Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Search
2.2. Study Selection
2.3. Data Abstraction
2.4. Statistical Analyses
3. Results
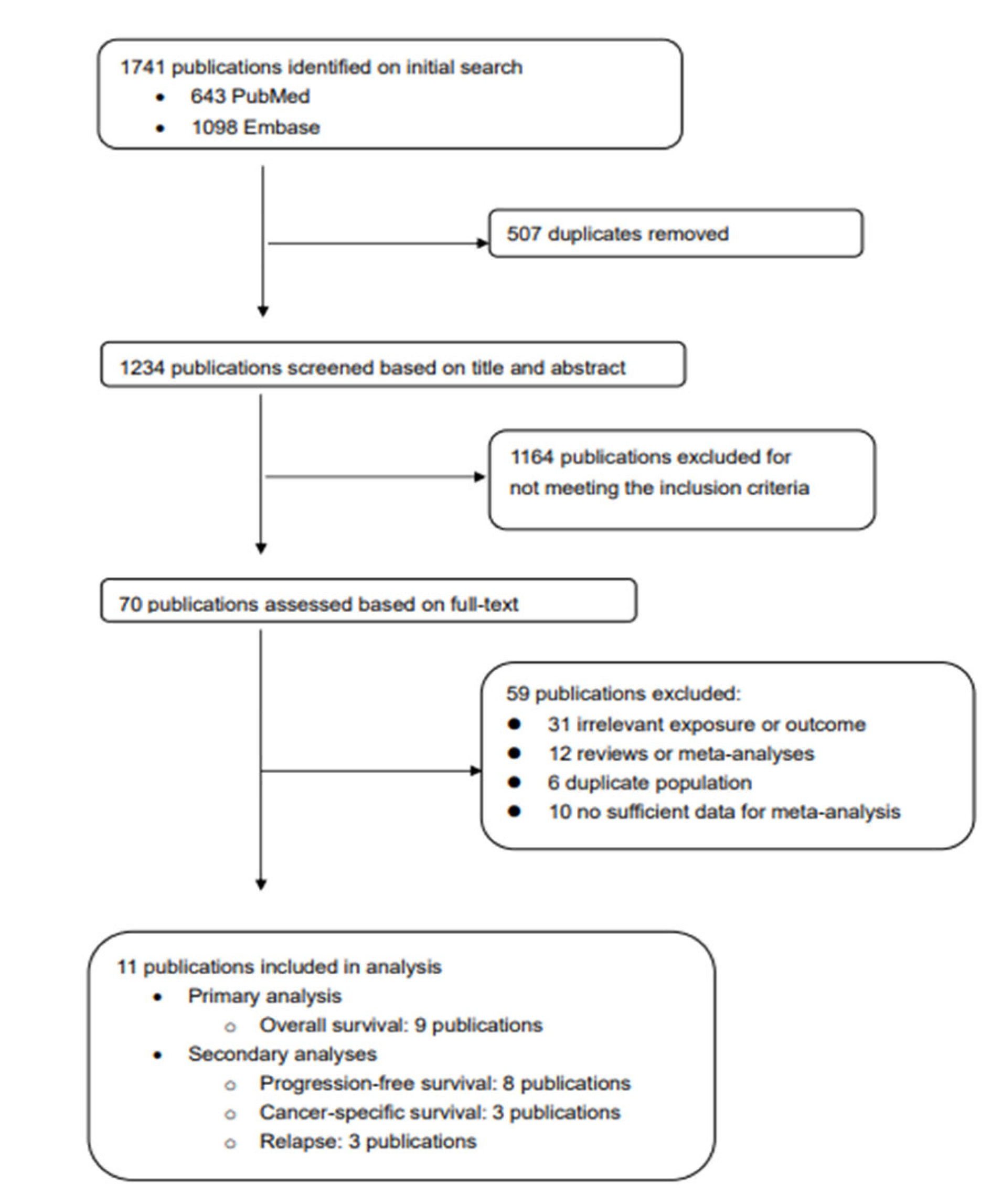
3.1. Description of Included Studies
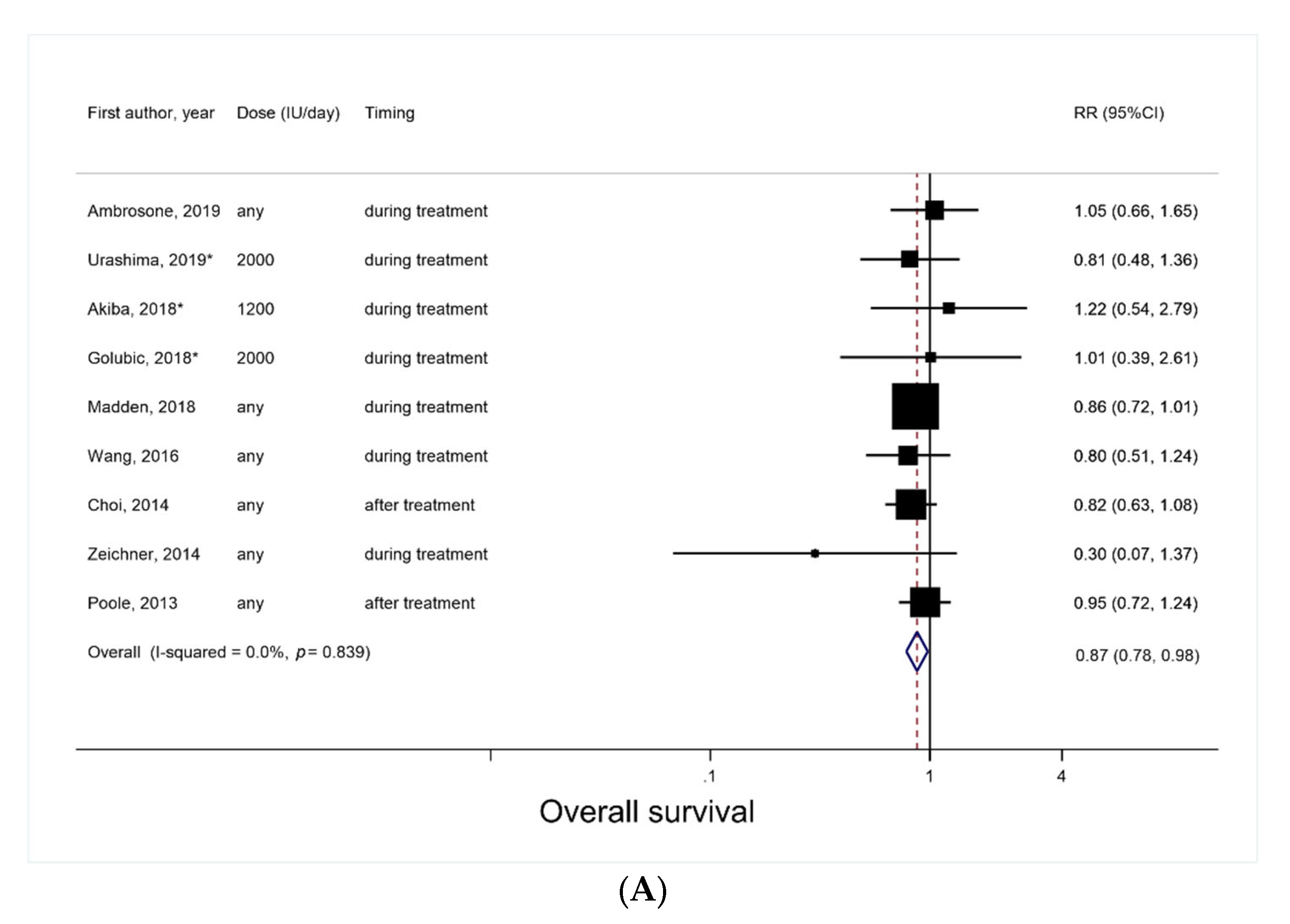
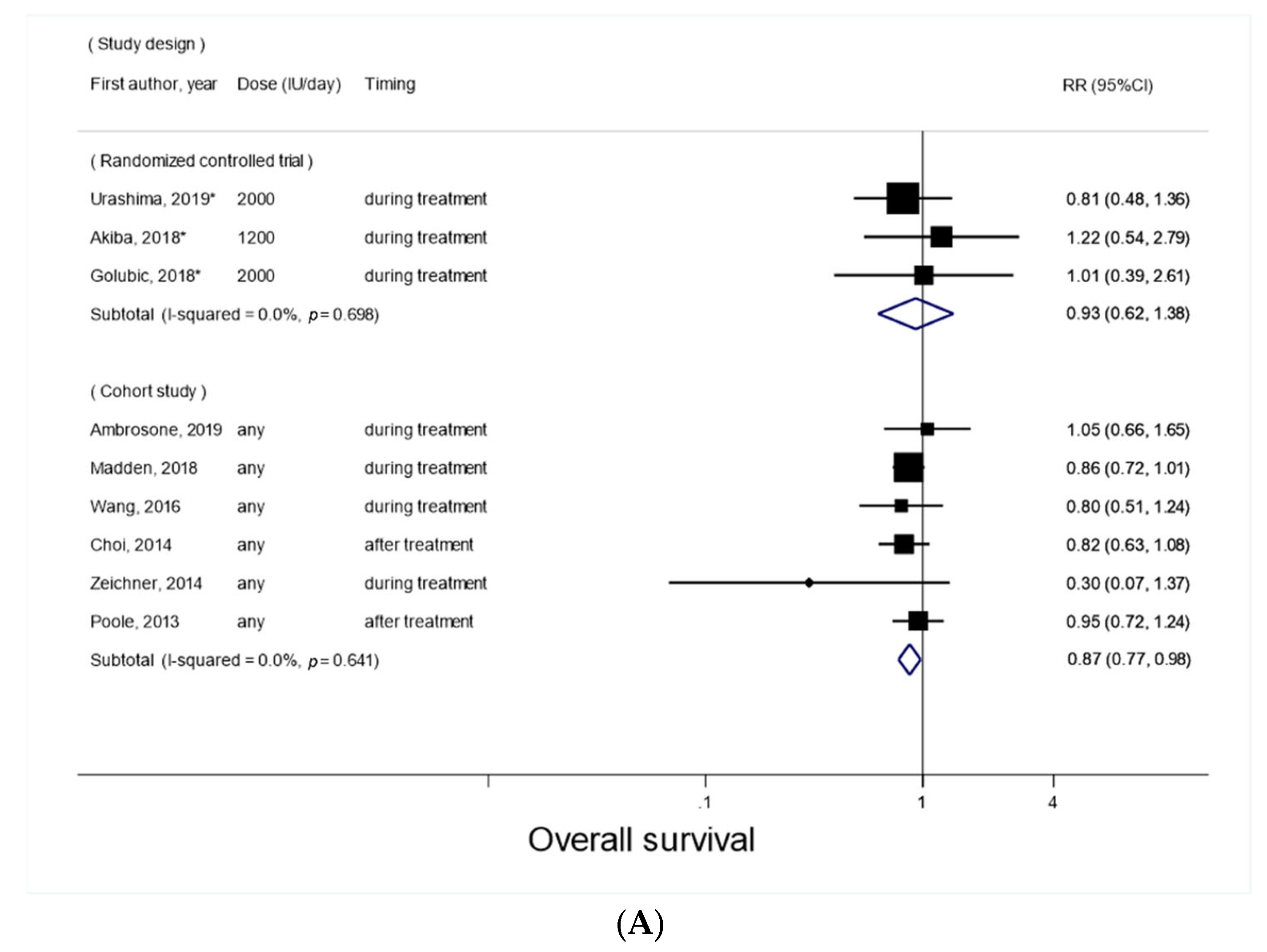
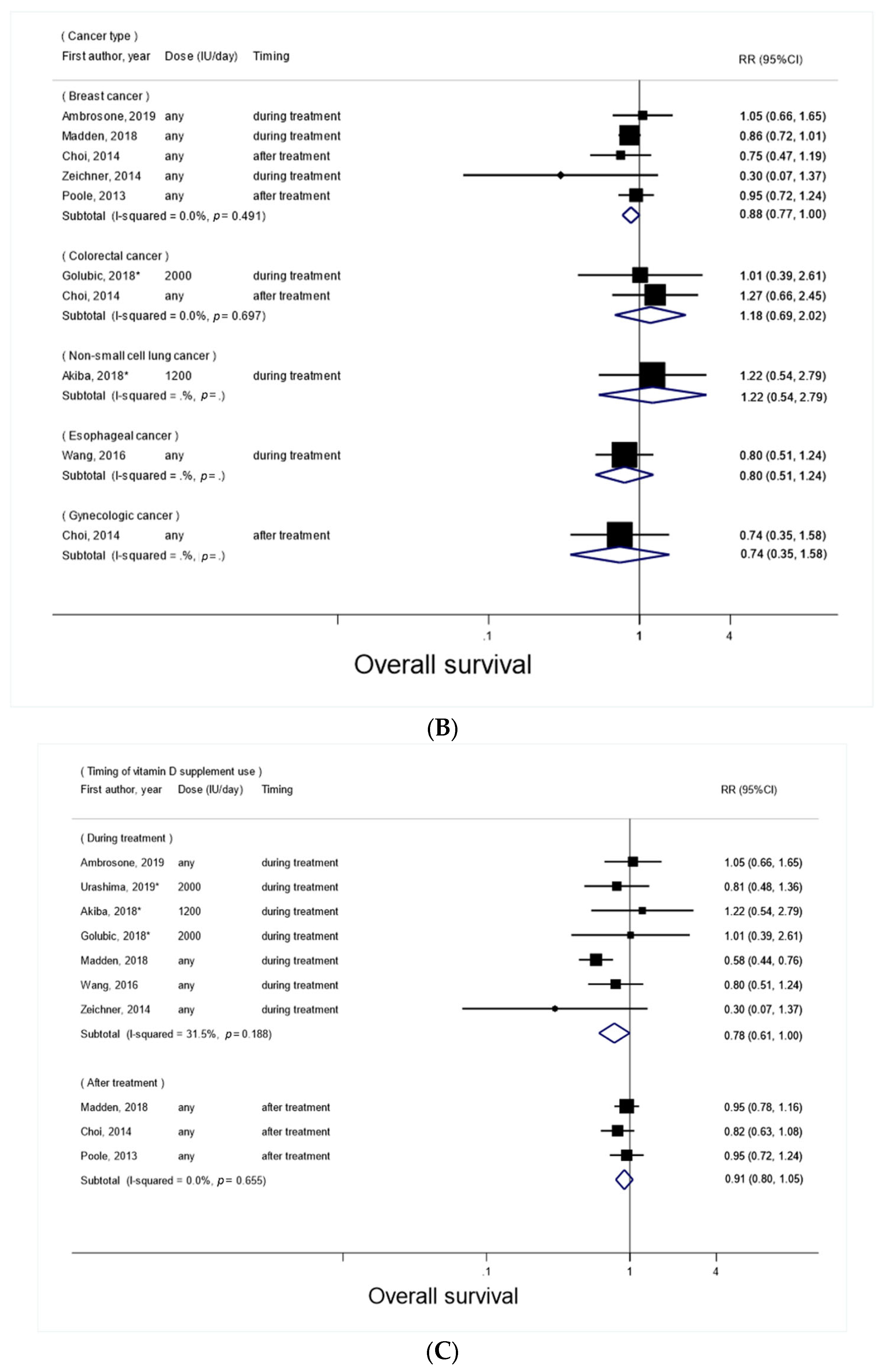
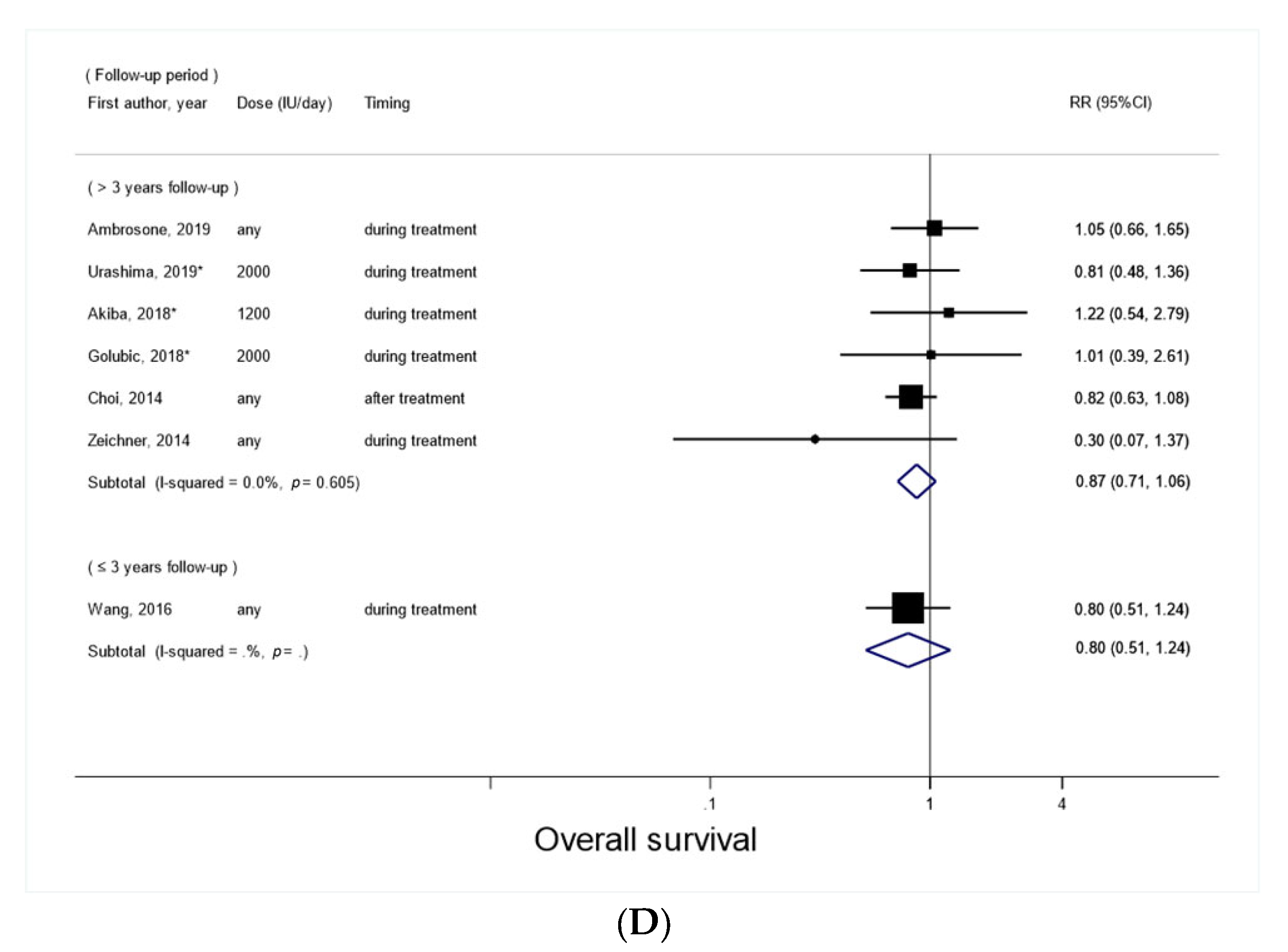
3.2. Vitamin D Supplement Use after Cancer Diagnosis and Overall Survival
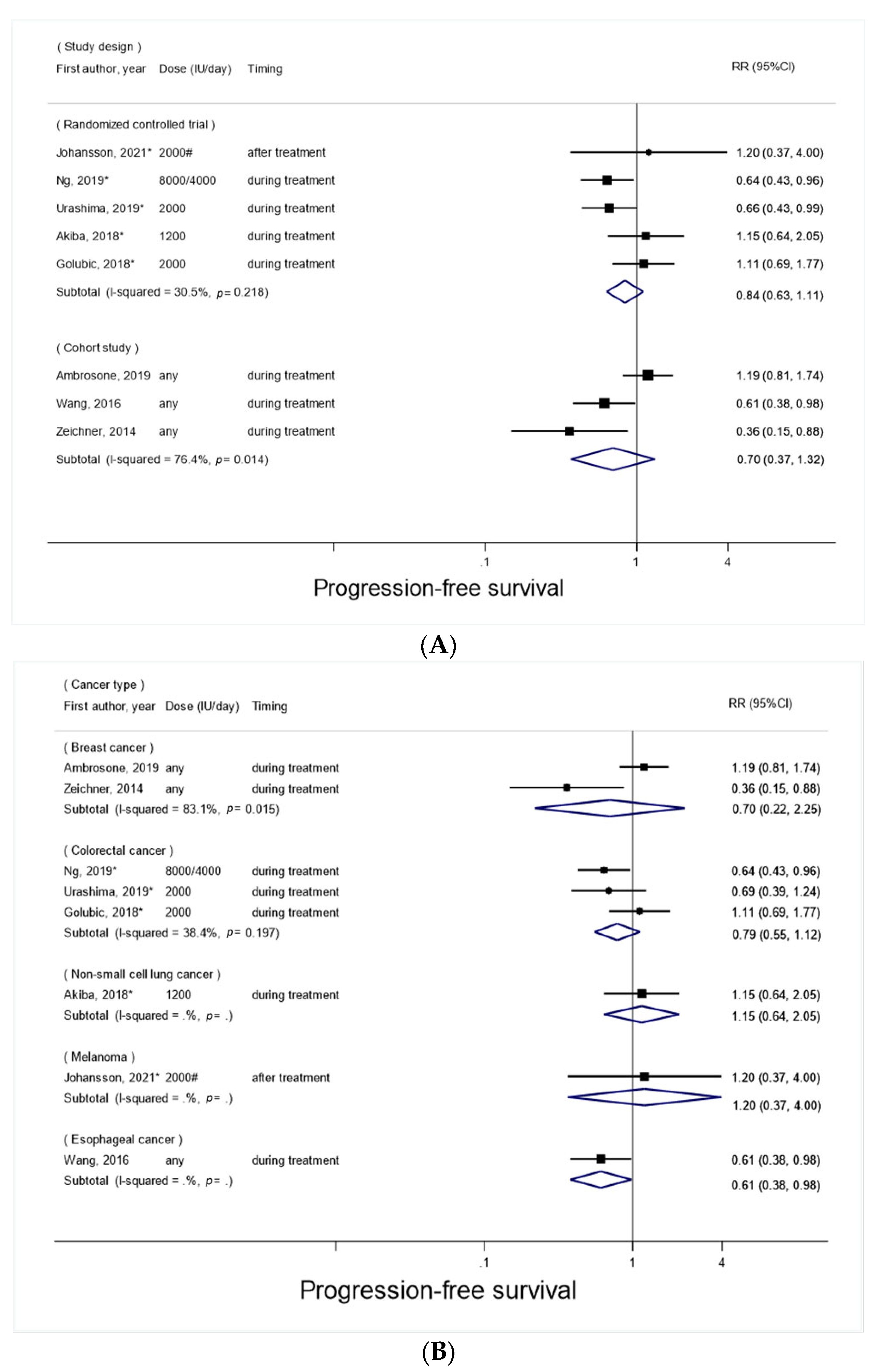
