.016, −0.002) | 8.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, R.; Hou, X.; Wang, Y.; Pan, M.; Kang, N.; Yuchi, Y.; Liao, W.; Liu, X.; Mao, Z.; et al. Mediation Effect of Platelet Traits on Associations of Central Obesity with Aging Biomarkers in Rural Adults of Henan, China. Nutrients 2022, 14, 3597. https://doi.org/10.3390/nu14173597
Chen X, Li R, Hou X, Wang Y, Pan M, Kang N, Yuchi Y, Liao W, Liu X, Mao Z, et al. Mediation Effect of Platelet Traits on Associations of Central Obesity with Aging Biomarkers in Rural Adults of Henan, China. Nutrients. 2022; 14(17):3597. https://doi.org/10.3390/nu14173597
Chicago/Turabian StyleChen, Xinwei, Ruiying Li, Xiaoyu Hou, Yuqin Wang, Mingming Pan, Ning Kang, Yinghao Yuchi, Wei Liao, Xiaotian Liu, Zhenxing Mao, and et al. 2022. "Mediation Effect of Platelet Traits on Associations of Central Obesity with Aging Biomarkers in Rural Adults of Henan, China" Nutrients 14, no. 17: 3597. https://doi.org/10.3390/nu14173597
APA StyleChen, X., Li, R., Hou, X., Wang, Y., Pan, M., Kang, N., Yuchi, Y., Liao, W., Liu, X., Mao, Z., Huo, W., Wang, C., & Hou, J. (2022). Mediation Effect of Platelet Traits on Associations of Central Obesity with Aging Biomarkers in Rural Adults of Henan, China. Nutrients, 14(17), 3597. https://doi.org/10.3390/nu14173597








