Maternal Anemia during the First Trimester and Its Association with Psychological Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
2.3. Statistical Methods
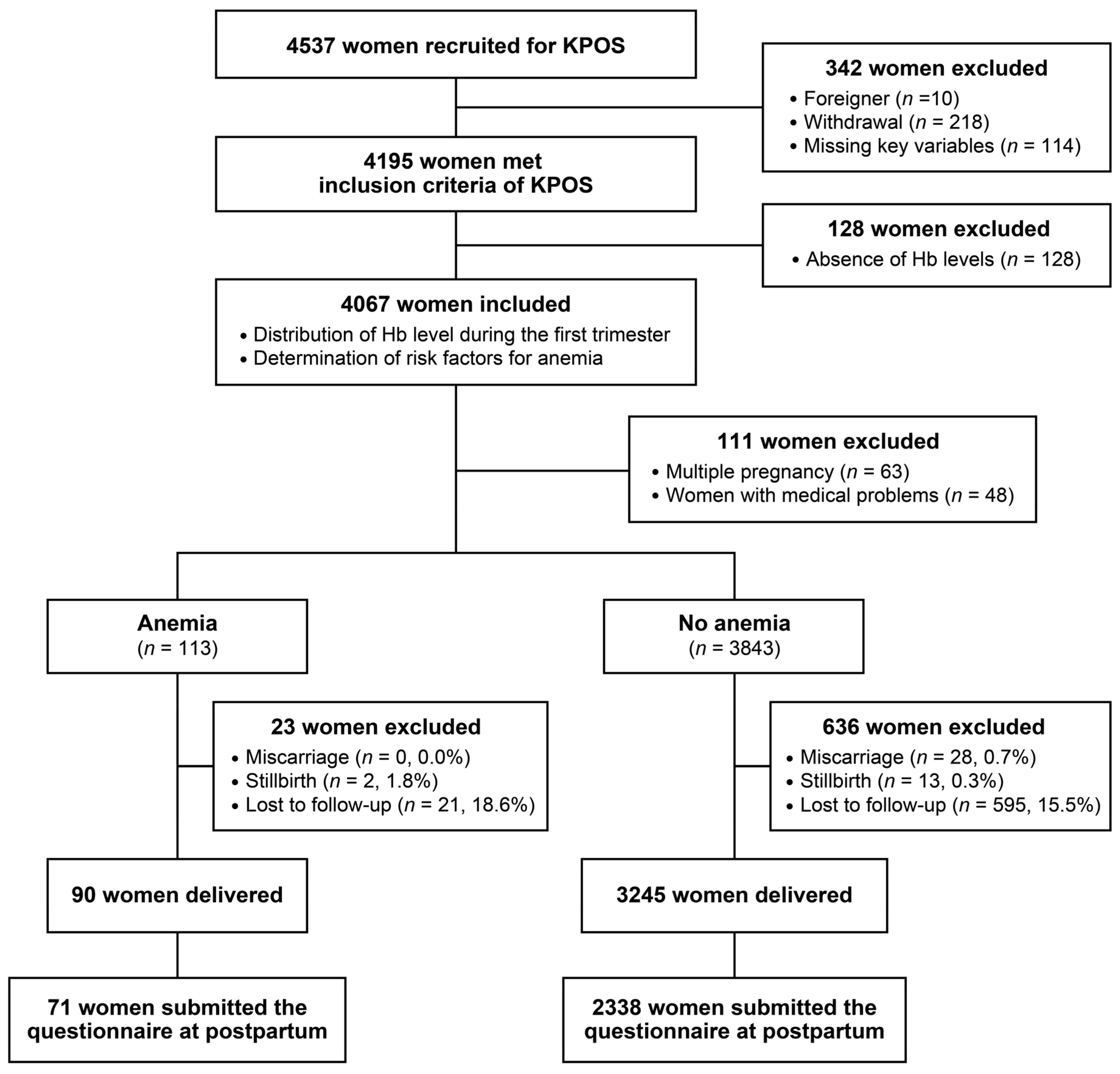
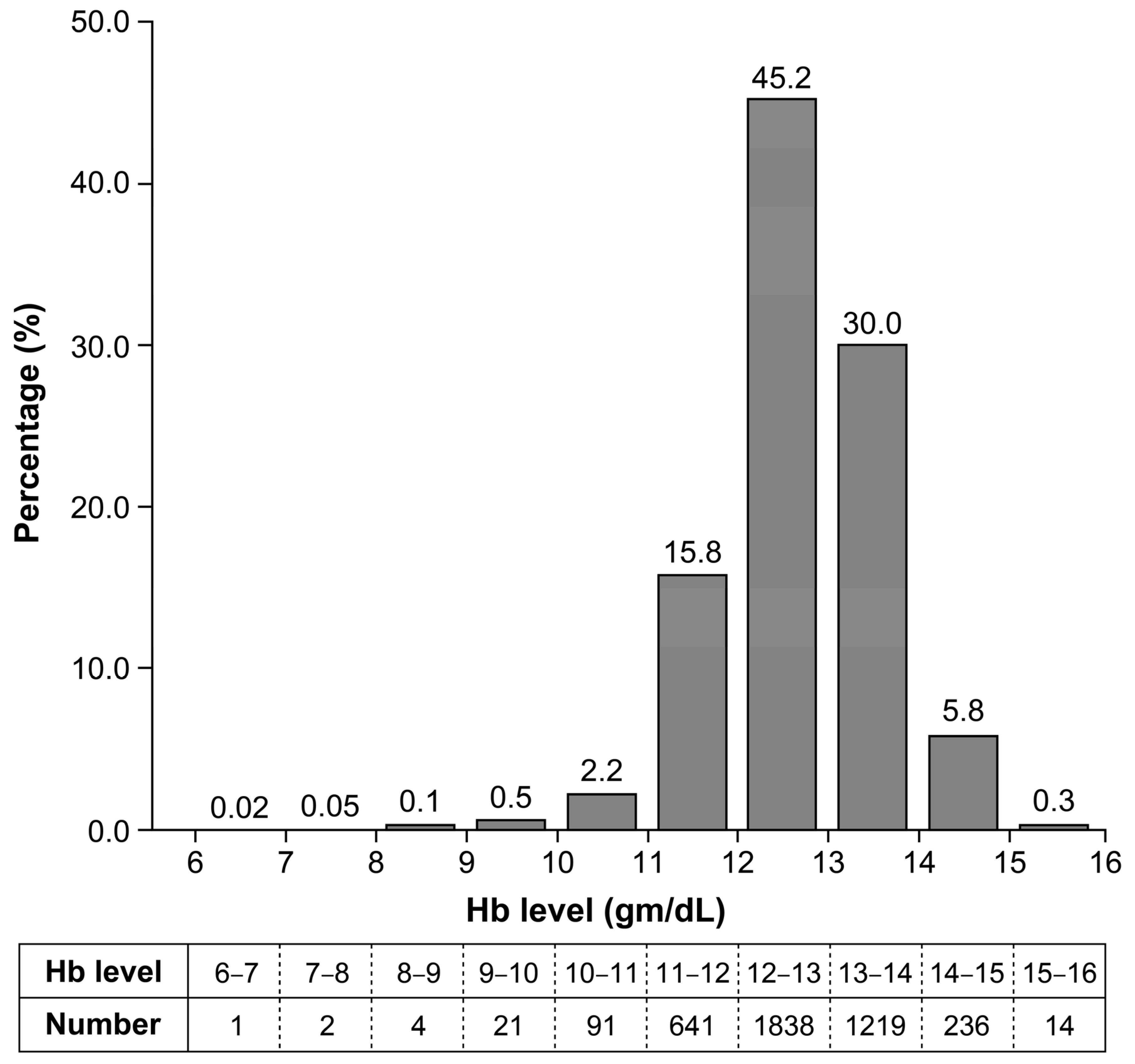
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Anemia in pregnancy: ACOG Practice Bulletin, Number 233. Obstet. Gynecol. 2021, 138, e55–e64. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Buekens, P.; Alexander, S.; Demianczuk, N.; Wollast, E. Anemia during pregnancy and birth outcome: A meta-analysis. Am. J. Perinatol. 2000, 17, 137–146. [Google Scholar] [CrossRef]
- Ren, A.; Wang, J.; Ye, R.W.; Li, S.; Liu, J.M.; Li, Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int. J. Gynaecol. Obstet. 2007, 98, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sukrat, B.; Wilasrusmee, C.; Siribumrungwong, B.; McEvoy, M.; Okascharoen, C.; Attia, J.; Thakkinstian, A. Hemoglobin concentration and pregnancy outcomes: A systematic review and meta-analysis. BioMed Res. Int. 2013, 2013, 769057. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Lowry, E.; Heiskala, A.; Uusitalo, I.; Koivunen, P.; Kajantie, E.; Vääräsmäki, M.; Järvelin, M.R.; Sebert, S. Maternal hemoglobin associates with preterm delivery and small for gestational age in two Finnish birth cohorts. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Woldetensay, Y.K.; Belachew, T.; Biesalski, H.K.; Ghosh, S.; Lacruz, M.E.; Scherbaum, V.; Kantelhardt, E.J. The role of nutrition, intimate partner violence and social support in prenatal depressive symptoms in rural Ethiopia: Community-based birth cohort study. BMC Pregnancy Childbirth 2018, 18, 374. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.R.; Murthy, G.V.S.; Singh, N.; Nath, A.; Rathnaiah, M.; Saldanha, N.; Deepa, R.; Kinra, S. Sociodemographic and medical risk factors associated with antepartum depression. Front. Public Health 2018, 6, 127. [Google Scholar] [CrossRef]
