The Influence of Sociodemographic Factors, Lifestyle, and Risk Perception on Dietary Patterns in Pregnant Women Living in Highly Contaminated Areas: Data from the NEHO Birth Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Assessment
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
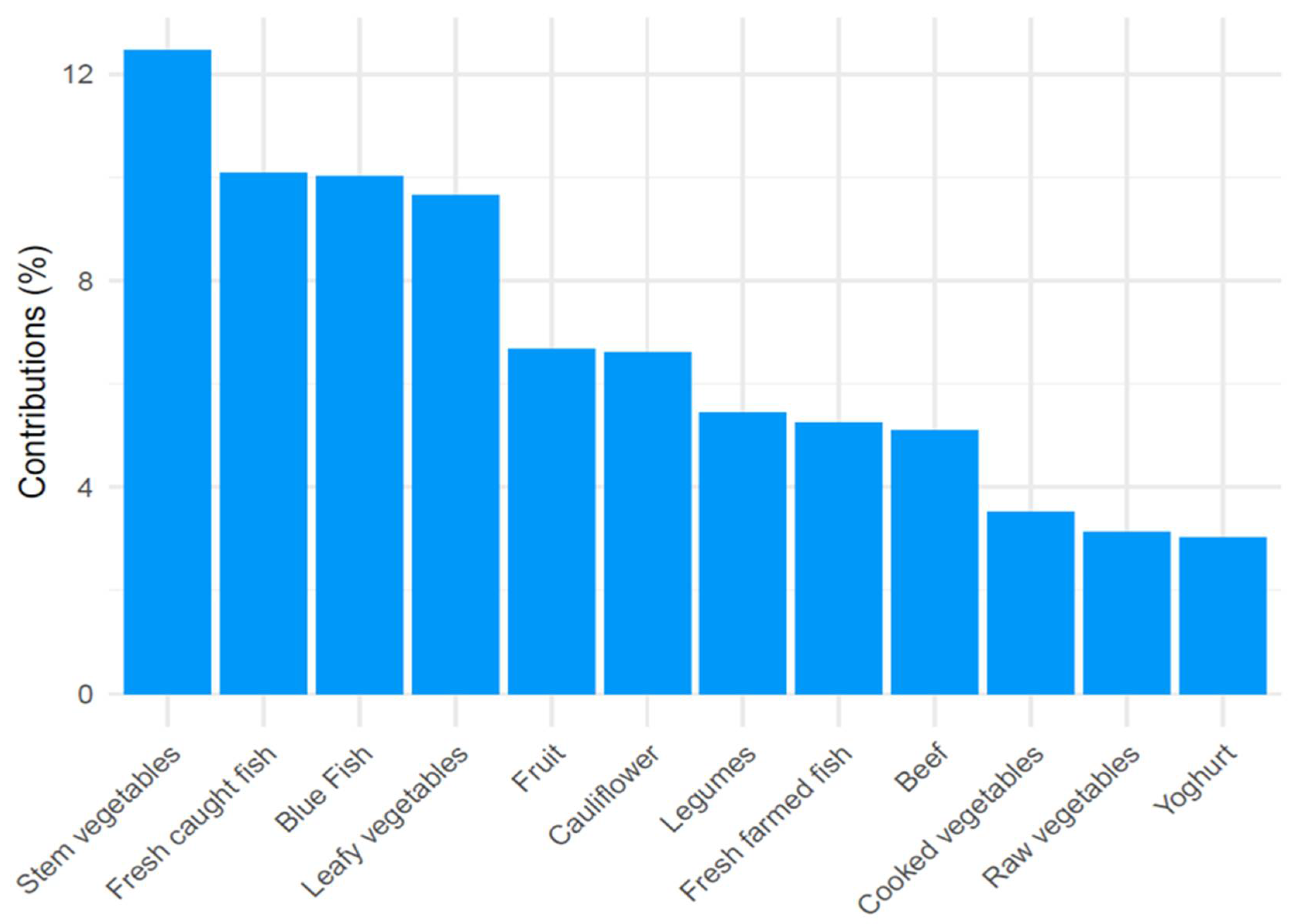
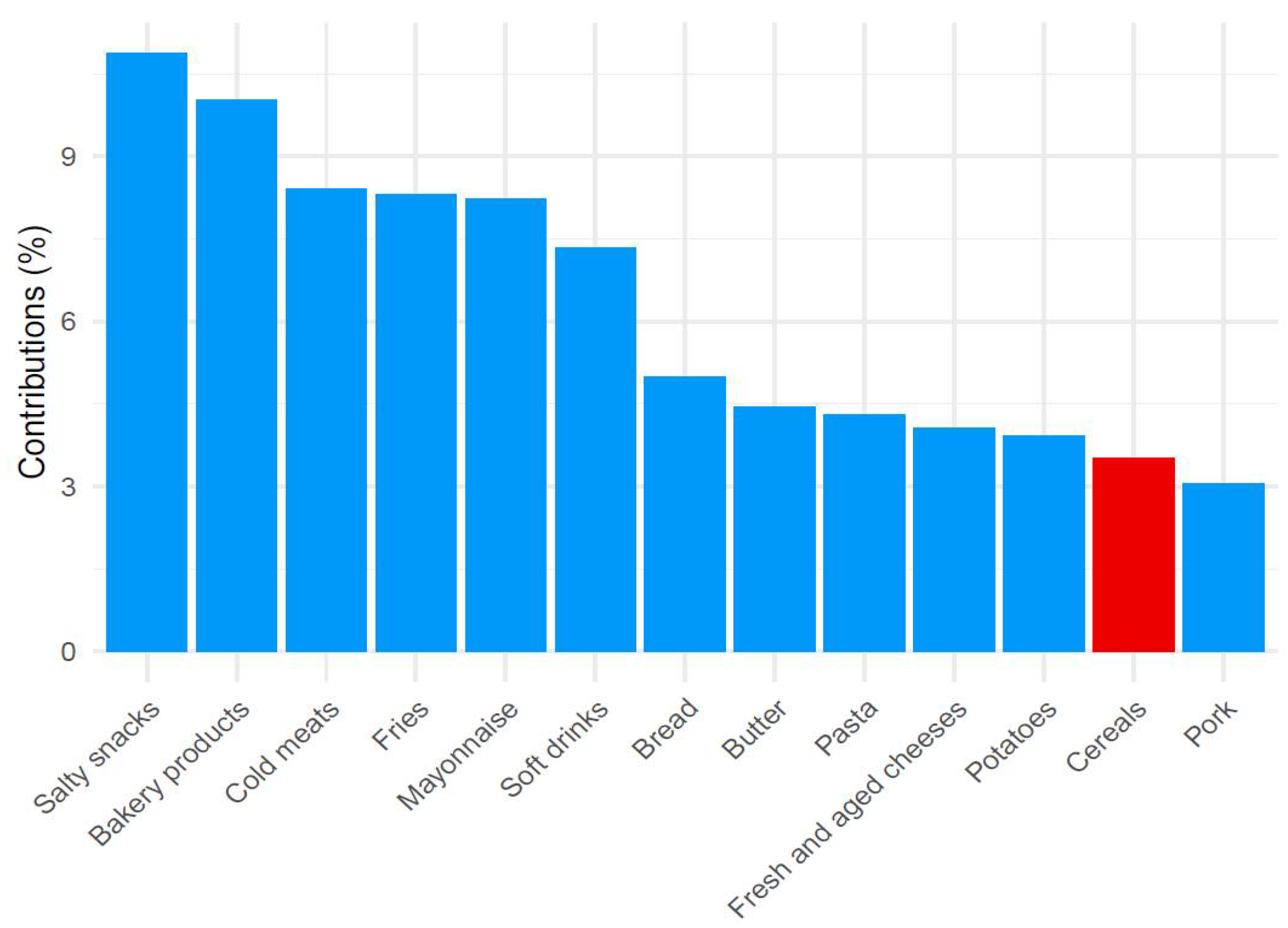
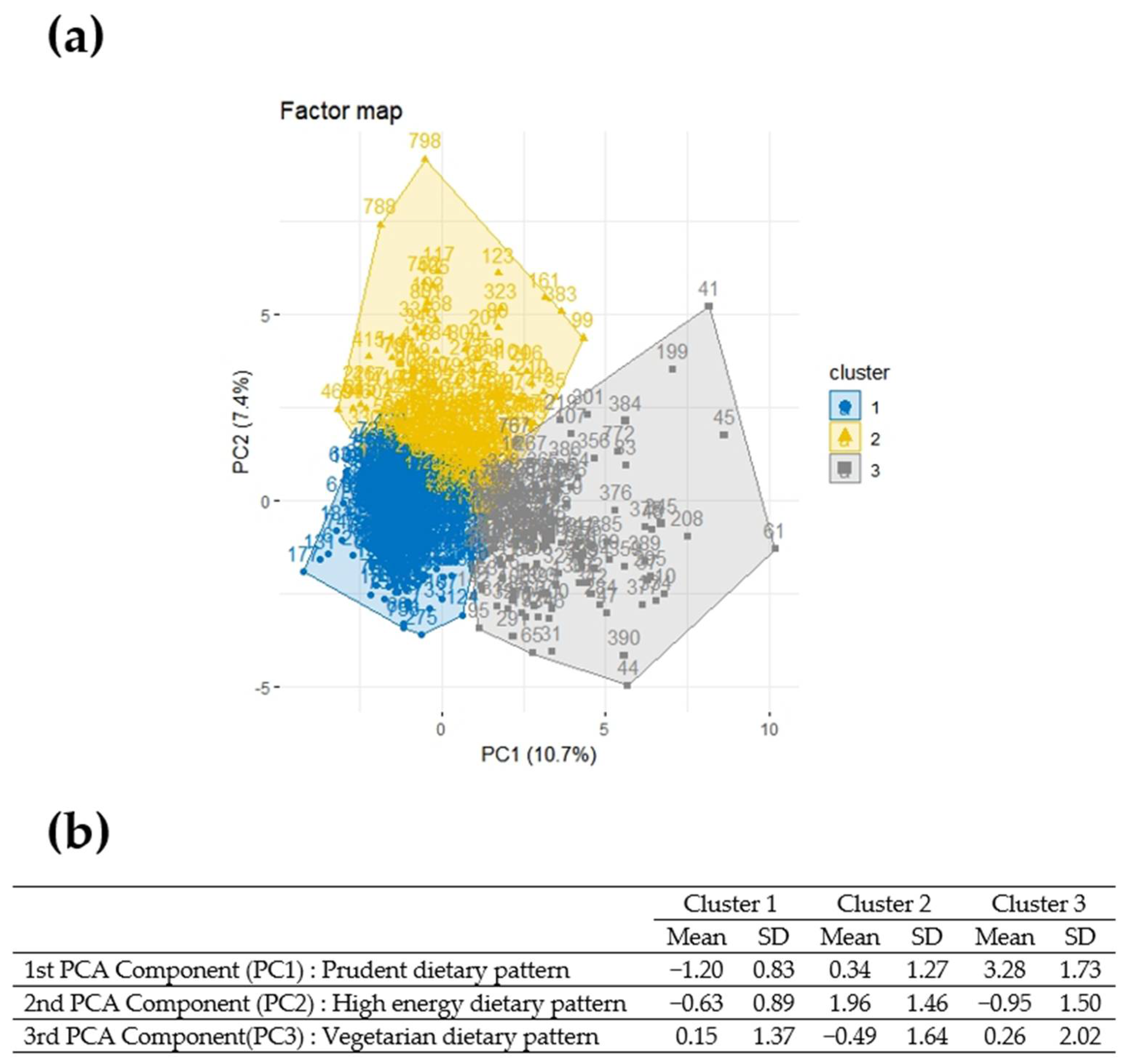
3.2. Principal Component Analysis Subsection
3.3. Hierarchical Clustering on Principle Components
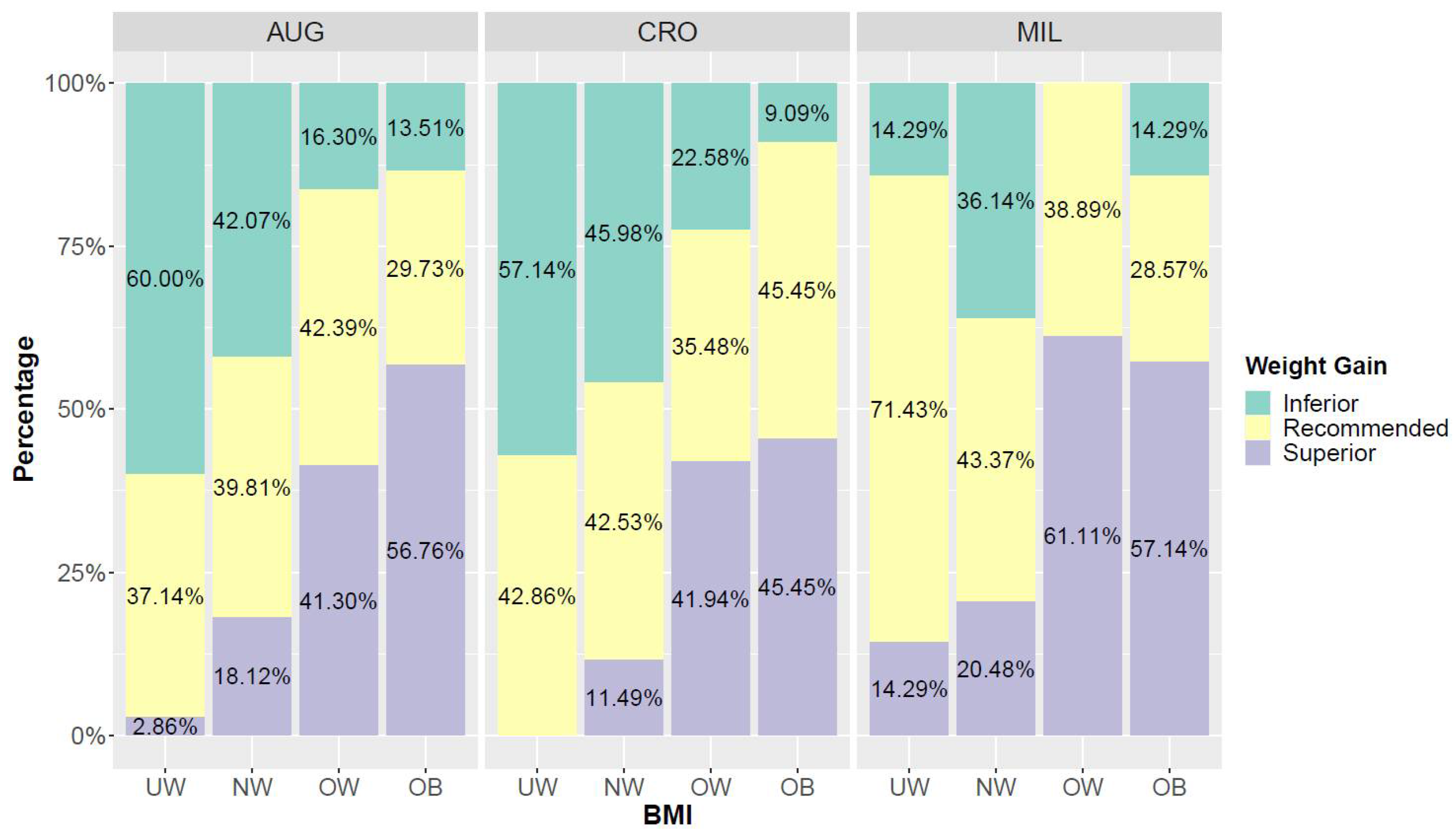
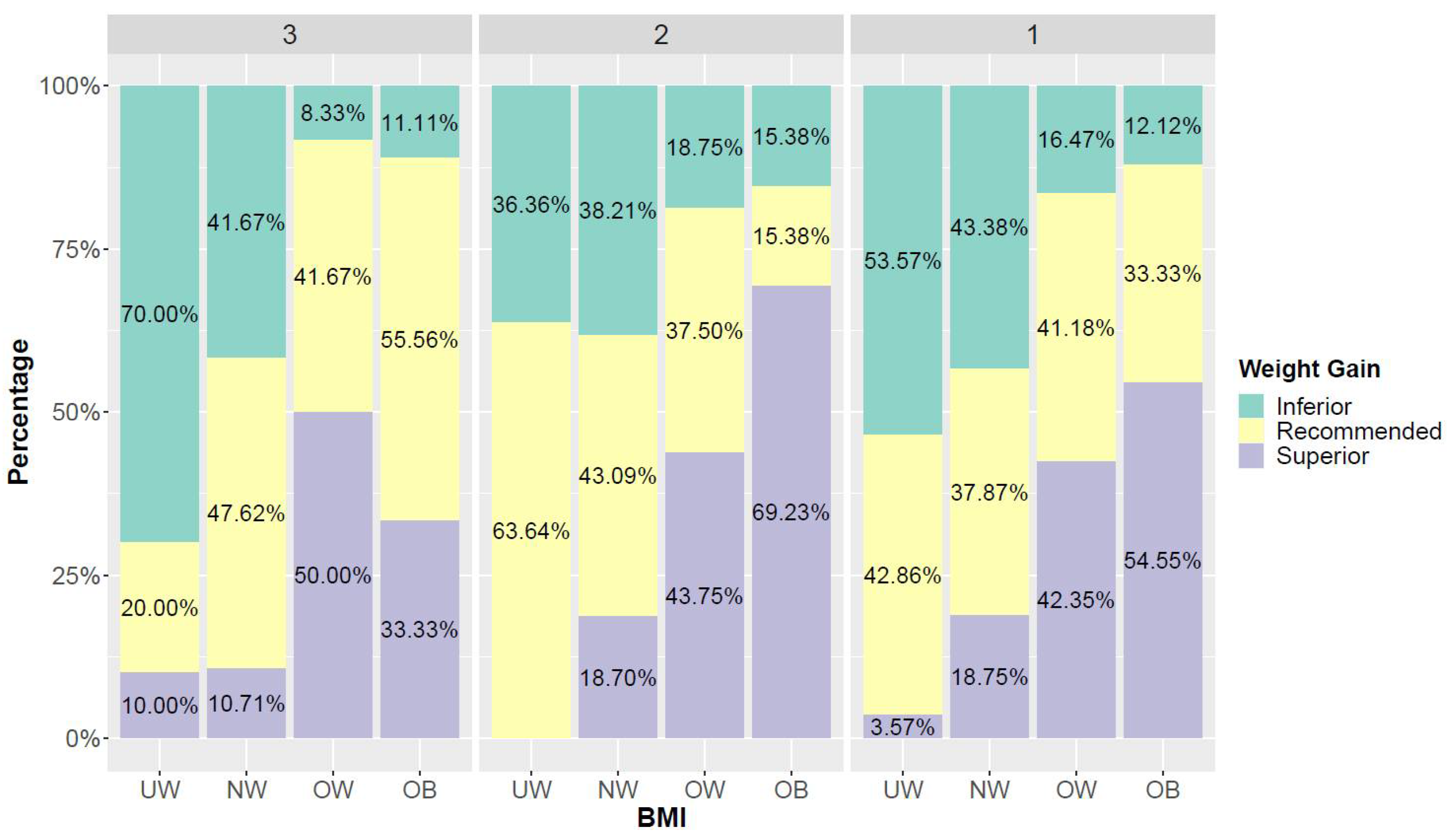
3.4. BMI and Weight Gain
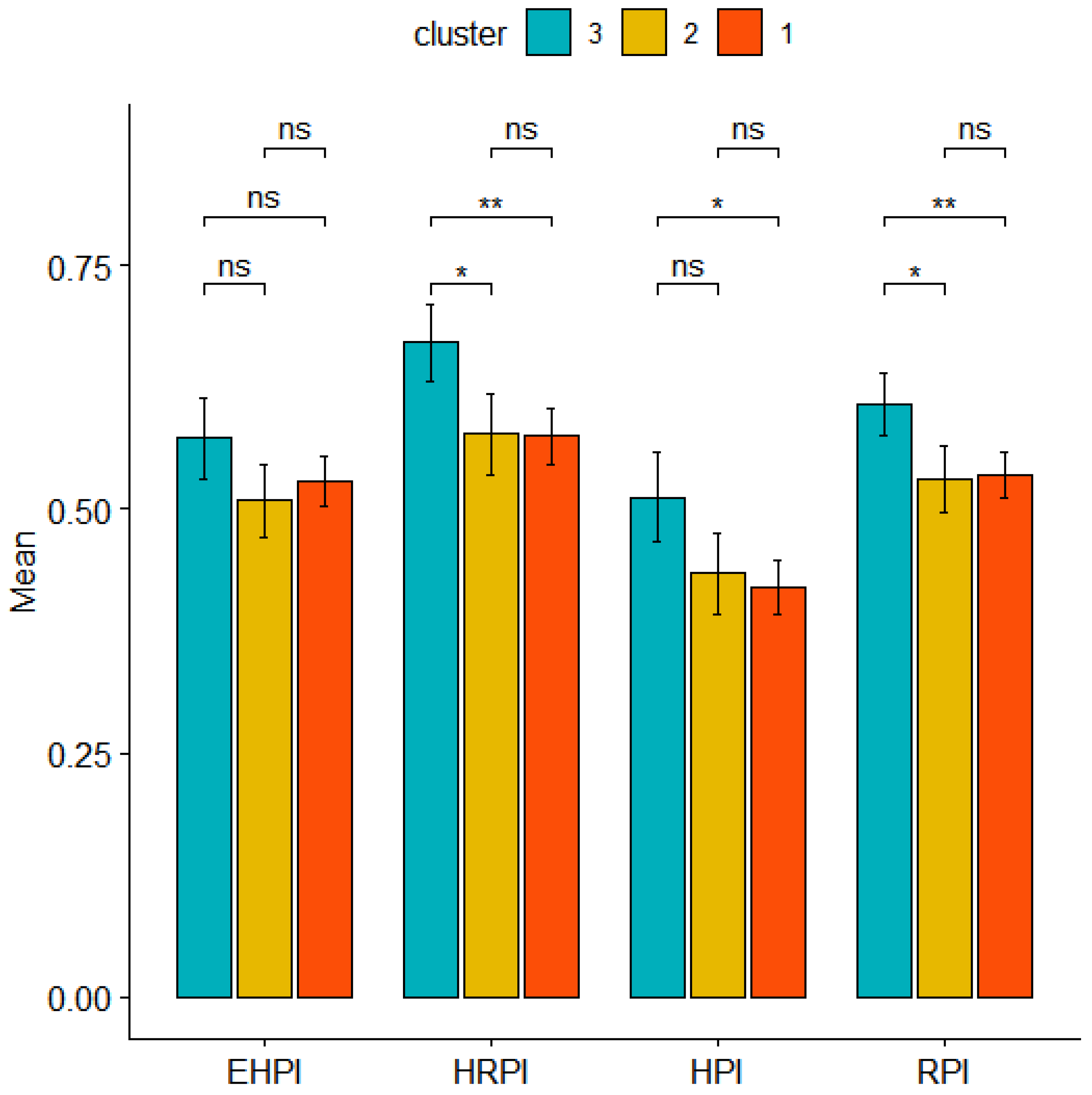
3.5. Risk Perception
