Association of Pyridoxal 5′-Phosphate with Sleep-Related Problems in a General Population
Abstract
1. Introduction
2. Materials and Methods
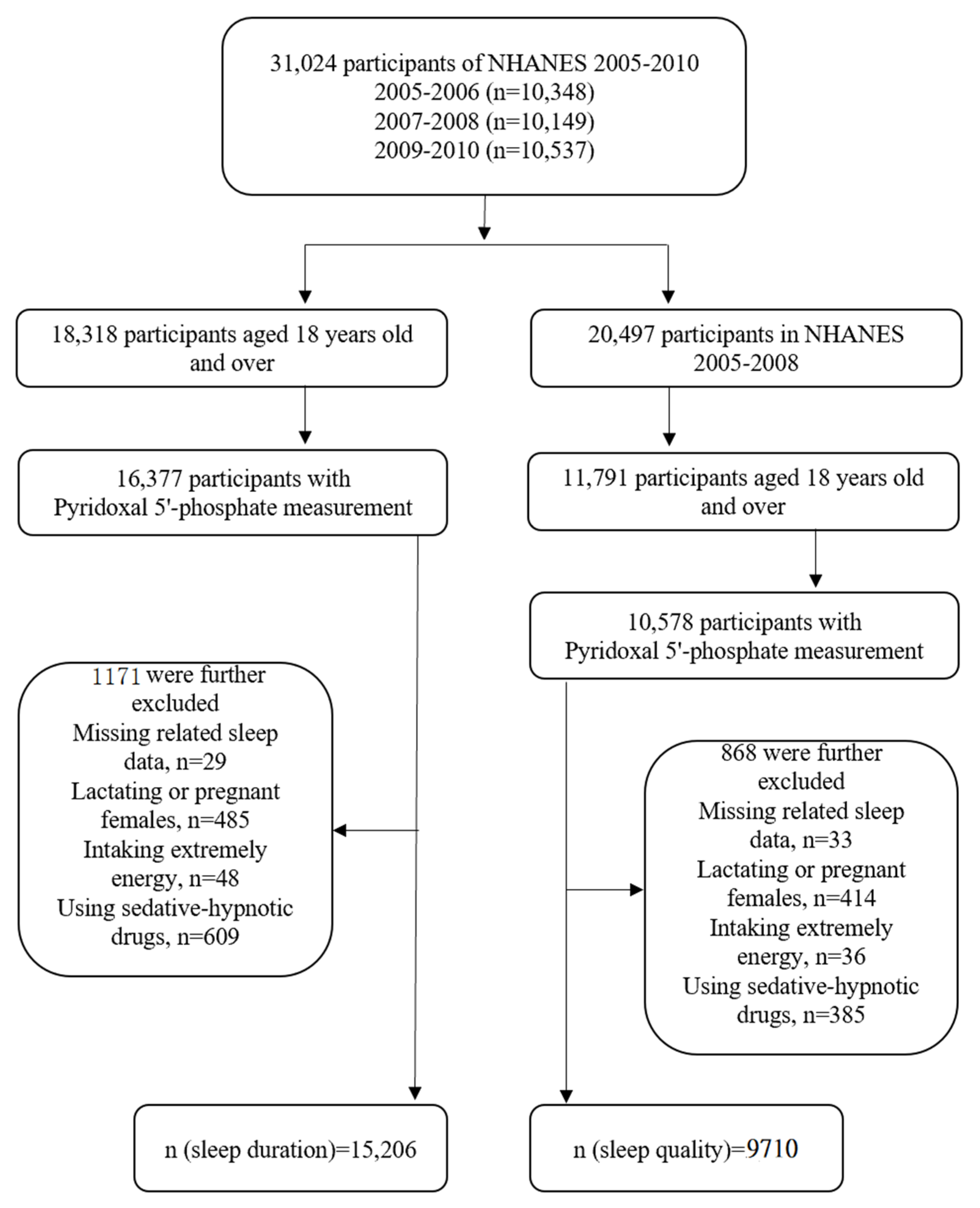
2.1. Participants
2.2. Sleep-Related Problems
2.3. Serum Pyridoxal 5′-Phosphate Measurement
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tempesta, D.; Socci, V.; de Gennaro, L.; Ferrara, M. Sleep and emotional processing. Sleep Med. Rev. 2018, 40, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Gaisl, T.; Miladinovic, D.; Marcinkevics, R.; Osswald, M.; Bauer, S.; Buhmann, J.; Zenobi, R.; Sinues, P.; Brown, S.A.; et al. Rapid and reversible control of human metabolism by individual sleep states. Cell Rep. 2021, 37, 109903. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Albrecht, U.; Ripperger, J.A. Circadian Clocks and Sleep: Impact of Rhythmic Metabolism and Waste Clearance on the Brain. Trends Neurosci. 2018, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Haspel, J.A.; Anafi, R.; Brown, M.K.; Cermakian, N.; Depner, C.; Desplats, P.; Gelman, A.E.; Haack, M.; Jelic, S.; Kim, B.S.; et al. Perfect timing: Circadian rhythms, sleep, and immunity—An NIH workshop summary. JCI Insight 2020, 5, e131487. [Google Scholar] [CrossRef]
- Partinen, M. Sleep research in 2020: COVID-19-related sleep disorders. Lancet Neurol. 2021, 20, 15–17. [Google Scholar] [CrossRef]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef]
- Orr, W.C.; Fass, R.; Sundaram, S.S.; Scheimann, A.O. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 616–624. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Tall, A.R.; Jelic, S. How broken sleep promotes cardiovascular disease. Nature 2019, 566, 329–330. [Google Scholar] [CrossRef]
- Lee, S.W.H.; Ng, K.Y.; Chin, W.K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Med. Rev. 2017, 31, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, H.; Zhang, D. Sleep Duration and Depression Among Adults: A Meta-Analysis of Prospective Studies. Depress. Anxiety 2015, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Lysen, T.S.; Darweesh, S.K.L.; Ikram, M.K.; Luik, A.I.; Ikram, M.A. Sleep and risk of parkinsonism and Parkinson’s disease: A population-based study. Brain A J. Neurol. 2019, 142, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016, 39, 552–566. [Google Scholar] [CrossRef]
- Ashbrook, L.H.; Krystal, A.D.; Fu, Y.H.; Ptacek, L.J. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology 2020, 45, 45–54. [Google Scholar] [CrossRef]