- Yılmaz, E.; Yılmaz, Z.; Çakmak, B.; Gültekin, İ.B.; Çekmez, Y.; Mahmutoğlu, S.; Küçüközkan, T. Relationship between anemia and depressive mood in the last trimester of pregnancy. J. Matern.-Fetal Neonatal Med. 2017, 30, 977–982. [Google Scholar] [CrossRef]
- Xu, F.; Roberts, L.; Binns, C.; Sullivan, E.; Homer, C.S.E. Anaemia and depression before and after birth: A cohort study based on linked population data. BMC Psychiatry 2018, 18, 224. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Abdulghani, H.M. Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr. Dis. Treat. 2014, 10, 311–316. [Google Scholar] [CrossRef]
- Goshtasebi, A.; Alizadeh, M.; Gandevani, S.B. Association between maternal anaemia and postpartum depression in an urban sample of pregnant women in Iran. J. Health Popul. Nutr. 2013, 31, 398–402. [Google Scholar] [CrossRef]
- Azami, M.; Badfar, G.; Khalighi, Z.; Qasemi, P.; Shohani, M.; Soleymani, A.; Abbasalizadeh, S. The association between anemia and postpartum depression: A systematic review and meta-analysis. Casp. J. Intern. Med. 2019, 10, 115–124. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, H.B.; Sunwoo, S. Association between anemia and maternal depression: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 122, 88–96. [Google Scholar] [CrossRef]
- Paterson, J.A.; Davis, J.; Gregory, M.; Holt, S.J.R.; Pachulski, A.; Stamford, D.E.C.; Wothers, J.B.; Jarrett, A. A study on the effects of low haemoglobin on postnatal women. Midwifery 1994, 10, 77–86. [Google Scholar] [CrossRef]
- Armony-Sivan, R.; Shao, J.; Li, M.; Zhao, G.; Zhao, Z.; Xu, G.; Zhou, M.; Zhan, J.; Bian, Y.; Ji, C.; et al. No relationship between maternal iron status and postpartum depression in two samples in China. J. Pregnancy 2012, 2012, 521431. [Google Scholar] [CrossRef]