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacks, D.A. Determinants of Fetal Growth. Curr. Diabetes Rep. 2004, 4, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Vorherr, H. Factors Influencing Fetal Growth. Am. J. Obstet. Gynecol. 1982, 142, 577–588. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Clark, P.M. Fetal Undernutrition and Disease in Later Life. Rev. Reprod. 1997, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Koemel, N.A.; Skilton, M.R. Epigenetic Aging in Early Life: Role of Maternal and Early Childhood Nutrition. Curr. Nutr. Rep. 2022, 11, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Buklijas, T.; Hanson, M.A. The Developmental Origins of Health and Disease (DOHaD) Concept: Past, Present, and Future. In The Epigenome and Developmental Origins of Health and Disease; Rosenfeld, C.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–15. ISBN 9780128013830. [Google Scholar] [CrossRef]
- Good Maternal Nutrition the Best Start in Life; WHO: Copenhagen, Denmark, 2016; ISBN 978-92-890-5154-5.
- De Castro, M.B.T.; Freitas Vilela, A.A.; de Oliveira, A.S.D.; Cabral, M.; de Souza, R.A.G.; Kac, G.; Sichieri, R. Sociodemographic Characteristics Determine Dietary Pattern Adherence during Pregnancy. Public Health Nutr. 2016, 19, 1245–1251. [Google Scholar] [CrossRef]
- Teixeira, J.A.; Castro, T.G.; Grant, C.C.; Wall, C.R.; da Silva Castro, A.L.; Francisco, R.P.V.; Vieira, S.E.; Saldiva, S.R.D.M.; Marchioni, D.M. Dietary Patterns Are Influenced by Socio-Demographic Conditions of Women in Childbearing Age: A Cohort Study of Pregnant Women. BMC Public Health 2018, 18, 301. [Google Scholar] [CrossRef]
- Arkkola, T.; Uusitalo, U.; Kronberg-Kippilä, C.; Männistö, S.; Virtanen, M.; Kenward, M.G.; Veijola, R.; Knip, M.; Ovaskainen, M.-L.; Virtanen, S.M. Seven Distinct Dietary Patterns Identified among Pregnant Finnish Women—Associations with Nutrient Intake and Sociodemographic Factors. Public Health Nutr. 2008, 11, 176–182. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, N.; Joisten, C. Impact of Physical Activity on Course and Outcome of Pregnancy from Pre- to Postnatal. Eur. J. Clin. Nutr. 2021, 75, 1698–1709. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health. Joint WHO/FAO Expert Consultation; WHO Technical Report Series No. 916. WHO: Geneva, Switzerland, 2004; Available online: http://www.who.int/dietphysicalactivity/goals/en/index.html (accessed on 25 May 2022).