- Billings, M.E.; Hale, L.; Johnson, D.A. Physical and Social Environment Relationship With Sleep Health and Disorders. Chest 2020, 157, 1304–1312. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wang, W.; Dong, X.; Zhang, D. Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults. Nutrients 2020, 12, 2103. [Google Scholar] [CrossRef]
- He, L.; Biddle, S.J.H.; Lee, J.T.; Duolikun, N.; Zhang, L.; Wang, Z.; Zhao, Y. The prevalence of multimorbidity and its association with physical activity and sleep duration in middle aged and elderly adults: A longitudinal analysis from China. Int. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 77. [Google Scholar] [CrossRef]
- Bourke, S.C.; Gibson, G.J. Sleep and breathing in neuromuscular disease. Eur. Respir. J. 2002, 19, 1194–1201. [Google Scholar] [CrossRef]
- Abad, V.C.; Sarinas, P.S.; Guilleminault, C. Sleep and rheumatologic disorders. Sleep Med. Rev. 2008, 12, 211–228. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ge, H.; Sun, J.; Hao, K.; Yao, W.; Zhang, D. Associations of Dietary omega-3, omega-6 Fatty Acids Consumption with Sleep Disorders and Sleep Duration among Adults. Nutrients 2021, 13, 1475. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Bilski, P.; Li, M.Y.; Chignell, C.F.; Daub, M.E. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 9374–9378. [Google Scholar] [CrossRef]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef]
- Coburn, S.P.; Slominski, A.; Mahuren, J.D.; Wortsman, J.; Hessle, L.; Millan, J.L. Cutaneous metabolism of vitamin B-6. J. Investig. Dermatol. 2003, 120, 292–300. [Google Scholar] [CrossRef][Green Version]
- Mitchell, E.S.; Conus, N.; Kaput, J. B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci. Biobehav. Rev. 2014, 47, 307–320. [Google Scholar] [CrossRef]
- Rall, L.C.; Meydani, S.N. Vitamin B6 and immune competence. Nutr. Rev. 1993, 51, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Tullidge, G.M. Pyridoxine (vitamin B6) in epilepsy; a clinical trial. Lancet 1946, 2, 345. [Google Scholar] [CrossRef]
- Darin, N.; Reid, E.; Prunetti, L.; Samuelsson, L.; Husain, R.A.; Wilson, M.; El Yacoubi, B.; Footitt, E.; Chong, W.K.; Wilson, L.C.; et al. Mutations in PROSC Disrupt Cellular Pyridoxal Phosphate Homeostasis and Cause Vitamin-B6-Dependent Epilepsy. Am. J. Hum. Genet. 2016, 99, 1325–1337. [Google Scholar] [CrossRef]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, O.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef]
- Knott, P.J.; Curzon, G. Free tryptophan in plasma and brain tryptophan metabolism. Nature 1972, 239, 452–453. [Google Scholar] [CrossRef]