- Eckerdal, P.; Kollia, N.; Löfblad, J.; Hellgren, C.; Karlsson, L.; Högberg, U.; Wikström, A.K.; Skalkidou, A. Delineating the association between heavy postpartum haemorrhage and postpartum depression. PLoS ONE 2016, 11, e0144274. [Google Scholar] [CrossRef]
- Maeda, Y.; Ogawa, K.; Morisaki, N.; Tachibana, Y.; Horikawa, R.; Sago, H. Association between perinatal anemia and postpartum depression: A prospective cohort study of Japanese women. Int. J. Gynaecol. Obstet. 2020, 148, 48–52. [Google Scholar] [CrossRef]
- Okagbue, H.I.; Adamu, P.I.; Bishop, S.A.; Oguntunde, P.E.; Opanuga, A.A.; Akhmetshin, E.M. Systematic review of prevalence of antepartum depression during the trimesters of pregnancy. Open Access Maced. J. Med. Sci 2019, 7, 1555–1560. [Google Scholar] [CrossRef]
- Choi, H.; Kwak, D.W.; Kim, M.H.; Lee, S.Y.; Chung, J.H.; Han, Y.J.; Park, H.J.; Kim, M.Y.; Cha, D.H.; Koo, S.; et al. The Korean Pregnancy Outcome Study (KPOS): Study design and participants. J. Epidemiol. 2021, 31, 392–400. [Google Scholar] [CrossRef]
- WHO/IASO; IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment, Health Communications; Health Communications Australia: Melbourne, Australia, 2000.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the hospital anxiety and depression scale-an updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hur, J.W.; Kim, K.H.; Oh, K.S.; Shin, Y.C. Clinical application of Korean version of Edinburgh Postnatal Depression Scale. J. Korean Neuropsychiatr. Assoc. 2008, 47, 36–44. [Google Scholar]
- Lim, J.S.; Lim, S.W.; Ahn, J.H.; Song, B.S.; Shim, K.S.; Hwang, I.T. New Korean reference for birth weight by gestational age and sex: Data from the Korean Statistical Information Service (2008–2012). Ann. Pediatric Endocrinol. Metab. 2014, 19, 146–153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 19 December 2020).