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental Pollutants and Child Health—A Review of Recent Concerns. Int. J. Hyg. Environ. Health 2016, 219, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Duan, X.; Feng, W.; He, J.; Cao, S.; Liu, S.; Shi, D.; Wang, H.; Wu, F. Health Risks to Metals in Multimedia via Ingestion Pathway for Children in a Typical Urban Area of China. Chemosphere 2019, 226, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Röösli, M.; Frey, U.; Latzin, P. Air Pollution During Pregnancy and Neonatal Outcome: A Review. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-T. Review of Epidemiological Studies on Air Pollution and Health Effects in Children. Clin. Exp. Pediatr. 2021, 64, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Maltese, S.; Drago, G.; Panunzi, S.; Cibella, F.; Bianchi, F.; Minichilli, F.; Cori, L. Measuring Risk Perception in Pregnant Women in Heavily Polluted Areas: A New Methodological Approach from the NEHO Birth Cohort. Int. J. Environ. Res. Public Health 2021, 18, 10616. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood from Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Drago, G.; Colombo, P.; Alesci, A.; Augello, P.; Bisbano, A.; Bucolo, A.; Dattoli, P.; De Sole, R.; La Runa, V.; et al. Three Contaminated Sites in Southern Italy. The Neonatal Environment and Health Outcomes Cohort: Protocol for a Longitudinal Birth Cohort Study. BMJ Open 2019, 9, e029471. [Google Scholar] [CrossRef]
- Ruggieri, S.; Maltese, S.; Drago, G.; Cibella, F.; Panunzi, S. The Neonatal Environment and Health Outcomes (NEHO) Birth Cohort Study: Behavioral and Socioeconomic Characteristics and Drop-Out Rate from a Longitudinal Birth Cohort in Three Industrially Contaminated Sites in Southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 1252. [Google Scholar] [CrossRef]
- Drago, G.; Ruggieri, S.; Bianchi, F.; Sampino, S.; Cibella, F. Birth Cohorts in Highly Contaminated Sites: A Tool for Monitoring the Relationships Between Environmental Pollutants and Children’s Health. Front. Public Health 2020, 8, 125. [Google Scholar] [CrossRef]
- Longo, V.; Longo, A.; Di Sano, C.; Cigna, D.; Cibella, F.; Di Felice, G.; Colombo, P. In Vitro Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE-47) Impairs Innate Inflammatory Response. Chemosphere 2019, 219, 845–854. [Google Scholar] [CrossRef]
- Albano, G.D.; Moscato, M.; Montalbano, A.M.; Anzalone, G.; Gagliardo, R.; Bonanno, A.; Giacomazza, D.; Barone, R.; Drago, G.; Cibella, F.; et al. Can PBDEs Affect the Pathophysiologic Complex of Epithelium in Lung Diseases? Chemosphere 2020, 241, 125087. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bustaffa, E.; Bolignano, D.; Cori, L.; Faita, F.; Gastaldelli, A.; Interdonato, M.; Minichilli, F.; Quattrone, G.; Squadrito, F.; et al. Biomarkers of Exposure and Early Effect in Three Contaminated Sites of Southern Italy: Protocols for Etiological Epidemiological Studies. BMJ Open 2020, 10, e036160. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and Genotoxic Effects of the Flame Retardants (PBDE-47, PBDE-99 and PBDE-209) in Human Bronchial Epithelial Cells. Chemosphere 2020, 245, 125600. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Bonanno, A.; Montalbano, A.M.; Di Sano, C.; Anzalone, G.; Gagliardo, R.; Ruggieri, S.; Profita, M. Cadmium and Cadmium/BDE (47 or 209) Exposure Affect Mitochondrial Function, DNA Damage/Repair Mechanisms and Barrier Integrity in Airway Epithelial Cells. Atmosphere 2022, 13, 201. [Google Scholar] [CrossRef]
- Farchi, S.; Forastiere, F.; Vecchi Brumatti, L.; Alviti, S.; Arnofi, A.; Bernardini, T.; Bin, M.; Brescianini, S.; Colelli, V.; Cotichini, R.; et al. Piccolipiù, a multicenter birth cohort in Italy: Protocol of the study. BMC Pediatr. 2014, 14, 36. [Google Scholar] [CrossRef]
- CREA. Centro di Ricerca Alimenti e la Nutrizione. Linee Guida per Una Sana Alimentazione. (2018) December 2019. Available online: https://www.crea.gov.it/en/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018 (accessed on 25 May 2022).
- WHO. Regional Office for Europe, BMI Definition. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 25 May 2022).
- Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; p. 12584. ISBN 978-0-309-13113-1.
- Cespedes, E.M.; Hu, F.B. Dietary Patterns: From Nutritional Epidemiologic Analysis to National Guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 25 May 2022).