- Bernstein, A.L. Vitamin B6 in clinical neurology. Ann. N. Y. Acad. Sci. 1990, 585, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Coon, S.L.; Roseboom, P.H.; Weller, J.L.; Bernard, M.; Gastel, J.A.; Zatz, M.; Iuvone, P.M.; Rodriguez, I.R.; Bégay, V.; et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 1997, 52, 307–357; discussion 357–358. [Google Scholar]
- Lee, B.H.; Hille, B.; Koh, D.S. Serotonin modulates melatonin synthesis as an autocrine neurotransmitter in the pineal gland. Proc. Natl. Acad. Sci. USA 2021, 118, e2113852118. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wahlqvist, M.L.; Lee, M.S. Sleep quality in the survival of elderly taiwanese: Roles for dietary diversity and pyridoxine in men and women. J. Am. Coll. Nutr. 2013, 32, 417–427. [Google Scholar] [CrossRef]
- Lemoine, P.; Bablon, J.C.; da Silva, C. A combination of melatonin, vitamin B6 and medicinal plants in the treatment of mild-to-moderate insomnia: A prospective pilot study. Complement. Ther. Med. 2019, 45, 104–108. [Google Scholar] [CrossRef]
- Scholey, A.; Benson, S.; Gibbs, A.; Perry, N.; Sarris, J.; Murray, G. Exploring the Effect of Lactium and Zizyphus Complex on Sleep Quality: A Double-Blind, Randomized Placebo-Controlled Trial. Nutrients 2017, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Aspy, D.J.; Madden, N.A.; Delfabbro, P. Effects of Vitamin B6 (Pyridoxine) and a B Complex Preparation on Dreaming and Sleep. Percept. Mot. Ski. 2018, 125, 451–462. [Google Scholar] [CrossRef] [PubMed]
- CDC-NCHS. NHANES. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 19 April 2022).
- Fantus, R.J.; Packiam, V.T.; Wang, C.H.; Erickson, B.A.; Helfand, B.T. The Relationship between Sleep Disorders and Lower Urinary Tract Symptoms: Results from the NHANES. J. Urol. 2018, 200, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Roxbury, C.R.; Qiu, M.; Shargorodsky, J.; Lin, S.Y. Association between allergic rhinitis and poor sleep parameters in U.S. adults. Int. Forum Allergy Rhinol. 2018, 8, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, L.; Rao, M.N.; Schillinger, D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005–2008. Prev. Chronic. Dis. 2012, 9, E76. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C.; Feroe, A.G.; Attanasio, R. Antimony and sleep-related disorders: NHANES 2005-2008. Environ Res 2017, 156, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, H.A.; Beydoun, M.A.; Jeng, H.A.; Zonderman, A.B.; Eid, S.M. Bisphenol-A and Sleep Adequacy among Adults in the National Health and Nutrition Examination Surveys. Sleep 2016, 39, 467–476. [Google Scholar] [CrossRef]
- NHANES. Measurement of Vitamin B6. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/labmethods/vit_b6_e_met.pdf (accessed on 19 April 2022).