- World Health Organization. Anaemia in Women and Children: WHO Global Anaemia Estimates; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 29 September 2020).
- Masukume, G.; Khashan, A.S.; Kenny, L.C.; Baker, P.N.; Nelson, G.; SCOPE Consortium. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS ONE 2015, 10, e0122729. [Google Scholar] [CrossRef]
- Lin, L.; Wei, Y.; Zhu, W.; Wang, C.; Su, R.; Feng, H.; Yang, H.; Gestational diabetes mellitus Prevalence Survey (GPS) study Group. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: A multicentre retrospective study. BMC Pregnancy Childbirth 2018, 18, 111. [Google Scholar] [CrossRef]
- Ouzounian, J.G.; Elkayam, U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar] [CrossRef]
- Hamalainen, H.; Hakkarainen, K.; Heinonen, S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin. Nutr. 2003, 22, 271–275. [Google Scholar] [CrossRef]
- Zhang, Q.; Ananth, C.V.; Li, Z.; Smulian, J.C. Maternal anaemia and preterm birth: A prospective cohort study. Int. J. Epidemiol. 2009, 38, 1380–1389. [Google Scholar] [CrossRef]
- Kumar, K.J.; Asha, N.; Murthy, D.S.; Sujatha, M.; Manjunath, V. Maternal anemia in various trimesters and its effect on newborn weight and maturity: An observational study. Int. J. Prev. Med. 2013, 4, 193–199. [Google Scholar]
- Balarajan, Y.; Ramakrishnan, U.; Ozaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Gebre, A.; Mulugeta, A. Prevalence of anemia and associated factors among pregnant women in north western zone of Tigray, Northern Ethiopia: A cross-sectional study. J. Nutr. Metab 2015, 2015, 165430. [Google Scholar] [CrossRef]
- Adebisi, O.Y.; Strayhorn, G. Anemia in pregnancy and race in the United States: Blacks at risk. Fam. Med. 2005, 37, 655–662. [Google Scholar]
- Imai, K. Parity-based assessment of anemia and iron deficiency in pregnant women. Taiwan J. Obstet. Gynecol. 2020, 59, 838–841. [Google Scholar] [CrossRef]
- Liu, X.; Du, J.; Wang, G.; Chen, Z.; Wang, W.; Xi, Q. Effect of pre-pregnancy body mass index on adverse pregnancy outcome in north of China. Arch. Gynecol. Obstet. 2011, 283, 65–70. [Google Scholar] [CrossRef]
- Sebire, N.J.; Jolly, M.; Harris, J.; Regan, L.; Robinson, S. Is maternal underweight really a risk factor for adverse pregnancy outcome? A population-based study in London. BJOG 2001, 108, 61–66. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, H.; Liu, J.; Ma, Q.; Yuan, Y.; Pang, Q.; Liu, J.; Kong, C.; Liu, M. Prevalence of anemia and sociodemographic characteristics among pregnant and non-pregnant women in southwest China: A longitudinal observational study. BMC Pregnancy Childbirth 2020, 20, 535. [Google Scholar] [CrossRef]
- Ru, Y.; Pressman, E.K.; Cooper, E.M.; Guillet, R.; Katzman, P.J.; Kent, T.R.; Bacak, S.J.; O’Brien, K.O. Iron deficiency and anemia are prevalent in women with multiple gestations. Am. J. Clin. Nutr. 2016, 104, 1052–1060. [Google Scholar] [CrossRef]