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The Importance of Nutrition in Pregnancy and Lactation: Lifelong Consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Starling, P.; Charlton, K.; McMahon, A.T.; Lucas, C. Fish intake during pregnancy and foetal neurodevelopment—A systematic review of the evidence. Nutrients 2015, 7, 2001–2014. [Google Scholar] [CrossRef]
- Kofoed, A.B.; Deen, L.; Hougaard, K.S.; Petersen, K.U.; Meyer, H.W.; Pedersen, E.B.; Ebbehøj, N.E.; Heitmann, B.L.; Bonde, J.P.; Tøttenborg, S.S. Maternal exposure to airborne polychlorinated biphenyls (PCBs) and risk of adverse birth outcomes. Eur. J. Epidemiol. 2021, 36, 861–872. [Google Scholar] [CrossRef]
- Doyle, I.-M.; Borrmann, B.; Grosser, A.; Razum, O.; Spallek, J. Determinants of Dietary Patterns and Diet Quality during Pregnancy: A Systematic Review with Narrative Synthesis. Public Health Nutr. 2017, 20, 1009–1028. [Google Scholar] [CrossRef]
- Slattery, M.L. Defining Dietary Consumption: Is the Sum Greater than Its Parts? Am. J. Clin. Nutr. 2008, 88, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.A.; Rinaldi, A.E.; Conde, W.L.; Mainardi, G.M.; Behar, D.; Slater, B. Dietary patterns: A literature review of the methodological characteristics of the main steps of the multivariate analyzes. Rev. Bras. Epidemiol. 2015, 18, 837–857. [Google Scholar] [CrossRef] [PubMed]
- Ocké, M.C. Evaluation of Methodologies for Assessing the Overall Diet: Dietary Quality Scores and Dietary Pattern Analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Trijsburg, L.; Talsma, E.F.; de Vries, J.H.M.; Kennedy, G.; Kuijsten, A.; Brouwer, I.D. Diet Quality Indices for Research in Low- and Middle-Income Countries: A Systematic Review. Nutr. Rev. 2019, 77, 515–540. [Google Scholar] [CrossRef]
- Waijers, P.M.C.M.; Feskens, E.J.M.; Ocké, M.C. A Critical Review of Predefined Diet Quality Scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [CrossRef]
- Hearty, Á.P.; Gibney, M.J. Comparison of Cluster and Principal Component Analysis Techniques to Derive Dietary Patterns in Irish Adults. Br. J. Nutr. 2008, 101, 598–608. [Google Scholar] [CrossRef]
- Wall, C.; Gammon, C.; Bandara, D.; Grant, C.; Atatoa Carr, P.; Morton, S. Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients 2016, 8, 300. [Google Scholar] [CrossRef]
- Jarman, M.; Mathe, N.; Ramazani, F.; Pakseresht, M.; Robson, P.; Johnson, S.; Bell, R.; the APrON and ENRICH study teams. Dietary Patterns Prior to Pregnancy and Associations with Pregnancy Complications. Nutrients 2018, 10, 914. [Google Scholar] [CrossRef] [Green Version]
- Boylan, S.; Lallukka, T.; Lahelma, E.; Pikhart, H.; Malyutina, S.; Pajak, A.; Kubinova, R.; Bragina, O.; Stepaniak, U.; Gillis-Januszewska, A.; et al. Socio-Economic Circumstances and Food Habits in Eastern, Central and Western European Populations. Public Health Nutr. 2011, 14, 678–687. [Google Scholar] [CrossRef]
- Stefler, D.; Pajak, A.; Malyutina, S.; Kubinova, R.; Bobak, M.; Brunner, E.J. Comparison of Food and Nutrient Intakes between Cohorts of the HAPIEE and Whitehall II Studies. Eur. J. Public Health 2016, 26, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Olinto, M.T.A.; Dias-da-Costa, J.S.; Alves, A.L.; Balbinotti, M.; Pattussi, M.P.; Bassani, D.G. Socioeconomic, Demographic and Lifestyle Factors Associated with Dietary Patterns of Women Living in Southern Brazil. Cad. Saúde Pública 2009, 25, 1297–1306. [Google Scholar] [CrossRef]
- Osorio-Yáñez, C.; Gelaye, B.; Enquobahrie, D.A.; Qiu, C.; Williams, M.A. Dietary intake and urinary metals among pregnant women in the Pacific Northwest. Environ. Pollut. 2018, 236, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, E.; Grimalt, J.O.; Lopez-Espinosa, M.J.; Llop, S.; Rebagliato, M.; Ballester, F. Maternal origin and other determinants of cord serum organochlorine compound concentrations in infants from the general population. Environ. Sci. Technol. 2010, 44, 6488–6495. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Murcia, M.; Soler-Blasco, R.; Casas, M.; Zubero, B.; Riutort-Mayol, G.; Gil, F.; Olmedo, P.; Grimalt, J.O.; Amorós, R.; et al. Exposure to metals and metalloids among pregnant women from Spain: Levels and associated factors. Chemosphere 2022, 286 Pt 2, 131809. [Google Scholar] [CrossRef]
- Wesołowska, E.; Jankowska, A.; Trafalska, E.; Kałużny, P.; Grzesiak, M.; Dominowska, J.; Hanke, W.; Calamandrei, G.; Polańska, K. Sociodemographic, Lifestyle, Environmental and Pregnancy-Related Determinants of Dietary Patterns during Pregnancy. Int. J. Environ. Res. Public Health 2019, 16, 754. [Google Scholar] [CrossRef]
- Laraia, B.A.; Bodnar, L.M.; Siega-Riz, A.M. Pregravid Body Mass Index Is Negatively Associated with Diet Quality during Pregnancy. Public Health Nutr. 2007, 10, 920–926. [Google Scholar] [CrossRef]
- Parker, H.W.; Tovar, A.; McCurdy, K.; Vadiveloo, M. Associations between Pre-Pregnancy BMI, Gestational Weight Gain, and Prenatal Diet Quality in a National Sample. PLoS ONE 2019, 14, e0224034. [Google Scholar] [CrossRef]