- Burgard, S.A.; Ailshire, J.A. Gender and Time for Sleep among U.S. Adults. Am. Sociol. Rev. 2013, 78, 51–69. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann. Med. 1999, 31, 130–140. [Google Scholar] [CrossRef]
- Garcia-Garcia, C.; Baik, I. Effects of poly-gamma-glutamic acid and vitamin B6 supplements on sleep status: A randomized intervention study. Nutr. Res. Pract. 2021, 15, 309–318. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Ophir, U.; Nave, R.; Epstein, R.; Shen-Orr, Z.; Herer, P. The effect of pyridoxine administration on melatonin secretion in normal men. Neuro Endocrinol. Lett. 2002, 23, 213–217. [Google Scholar] [PubMed]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef]
- Dakshinamurti, K.; Paulose, C.S.; Viswanathan, M.; Siow, Y.L.; Sharma, S.K.; Bolster, B. Neurobiology of pyridoxine. Ann. N.Y. Acad. Sci. 1990, 585, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Bowling, F.G. Pyridoxine supply in human development. Semin. Cell Dev. Biol. 2011, 22, 611–618. [Google Scholar] [CrossRef]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.L.; Licinio, J.; Gold, P.W.; et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Paul, L.; Ueland, P.M.; Selhub, J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr. Rev. 2013, 71, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Stubbs, B.; Kohler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med. Rev. 2018, 41, 255–265. [Google Scholar] [CrossRef]
- Mahfouz, M.M.; Zhou, S.Q.; Kummerow, F.A. Vitamin B6 compounds are capable of reducing the superoxide radical and lipid peroxide levels induced by H2O2 in vascular endothelial cells in culture. International J. Vitam. Nutr. Res. 2009, 79, 218–229. [Google Scholar] [CrossRef]
- Danielyan, K.E.; Simonyan, A.A. Protective abilities of pyridoxine in experimental oxidative stress settings in vivo and in vitro. Biomed. Pharmacother. 2017, 86, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Hustad, S.; Schneede, J.; Vollset, S.E.; Ueland, P.M. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am. J. Clin. Nutr. 2007, 86, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, A.; de Lecea, L.; Jennings, K.J. Neurobiological and Hormonal Mechanisms Regulating Women’s Sleep. Front Neurosci. 2020, 14, 625397. [Google Scholar] [CrossRef]