- Tan, J.; He, G.; Qi, Y.; Yang, H.; Xiong, Y.; Liu, C.; Wang, W.; Zou, K.; Lee, A.H.; Sun, X.; et al. Prevalence of anemia and iron deficiency anemia in Chinese pregnant women (IRON WOMEN): A national cross-sectional survey. BMC Pregnancy Childbirth 2020, 20, 670. [Google Scholar] [CrossRef]
- Suzuki, S.; Kikuchi, F.; Ouchi, N.; Nagayama, C.; Nakagawa, M.; Inde, Y.; Igarashi, M.; Miyake, H. Risk factors for postpartum hemorrhage after vaginal delivery of twins. J. Nippon. Med. Sch. 2007, 74, 414–417. [Google Scholar] [CrossRef][Green Version]
- Blitz, M.J.; Yukhayev, A.; Pachtman, S.L.; Reisner, J.; Moses, D.; Sison, C.P.; Greenberg, M.; Rochelson, B. Twin pregnancy and risk of postpartum hemorrhage. J. Matern.-Fetal Neonatal Med. 2020, 33, 3740–3745. [Google Scholar] [CrossRef]
- Scanlon, K.S.; Yip, R.; Schieve, L.A.; Cogswell, M.E. High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstet. Gynecol. 2000, 96, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Rahman, M.M.; Rahman, M.S.; Swe, K.T.; Islam, M.R.; Rahman, M.O.; Akter, S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.; Hoffman, M.K.; Goudar, S.S.; Patel, A.; Saleem, S.; Ali, S.A.; Goldenberg, R.L.; Hibberd, P.L.; Moore, J.; Wallace, D.; et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG 2019, 126, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Sharma, J.B.; Batra, S.; Sharma, S.; Murthy, N.S.; Arora, R. Maternal and perinatal outcome in varying degrees of anemia. Int. J. Gynaecol. Obstet. 2002, 79, 93–100. [Google Scholar] [CrossRef]
- Zhang, Q.; Ananth, C.V.; Rhoads, G.G.; Li, Z. The impact of maternal anemia on perinatal mortality: A population-based, prospective cohort study in China. Ann. Epidemiol. 2009, 19, 793–799. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Wang, Y.; Sun, M.; Guo, Y.; Ma, S.; Wang, X.; Jiang, H.; Wang, X.; Lu, J.; et al. Severity of anemia during pregnancy and adverse maternal and fetal outcomes. JAMA Netw. Open 2022, 5, e2147046. [Google Scholar] [CrossRef]
- Jwa, S.C.; Fujiwara, T.; Yamanobe, Y.; Kozuka, K.; Sago, H. Changes in maternal hemoglobin during pregnancy and birth outcomes. BMC Pregnancy Childbirth 2015, 15, 80. [Google Scholar] [CrossRef]
- Steer, P.J. Maternal hemoglobin concentration and birth weight. Am. J. Clin. Nutr. 2000, 71, 1285S–1287S. [Google Scholar] [CrossRef]
- Whittaker, P.G.; Macphail, S.; Lind, T. Serial hematologic changes and pregnancy outcome. Obstet. Gynecol. 1996, 88, 33–39. [Google Scholar] [CrossRef]
- Vricella, L.K. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am. J. Clin. Nutr. 2017, 106, 1620S–1625S. [Google Scholar] [CrossRef]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung e.V. Eisen. Deutsche Gesellschaft für Ernährung (DGE); Österreichische Gesellschaft für Ernährung (ÖGE), Schweizerische Gesellschaft für Ernährung (SGE), Eds.; Referenzwerte für die Nährstoffzufuhr: Bonn, Australia, 2017. [Google Scholar]
| Maternal Anemia during the First Trimester | p-Value | ||