- Yong, H.Y.; Mohd Shariff, Z.; Mohd Yusof, B.N.; Rejali, Z.; Tee, Y.Y.S.; Bindels, J.; van der Beek, E.M. Pre-Pregnancy BMI Influences the Association of Dietary Quality and Gestational Weight Gain: The SECOST Study. Int. J. Environ. Res. Public Health 2019, 16, 3735. [Google Scholar] [CrossRef] [Green Version]
- Pouchieu, C.; Lévy, R.; Faure, C.; Andreeva, V.A.; Galan, P.; Hercberg, S.; Touvier, M. Socioeconomic, Lifestyle and Dietary Factors Associated with Dietary Supplement Use during Pregnancy. PLoS ONE 2013, 8, e70733. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of Women’s Nutrition before and during Early Pregnancy on Maternal and Infant Outcomes: A Systematic Review: Periconceptual Nutrition and Maternal and Infant Outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Caut, C.; Leach, M.; Steel, A. Dietary Guideline Adherence during Preconception and Pregnancy: A Systematic Review. Matern. Child Nutr. 2020, 16, e12916. [Google Scholar] [CrossRef] [PubMed]
- Samura, T.; Steer, J.; Michelis, L.D.; Carroll, L.; Holland, E.; Perkins, R. Factors Associated with Excessive Gestational Weight Gain: Review of Current Literature. Glob. Adv. Health Med. 2016, 5, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Akgun, N.; Keskin, H.L.; Ustuner, I.; Pekcan, G.; Avsar, A.F. Factors Affecting Pregnancy Weight Gain and Relationships with Maternal/Fetal Outcomes in Turkey. SMJ 2017, 38, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Y.; Li, S.; Zhang, D. Maternal prepregnancy obesity and the risk of shoulder dystocia: A meta-analysis. BJOG 2018, 125, 407–413. [Google Scholar] [CrossRef]
- Balsells, M.; García-Patterson, A.; Corcoy, R. Systematic Review and Meta-Analysis on the Association of Prepregnancy Underweight and Miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 73–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations; University UN. Human Energy Requirements, Report of a Joint FAO/WHO/UNU Expert Consultation, Rome, Italy, 17–24 October 2001; WHO: Rome, Italy, 2004. [Google Scholar]
- Goldberg, G.R. Nutrition in pregnancy: The facts and fallacies. Nurs. Stand. 2003, 17, 39–42. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA 2017, 317, 2207. [Google Scholar] [CrossRef]
- Margerison Zilko, C.E.; Rehkopf, D.; Abrams, B. Association of Maternal Gestational Weight Gain with Short- and Long-Term Maternal and Child Health Outcomes. Am. J. Obstet. Gynecol. 2010, 202, e1–e574. [Google Scholar] [CrossRef]
- Vizcaino, E.; Grimalt, J.O.; Glomstad, B.; Fernández-Somoano, A.; Tardón, A. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environ. Health Perspect. 2014, 122, 873–879. [Google Scholar] [CrossRef]
- Bagherzadeh, R.; Gharibi, T.; Safavi, B.; Mohammadi, S.Z.; Karami, F.; Keshavarz, S. Pregnancy; an Opportunity to Return to a Healthy Lifestyle: A Qualitative Study. BMC Pregnancy Childbirth 2021, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Almalik, M.M.A.; Mosleh, S.M. Pregnant Women: What Do They Need to Know during Pregnancy? A Descriptive Study. Women Birth 2017, 30, 100–106. [Google Scholar] [CrossRef]
- Lennon, S.L. Risk Perception in Pregnancy: A Concept Analysis. J. Adv. Nurs. 2016, 72, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Phelan, S. Pregnancy: A “Teachable Moment” for Weight Control and Obesity Prevention. Am. J. Obstet. Gynecol. 2010, 202, e1–e135. [Google Scholar] [CrossRef] [PubMed]
- Cori, L.; Bianchi, F.; Sprovieri, M.; Cuttitta, A.; Ruggieri, S.; Alessi, A.L.; Biondo, G.; Gorini, F. Communication and Community Involvement to Support Risk Governance. Int. J. Environ. Res. Public Health 2019, 16, 4356. [Google Scholar] [CrossRef] [Green Version]

| Maternal Characteristics | AUGUSTA-PRIOLO (n = 534) | CROTONE (n = 165) | MILAZZO (n = 117) | p Value | COHORT (n = 816) |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 30.4 (±5.1) | 30.5 (±5.4) | 31.5 (±4.5) | 0.12 * | 30.6 (±5.1) |
| (n = 533) | (n = 164) | (n = 116) | (n = 813) | ||
| Pre-gravidic BMI, kg/m2 (mean ± SD) | 23.6 (±4.9) | 23.7 (±5.1) | 22.9 (±3.7) | 0.30 * | 23.5 (±4.8) |
| Educational level | <0.001 ** | ||||
| Second school or lower classification | 153 (28.7%) | 40 (24.2%) | 12 (10.3%) | 205 (25.1%) | |
| High school | 264 (49.4%) | 83 (50.3%) | 62 (53.0%) | 409 (50.1%) | |
| Degree or higher qualification | 117 (21.9%) | 42 (25.5%) | 43 (36.7%) | 202 (24.8%) | |
| (n = 532) | (n = 165) | (n = 117) | (n = 814) | ||
| Marital status | 0.11 ** | ||||
| Unmarried | 208 (39.1%) | 50 (30.3%) | 41 (35.0%) | 299 (36.7%) | |
| Married | 324 (60.9%) | 115 (69.7%) | 76 (65.0%) | 515 (63.3%) |
| Pregnancy Behaviors | AUGUSTA-PRIOLO (n = 534) | CROTONE (n = 165) | MILAZZO (n = 117) | p Value | COHORT (n = 816) |
|---|---|---|---|---|---|
| (n = 473) | (n = 137) | (n = 116) | (n = 726) | ||
| Weight gain, kg (mean ± SD) | 12.1 (±4.0) | 11.2 (±4.4) | 12.9 (±3.8) | <0.01 * | 12.8 (±) |
| (n = 532) | (n = 164) | (n = 117) | (n = 813) | ||
| Smoking | 0.60 ** | ||||
| No | 471 (88.5%) | 144 (87.8%) | 107 (91.5%) | 722 (88.8%) | |
| Yes | 61 (11.5%) | 20 (12.2%) | 10 (8.5%) | 91 (11.2%) | |
| (n = 533) | (n = 158) | (n = 117) | (n = 808) | ||
| Alcohol consumption | <0.001 ** | ||||
| No | 526 (98.7%) | 142 (89.9%) | 101 (86.3%) | 769 (95.2%) | |
| Yes | 7 (1.3%) | 16 (10.1%) | 16 (13.7%) | 39 (4.8%) | |
| (n = 534) | (n = 165) | (n = 117) | (n = 816) | ||