- Mong, J.A.; Cusmano, D.M. Sex differences in sleep: Impact of biological sex and sex steroids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150110. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 2000, 17, 285–311. [Google Scholar] [CrossRef]


| Characteristics | Quartiles Plasma Pyridoxal 5′-Phosphate (PLP) (nmol/L) | p-Value | |||
|---|---|---|---|---|---|
| Q1 (<26.4) | Q2 (26.4–43.4) | Q3 (43.4–74.8) | Q4 (≥74.8) | ||
| n = 3804 | n = 3815 | n = 3790 | n = 3797 | ||
| Gender (%) a | |||||
| Male | 1533 (37.63) | 1891 (48.66) | 2209 (57.24) | 2120 (53.65) | <0.001 |
| Female | 2271 (62.37) | 1924 (51.33) | 1581 (42.76) | 1677 (46.35) | |
| Age (%) a | |||||
| 18–39 years | 1125 (31.83) | 1579 (42.66) | 1630 (43.01) | 1351 (37.15) | <0.001 |
| 40–59 years | 1222 (40.39) | 1212 (38.60) | 1157 (37.70) | 1103 (36.62) | |
| ≥60 year | 1457 (27.76) | 1024 (18.74) | 1003 (19.28) | 1343 (26.23) | |
| Race/ethnicity (%) a | |||||
| Mexican American | 610 (7.22) | 828 (10.00) | 836 (9.87) | 598 (6.43) | |
| Other Hispanic | 286 (3.83) | 372 (5.45) | 349 (4.98) | 294 (3.91) | |
| Non-Hispanic White | 1724 (67.54) | 1610 (65.17) | 1723 (69.11) | 2115 (76.13) | <0.001 |
| Non-Hispanic Black | 1058 (16.83) | 817 (12.33) | 683 (9.53) | 602 (7.45) | |
| Other races | 126 (4.58) | 188 (7.05) | 199 (6.51) | 188 (6.08) | |
| Educational level (%) a | |||||
| <high school | 1379 (25.26) | 1254 (22.43) | 1075 (18.57) | 814 (13.05) | <0.001 |
| High school | 1031 (29.87) | 932 (24.94) | 914 (24.18) | 816 (20.76) | |
| >high school | 1386 (44.87) | 1627 (52.63) | 1796 (57.26) | 2163 (66.19) | |
| Ratio of income to poverty (%) a | |||||
| <1 | 959 (18.21) | 808 (14.15) | 695 (11.92) | 503 (7.86) | <0.001 |
| ≥1 | 2845 (81.79) | 3007 (85.84) | 3095 (88.08) | 3294 (92.14) | |
| Marital status (%) a | |||||
| Married/Cohabiting | 1983 (58.91) | 2158 (63.91 | 2218 (66.19) | 2263 (66.23) | <0.001 |
| Windowed/Living alone | 1741 (41.09) | 1514 (36.09) | 1394 (33.81) | 1410 (33.77) | |
| Body mass index (%) a | |||||
| <25 kg/m2 | 971 (26.81) | 1098 (30.02) | 1204 (32.93) | 1371 (38.88) | <0.001 |
| 25 to <30 kg/m2 | 1026 (25.37) | 1218 (31.40) | 1372 (35.88) | 1430 (37.37) | |
| ≥30 kg/m2 | 1807 (47.81) | 1499 (38.58) | 1214 (31.20) | 996 (23.75) | |
| Physical activity (%) a | |||||
| Vigorous | 882 (26.80) | 1284 (37.29) | 1536 (44.33) | 1588 (45.78) | <0.001 |