|---|---|---|---|
| No (n = 3948) | Yes (n = 119) | ||
| Maternal age (years) | |||
| <35 | 2535 (64.2) | 70 (58.8) | 0.18 |
| 35–39 | 1194 (30.2) | 38 (31.9) | |
| >40 | 219 (5.5) | 11 (9.2) | |
| BMI (kg/m2) | |||
| <18.5 | 399 (10.1) | 14 (11.8) | 0.38 |
| 18.5–22.9 | 2510 (63.6) | 79 (66.4) | |
| 23.0–24.9 | 530 (13.4) | 17 (14.3) | |
| >25 | 509 (12.9) | 9 (7.6) | |
| Having a marital partner | |||
| No | 159 (4.0) | 12 (10.1) | 0.002 |
| Yes | 3789 (96.0) | 107 (89.9) | |
| Parity | |||
| 0 | 2449 (62.0) | 70 (58.8) | 0.74 |
| 1 | 1307 (33.1) | 42 (35.3) | |
| ≥2 | 192 (4.9) | 7 (5.9) | |
| Educational status | |||
| ≤High school | 340 (8.6) | 12 (10.1) | 0.54 |
| College | 2935 (74.3) | 91 (76.5) | |
| Graduate school | 673 (17.0) | 16 (13.4) | |
| Household income (won/month) | |||
| ≤3 million | 510 (12.9) | 20 (16.8) | 0.21 |
| 3–5 million | 1550 (39.3) | 51 (42.9) | |
| >5 million | 1888 (47.8) | 48 (40.3) | |
| Planned pregnancy | |||
| No | 1519 (38.5) | 53 (44.5) | 0.18 |
| Yes | 2428 (61.5) | 66 (55.5) | |
| Type of pregnancy | |||
| Natural | 3754 (95.1) | 109 (91.6) | 0.08 |
| Artificial | 193 (4.9) | 10 (8.4) | |
| Twin pregnancy | |||
| No | 3890 (98.6) | 113 (95.0) | 0.003 |
| Yes | 57 (1.4) | 6 (5.0) | |
| Cigarette smoking | |||
| Never smoked | 3516 (89.1) | 105 (88.2) | 0.80 * |
| Quit before pregnancy | 318 (8.1) | 8 (6.7) | |
| Quit after pregnancy | 108 (2.7) | 12 (5.0) | |
| Current smoker | 5 (0.1) | 0 (0.0) | |
| Alcohol drinking | |||
| Never drank | 768 (19.5) | 29 (24.4) | 0.31 * |
| Quit before pregnancy | 1900 (48.1) | 58 (48.7) | |
| Quit after pregnancy | 1274 (32.3) | 32 (26.9) | |
| Current drinker | 5 (0.1) | 0 (0) | |
| NVP | |||
| No | 977 (24.8) | 22 (18.5) | 0.12 |
| Yes | 2970 (75.2) | 97 (81.5) | |
| Threatened abortion | |||
| No | 3243 (82.2) | 101 (84.9) | 0.45 |
| Yes | 704 (17.8) | 18 (15.1) | |
| Folic acid intake | |||
| No intake | 449 (11.4) | 13 (10.9) | 0.47 |
| From recognition of pregnancy | 1651 (41.8) | 50 (42.0) | |
| Before pregnancy | 1643 (41.6) | 46 (38.7) | |
| - No response | 205 (5.2) | 10 (8.4) | |
| Iron or MM intake | |||
| No intake | 2268 (57.5) | 63 (56.7) | 0.16 |
| From recognition of pregnancy | 961 (24.3) | 35 (29.4) | |
| Before pregnancy | 514 (13.0) | 11 (9.2) | |
| - No response | 205 (5.2) | 10 (4.7) | |
| Anemia (%) | Unadjusted OR (95% CI) | p | Adjusted OR * (95% CI) | p | |
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| <35 | 2.7 | Ref. | - | Ref. | - |
| 35–40 | 3.1 | 1.13 (0.74–1.73) | 0.56 | 1.12 (0.72–1.74) | 0.60 |
| >40 | 4.8 | 1.94 (1.01–3.73) | 0.05 | 1.95 (0.98–3.86) | 0.06 |
| BMI (kg/m2) | |||||
| <18.5 | 3.4 | 1.26 (0.70–2.25) | 0.71 | 1.24 (0.69–2.25) | 0.46 |
| 18.5–22.9 | 3.1 | Ref. | - | Ref. | - |
| 23.0–24.9 | 3.1 | 1.15 (0.67–1.97) | 0.94 | 1.11 (0.64–1.92) | 0.69 |
| >25 | 1.7 | 0.64 (0.32–1.30) | 0.22 | 0.57 (0.28–1.17) | 0.13 |
| Having a marital partner | |||||
| No | 7.0 | 2.69 (1.41–5.12) | 0.002 | 2.84 (1.39–5.80) | 0.004 |