| Folic acid supplements | <0.001 ** | ||||
| No | 8 (1.5%) | 24 (14.5%) | 49 (41.9%) | 81 (9.9%) | |
| Yes | 526 (98.5%) | 141 (85.5%) | 68 (58.1%) | 735 (90.1%) | |
| (n = 534) | (n = 165) | (n = 117) | (n = 816) | ||
| Iron supplements | <0.001 ** | ||||
| No | 205 (38.4%) | 102 (61.8%) | 72 (61.5%) | 379 (46.4%) | |
| Yes | 329 (61.6%) | 63 (38.2%) | 45 (38.5%) | 437 (53.6%) | |
| (n = 534) | (n = 165) | (n = 117) | (n = 816) | ||
| Multivitamin supplements | <0.001 ** | ||||
| No | 211 (39.5%) | 149 (90.3%) | 42 (35.9%) | 402 (49.3%) | |
| Yes | 323 (60.5%) | 16 (9.7%) | 75 (64.1%) | 414 (50.7%) | |
| (n = 534) | (n = 165) | (n = 117) | (n = 816) | ||
| Natural supplements | <0.001 ** | ||||
| No | 532 (99.6%) | 162 (98.2%) | 74 (63.2%) | 768 (94.1%) | |
| Yes | 2 (0.4%) | 3 (1.8%) | 43 (36.8%) | 48 (5.9%) | |
| (n = 534) | (n = 165) | (n = 117) | (n = 816) | ||
| Sport activity | <0.001 ** | ||||
| No | 514 (96.3%) | 144 (87.3%) | 103 (88.0%) | 761 (93.3%) | |
| Yes | 20 (3.7%) | 21 (12.7%) | 14 (12.0%) | 55 (6.7%) |
| Maternal Characteristics | CLUSTER 3/ PRUDENT (n = 144) | CLUSTER 2/ HIGH ENERGY (n = 217) | CLUSTER 1/ VEGETARIAN (n = 455) | p Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 31.5 (±4.5) | 29.4 (±5.3) | 30.8 (±5.1) | <0.001 * |
| (n = 144) | (n = 215) | (n = 454) | ||
| Pre-gravidic BMI, kg/m2 (mean ± SD) | 23.1 (±4.0) | 23.3 (±4.6) | 23.7 (±5.0) | 0.40 * |
| SIN | <0.001 ** | |||
| Augusta-Priolo | 40 (27.8%) | 116 (53.5%) | 378 (83.1%) | |
| Crotone | 49 (34.0%) | 58 (26.7%) | 58 (12.7%) | |
| Milazzo | 55 (38.2%) | 43 (19.8%) | 19 (4.2%) | |
| Educational level | <0.001 ** | |||
| Second school or lower qualification | 15 (10.4%) | 66 (30.4%) | 124 (27.2%) | |
| High school | 77 (53.5%) | 110 (50.7%) | 222 (48.8%) | |
| Degree or higher qualification | 52 (36.1%) | 41 (18.9%) | 109 (24.0%) | |
| (n = 143) | (n = 217) | (n = 454) | ||
| Marital status | 0.08 ** | |||
| Unmarried | 42 (29.4%) | 89 (41.0%) | 168 (37.0%) | |
| Married | 101 (70.6%) | 128 (59.0%) | 286 (63.0%) |
| Pregnancy Behaviors | CLUSTER 3/ PRUDENT (n = 144) | CLUSTER 2/ HIGH ENERGY (n = 217) | CLUSTER 1/ VEGETARIAN (n = 455) | p Value |
|---|---|---|---|---|
| (n = 127) | (n = 180) | (n = 419) | ||
| Weight gain, kg (mean ± SD) | 11.8 (±3.8) | 12.4 (±4.3) | 12.0 (±4.0) | 0.40 * |
| Smoking | (n = 143) | (n = 217) | (n = 453) | 0.09 ** |
| No | 13 (90.9%) | 184 (84.8%) | 408 (90.1%) | |
| Yes | 13 (9.1%) | 33 (15.2%) | 45 (9.9%) | |
| (n = 141) | (n = 213) | (n = 454) | ||
| Alcohol consumption | <0.001 ** | |||
| No | 128 (90.8%) | 198 (93.0%) | 443 (97.6%) | |
| Yes | 13 (9.2%) | 15 (7.0%) | 11 (2.4%) | |
| Folic acid supplements | <0.001 ** | |||
| No | 31 (21.5%) | 27 (12.4%) | 23 (5.1%) | |
| Yes | 113 (78.5%) | 190 (87.6%) | 432 (94.9%) | |
| Iron supplements | <0.001 ** | |||
| No | 89 (61.8%) | 107 (49.3%) | 183 (40.2%) | |
| Yes | 55 (38.2%) | 110 (50.7%) | 272 (59.8%) | |
| Multivitamin supplements | <0.001 ** | |||
| No | 85 (59.0%) | 141 (65.0%) | 176 (38.7%) | |
| Yes | 59(41.0%) | 76 (35.0%) | 279 (61.3%) | |
| Natural supplements | <0.001 ** | |||
| No | 130 (90.3%) | 197 (90.8%) | 441 (96.9%) | |
| Yes | 14 (9.7%) | 20 (9.2%) | 14 (3.1%) | |
| Sport activity | <0.001 ** | |||
| No | 122 (84.7%) | 204 (94.0%) | 435 (95.6%) | |
| Yes | 22 (15.3%) | 13 (6.0%) | 20 (4.4%) |
| Variables | CLUSTER 3/PRUDENT | CLUSTER 1/VEGETARIAN | ||
|---|---|---|---|---|
| Coefficients | p Value | Coefficients | p Value | |
| Educational level | ||||
| High school | 1.05 | <0.01 | 0.02 | 0.93 |
| Degree or higher qualification | 1.45 | <0.001 | 0.19 | 0.44 |
| Age, years | 0.06 | <0.01 | 0.05 | <0.01 |
| Alcohol consumption (yes) | 0.13 | 0.74 | −1.20 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggieri, S.; Drago, G.; Panunzi, S.; Rizzo, G.; Tavormina, E.E.; Maltese, S.; Cibella, F. The Influence of Sociodemographic Factors, Lifestyle, and Risk Perception on Dietary Patterns in Pregnant Women Living in Highly Contaminated Areas: Data from the NEHO Birth Cohort. Nutrients 2022, 14, 3489. https://doi.org/10.3390/nu14173489
Ruggieri S, Drago G, Panunzi S, Rizzo G, Tavormina EE, Maltese S, Cibella F. The Influence of Sociodemographic Factors, Lifestyle, and Risk Perception on Dietary Patterns in Pregnant Women Living in Highly Contaminated Areas: Data from the NEHO Birth Cohort. Nutrients. 2022; 14(17):3489. https://doi.org/10.3390/nu14173489
Chicago/Turabian StyleRuggieri, Silvia, Gaspare Drago, Simona Panunzi, Giulia Rizzo, Elisa Eleonora Tavormina, Sabina Maltese, and Fabio Cibella. 2022. "The Influence of Sociodemographic Factors, Lifestyle, and Risk Perception on Dietary Patterns in Pregnant Women Living in Highly Contaminated Areas: Data from the NEHO Birth Cohort" Nutrients 14, no. 17: 3489. https://doi.org/10.3390/nu14173489
APA StyleRuggieri, S., Drago, G., Panunzi, S., Rizzo, G., Tavormina, E. E., Maltese, S., & Cibella, F. (2022). The Influence of Sociodemographic Factors, Lifestyle, and Risk Perception on Dietary Patterns in Pregnant Women Living in Highly Contaminated Areas: Data from the NEHO Birth Cohort. Nutrients, 14(17), 3489. https://doi.org/10.3390/nu14173489