| Moderate | 1142 (32.22) | 1080 (29.83) | 1086 (30.72) | 1197 (32.61) | |
| Other | 1780 (40.98) | 1450 (32.88) | 1168 (24.95) | 1012 (21.61) | |
| Depressive symptoms (%) a | 397 (10.95) | 279 (6.68) | 201 (4.85) | 179 (3.50) | <0.001 |
| Diabetes (%) a | 861 (18.52) | 616 (11.78) | 505 (10.20) | 492 (9.21) | <0.001 |
| Hypertension (%) a | 2194 (53.96) | 1786 (44.11) | 1726 (44.24) | 1847 (45.17) | <0.001 |
| Caffeine intake (mg/d) b | 103 (212) | 94 (172) | 86 (152) | 96.5 (173) | <0.001 |
| Total energy (kcal/day) b | 1772.5 (180.5) | 1891.5 (166) | 2591 (164) | 1977 (188) | <0.001 |
| Smoke at least 100 cigarettes in life (%) a | 2020 (56.11) | 1677 (48.63) | 1513 (44.21) | 1485 (40.02) | <0.001 |
| Had at least 12 alcohol drinks a year (%) a | 2133 (68.03) | 2261 (75.85) | 2387 (78.23) | 2539 (79.51) | <0.001 |
| Sampling season (%) a | |||||
| November to April | 1825 (42.90) | 1824 (40.04) | 1697 (39.29) | 1595 (37.30) | 0.081 |
| May to October | 1979 (57.10) | 1991 (59.96) | 2093 (60.71) | 2202 (62.70) | |
| Cases/ Participants | Crude | Model 1 a | Model 2 b | |
|---|---|---|---|---|
| Sleep disorders | ||||
| Q1 (<27.3) | 195/2428 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 153/2430 | 0.74 (0.54–1.03) | 0.77 (0.55–1.07) | 0.78 (0.53–1.16) |
| Q3 (44.0 to <76.3) | 141/2425 | 0.67 (0.49–0.90) ** | 0.67 (0.49–0.93) ** | 0.88 (0.61–1.28) |
| Q4 (≥76.3) | 150/2427 | 0.75 (0.55–1.03) | 0.74 (0.53–1.03) | 1.02 (0.66–1.58) |
| Trouble falling asleep | ||||
| Q1 (<27.3) | 447/2428 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 385/2430 | 0.77 (0.66–0.90) ** | 0.81 (0.69–0.96) * | 0.93 (0.76–1.14) |
| Q3 (44.0 to <76.3) | 334/2425 | 0.66 (0.54–0.79) ** | 0.72 (0.58–0.89) ** | 0.93 (0.72–1.19) |
| Q4 (≥76.3) | 342/2427 | 0.70 (0.58–0.84) ** | 0.76 (0.63–0.93) ** | 1.09 (0.87–1.36) |
| Wake up during the night | ||||
| Q1 (<27.3) | 541/2428 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 429/2430 | 0.72 (0.57–0.90) ** | 0.79 (0.63–0.98) ** | 0.92 (0.69–1.22) |
| Q3 (44.0 to <76.3) | 424/2425 | 0.68 (0.59–0.80) ** | 0.77 (0.66–0.90) ** | 0.98 (0.79–1.22) |
| Q4 (≥76.3) | 404/2427 | 0.63 (0.53–0.75) ** | 0.69 (0.58–0.82) ** | 0.89 (0.73–1.10) |
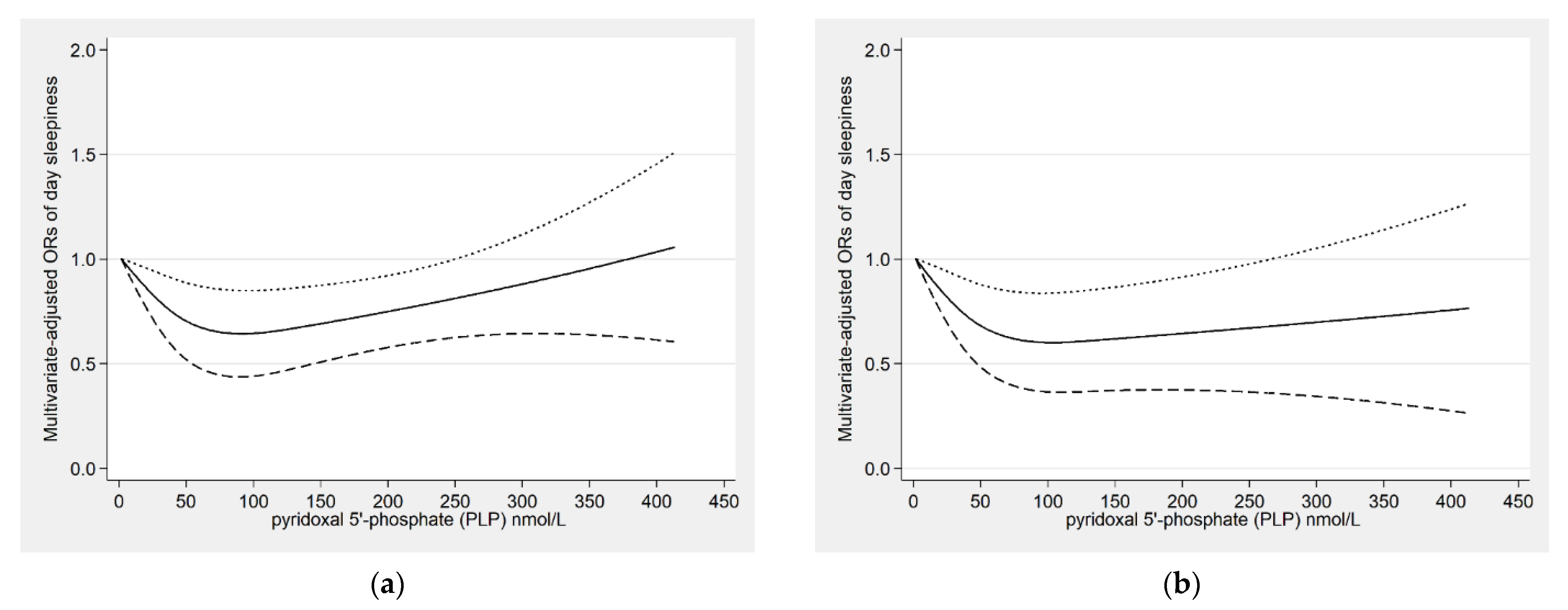
| Daytime sleepiness | ||||