| Yes | 2.7 | Ref. | - | Ref. | - |
| Parity | |||||
| 0 | 2.8 | Ref. | - | Ref. | - |
| 1 | 3.1 | 1.11 (0.74–1.67) | 0.62 | 1.28 (0.82–2.01) | 0.28 |
| ≥2 | 3.5 | 1.26 (0.54–2.96) | 0.59 | 1.23 (0.50–3.07) | 0.65 |
| Type of pregnancy | |||||
| Natural | 2.8 | Ref. | Ref. | - | |
| Artificial | 4.9 | 1.95 (1.00–3.80) | 0.049 | 1.55 (0.71–3.41) | 0.26 |
| Twin pregnancy | |||||
| No | 2.8 | Ref. | Ref. | - | |
| Yes | 9.5 | 3.77 (1.59–8.94) | 0.003 | 3.38 (1.26–9.04) | 0.015 |
| NVP | |||||
| No | 2.2 | Ref. | Ref. | - | |
| Yes | 3.2 | 1.47 (0.89–2.43) | 0.12 | 1.51 (0.91–2.51) | 0.11 |
| Maternal Anemia during the First Trimester | p-Value | ||
|---|---|---|---|
| Yes (n = 90) | No (n = 3245) | ||
| Hb measurements during the first trimester | |||
| GA at sampling (weeks) | 12.2 (12.0, 12.7) | 12.2 (12.0, 12.7) | 0.49 |
| Hb level in the first trimester (g/dL) | 10.5 (9.9, 10.7) | 12.7 (12.2, 13.2) | <0.001 |
| Iron or multiple micronutrients supplementation | |||
| Intake during the first trimester | 34/82 (41.4) | 1200/3081 (38.9) | 0.64 |
| - No response | 8/90 (8.9) | 164/3245 (5.1) | - |
| Intake during the second trimester | 86/88 (96.6) | 3017/3103 (97.2) | 0.91 |
| - No response | 2/90 (2.2) | 142/3245 (4.4) | - |
| Intake during the third trimester | 84/84 (100.0) | 2892/2942 (97.1) | 0.40 |
| - No response or not available | 6/90 (6.6) | 303/3245 (9.3) | - |
| Pregnancy outcomes | |||
| GA at birth (weeks) | 39.3 (38.5, 40.1) | 39.4 (38.5, 40.2) | 0.49 |
| Birth weight (kg) | 3.13 (2.91, 3.40) | 3.26 (3.01, 3.52) | 0.001 |
| Preterm delivery | 4 (4.4) | 157/3245 (4.8) | 0.86 |
| Low birth weight | 8 (8.9) | 119/3245 (3.7) | 0.02 |
| SGA less than 10th centile | 17 (18.9) | 265/3245 (8.2) | <0.001 |
| Cesarean section | 42 (46.7) | 1274/3245 (39.3) | 0.16 |
| Low Apgar score at 5 min < 7 | 3 (3.3) | 61/3245 (1.9) | 0.25 |
| Hypertensive disorders of pregnancy | 3 (3.3) | 37/3245 (1.1) | 0.09 |
| Maternal Anemia during the First Trimester | p-Value | ||
|---|---|---|---|
| Yes | No | ||
| Visit 1 (first trimester) | (n = 109) | (n = 3714) | |
| Depression score from K-EPDS | 6.00 (3.00, 9.00) | 6.00 (3.00, 9.00) | 0.96 |
| K-EPDS ≥ 10 | 22 (20.2) | 713 (19.2) | 0.79 |
| Anxiety score from HADS | 4.00 (2.00, 6.00) | 4.00 (2.00, 6.00) | 0.64 |
| HADS Anxiety ≥ 8 | 17 (15.6) | 398 (10.7) | 0.11 |
| Visit 2 (second trimester) | (n = 100) | (n = 3274) | |
| Depression score from K-EPDS | 4.50 (3.00, 7.00) | 5.00 (3.00, 7.00) | 0.99 |
| K-EPDS ≥ 10 | 14 (14.0) | 441 (13.5) | 0.87 |
| Anxiety score from HADS | 3.00 (2.00, 6.00) | 3.00 (2.00, 5.00) | 0.71 |
| HADS Anxiety ≥ 8 | 8 (8.0) | 247 (7.5) | 0.86 |
| Visit 3 (third trimester) | (n = 82) | (n = 2840) | |
| Depression score from K-EPDS | 5.00 (3.00, 7.00) | 5.00 (3.00, 8.00) | 0.69 |
| K-EPDS ≥ 10 | 9 (11.0) | 395 (13.9) | 0.45 |
| Anxiety score from HADS | 4.00 (2.00, 5.00) | 4.00 (2.00, 6.00) | 0.56 |
| HADS Anxiety ≥ 8 | 5 (6.1) | 254 (8.9) | 0.37 |