| Q1 (<27.3) | 503/2428 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 382/2430 | 0.66 (0.55–0.79) ** | 0.67 (0.55–0.80) ** | 0.76 (0.59–0.99) * |
| Q3 (44.0 to <76.3) | 368/2425 | 0.62 (0.51–0.75) ** | 0.64 (0.53–0.77) ** | 0.78 (0.62–0.98) * |
| Q4 (≥76.3) | 373/2427 | 0.59 (0.51–0.69) ** | 0.63 (0.54–0.72) ** | 0.80 (0.64–1.00) |
| Model 2 a | ||
|---|---|---|
| Males | Females | |
| Q1 (<27.3) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 0.68 (0.46–1.02) | 0.81 (0.58–1.13) |
| Q3 (44.0 to <76.3) | 0.72 (0.53–0.97) * | 0.82 (0.58–1.16) |
| Q4 (≥76.3) | 0.65 (0.45–0.93) * | 0.95 (0.70–1.30) |
| Model 2 a | |||
|---|---|---|---|
| 18 ≤ Age < 40 Years | 40 ≤ Age < 60 Years | Age ≥ 60 Years | |
| Q1 (<27.3) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (27.3 to <44.0) | 1.03 (0.64–1.64) | 0.93 (0.62–1.40) | 0.68 (0.48–0.97) * |
| Q3 (44.0 to <76.3) | 0.95 (0.66–1.37) | 1.01 (0.69–1.49) | 0.69 (0.49–0.96) * |
| Q4 (≥76.3) | 0.82 (0.52–1.30) | 1.56 (0.97–2.50) | 0.75 (0.51–1.10) |
| Pyridoxal 5′-Phosphate (PLP) (nmol/L) | Model 2 a | ||
|---|---|---|---|
| Very Short Sleep (<5 h/Night) | Short Sleep (5–<7 h/Night) | Long Sleep (≥9 h/Night) | |
| Q1 (<26.4) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (26.4 to <43.4) | 0.73 (0.54–1.00) | 0.79 (0.68–0.92) ** | 0.83 (0.53–1.30) |
| Q3 (43.4 to <74.8) | 0.58 (0.45–0.76) ** | 0.74 (0.65–0.85) ** | 0.62 (0.34–0.94) * |
| Q4 (≥74.8) | 0.58 (0.43–0.81) ** | 0.71 (0.61–0.83) ** | 0.67 (0.40–1.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Luo, J.; Zhang, L.; Kang, X.; Zhang, D. Association of Pyridoxal 5′-Phosphate with Sleep-Related Problems in a General Population. Nutrients 2022, 14, 3516. https://doi.org/10.3390/nu14173516
Ge L, Luo J, Zhang L, Kang X, Zhang D. Association of Pyridoxal 5′-Phosphate with Sleep-Related Problems in a General Population. Nutrients. 2022; 14(17):3516. https://doi.org/10.3390/nu14173516
Chicago/Turabian StyleGe, Lin, Jia Luo, Liming Zhang, Xiao Kang, and Dongfeng Zhang. 2022. "Association of Pyridoxal 5′-Phosphate with Sleep-Related Problems in a General Population" Nutrients 14, no. 17: 3516. https://doi.org/10.3390/nu14173516
APA StyleGe, L., Luo, J., Zhang, L., Kang, X., & Zhang, D. (2022). Association of Pyridoxal 5′-Phosphate with Sleep-Related Problems in a General Population. Nutrients, 14(17), 3516. https://doi.org/10.3390/nu14173516