| Visit 5 (postpartum) | (n = 71) | (n = 2338) | |
| Depression score from K-EPDS | 5.00 (2.00, 10.00) | 5.00 (2.00, 8.00) | 0.42 |
| K-EPDS ≥ 10 | 18 (25.4) | 380 (16.3) | 0.042 |
| Anxiety score from HADS | 3.00 (1.00, 6.00) | 3.00 (1.00, 5.00) | 0.16 |
| HADS Anxiety ≥ 8 | 12 (16.9) | 217 (9.3) | 0.031 |
| Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Low birth weight | 2.56 (1.12–5.42) | 0.013 | 2.29 (1.06–4.94) | 0.034 † |
| Small for gestational age | 2.62 (1.52–4.51) | <0.001 | 2.46 (1.41–4.29) | 0.001 † |
| Hypertensive disorders of pregnancy | 2.99 (0.90–3.02) | 0.07 | 2.69 (0.72–10.00) | 0.14 † |
| K-EPDS ≥ 10 (postpartum) | 1.75 (1.01–3.02) | 0.044 | 1.61 (0.93–2.80) | 0.092 ‡ |
| HADS-A ≥ 8 (postpartum) | 1.99 (1.05–3.76) | 0.034 | 1.81 (0.95–3.48) | 0.072 ‡ |
| Iron or Multiple Micronutrients Supplementation during the First Trimester | p-Value | ||
|---|---|---|---|
| Yes | No | ||
| Participants who had anemia during the first trimester | |||
| Low birth weight | 1/34 (2.9) | 7/48 (14.6) | 0.13 |
| Small for gestational age | 5/34 (14.7) | 10/48 (20.8) | 0.48 |
| Hypertensive disorders of pregnancy | 1/34 (2.9) | 2/48 (4.2) | 1.00 |
| K-EPDS ≥ 10 (postpartum) | 9/25 (36.0) | 9/41 (22.0) | 0.21 |
| HADS-A ≥ 8 (postpartum) | 6/25 (24.0) | 6/41 (14.6) | 0.33 |
| Participants who had normal hemoglobin levels during the first trimester | |||
| Low birth weight | 31/1200 (2.6) | 81/1881 (4.3) | 0.013 |
| Small for gestational age | 84/1200 (7.0) | 167/1881 (8.9) | 0.063 |
| Hypertensive disorders of pregnancy | 12/1200 (1.0) | 22/1881 (1.2) | 0.66 |
| K-EPDS ≥ 10 (postpartum) | 137/875 (15.7) | 229/1373 (16.7) | 0.52 |
| HADS-A ≥ 8 (postpartum) | 71/875 (8.1) | 138/1373 (10.1) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, D.-W.; Kim, S.; Lee, S.-Y.; Kim, M.-H.; Park, H.-J.; Han, Y.-J.; Cha, D.-H.; Kim, M.-Y.; Chung, J.-H.; Park, B.; et al. Maternal Anemia during the First Trimester and Its Association with Psychological Health. Nutrients 2022, 14, 3505. https://doi.org/10.3390/nu14173505
Kwak D-W, Kim S, Lee S-Y, Kim M-H, Park H-J, Han Y-J, Cha D-H, Kim M-Y, Chung J-H, Park B, et al. Maternal Anemia during the First Trimester and Its Association with Psychological Health. Nutrients. 2022; 14(17):3505. https://doi.org/10.3390/nu14173505
Chicago/Turabian StyleKwak, Dong-Wook, Seokyung Kim, Su-Young Lee, Min-Hyoung Kim, Hee-Jin Park, You-Jung Han, Dong-Hyun Cha, Moon-Young Kim, Jin-Hoon Chung, Bumhee Park, and et al. 2022. "Maternal Anemia during the First Trimester and Its Association with Psychological Health" Nutrients 14, no. 17: 3505. https://doi.org/10.3390/nu14173505
APA StyleKwak, D.-W., Kim, S., Lee, S.-Y., Kim, M.-H., Park, H.-J., Han, Y.-J., Cha, D.-H., Kim, M.-Y., Chung, J.-H., Park, B., & Ryu, H.-M. (2022). Maternal Anemia during the First Trimester and Its Association with Psychological Health. Nutrients, 14(17), 3505. https://doi.org/10.3390/nu14173505