3.3. Vitamin D Supplement Use after Cancer Diagnosis and Progression-Free Survival
3.4. Vitamin D Supplement Use after Cancer Diagnosis and Cancer-Specific Survival
3.5. Vitamin D Supplement use after Cancer Diagnosis and Relapse
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. Cancer. 2022. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 12 June 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulou, A.; Riza, E.; Samoli, E.; Benetou, V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Pouchieu, C.; Fassier, P.; Druesne-Pecollo, N.; Zelek, L.; Bachmann, P.; Touillaud, M.; Bairati, I.; Hercberg, S.; Galan, P.; Cohen, P.; et al. Dietary supplement use among cancer survivors of the NutriNet-Santé cohort study. Br. J. Nutr. 2015, 113, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.M.; McCorkle, R.; Smith, T.; Stein, K.D.; Cartmel, B. Factors related to the use of dietary supplements by cancer survivors. J. Altern. Complement. Med. 2009, 15, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Chen, Q.-Y.; Lee, D.H.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: A meta-analysis of randomised controlled trials. Br. J. Cancer 2022, 1–7. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef]
- Brown, J.C.; Rosenthal, M.H.; Ma, C.; Zhang, S.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; et al. Effect of High-Dose vs Standard-Dose Vitamin D(3) Supplementation on Body Composition among Patients with Advanced or Metastatic Colorectal Cancer: A Randomized Trial. Cancers 2020, 12, 3451. [Google Scholar] [CrossRef]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D Supplementation and Survival of Patients with Non-small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef]
- Golubić, Z.A.; Barsic, I.; Librenjak, N.; Plestina, S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer. Nutr. Cancer 2018, 70, 413–417. [Google Scholar] [CrossRef]
- Johansson, H.; Spadola, G.; Tosti, G.; Mandalà, M.; Minisini, A.; Queirolo, P.; Aristarco, V.; Baldini, F.; Cocorocchio, E.; Albertazzi, E.; et al. Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients 2021, 13, 1931. [Google Scholar] [CrossRef]
- Urashima, M.; Ohdaira, H.; Akutsu, T.; Okada, S.; Yoshida, M.; Kitajima, M.; Suzuki, Y. Effect of Vitamin D Supplementation on Relapse-Free Survival among Patients with Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA 2019, 321, 1361–1369. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use during Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Greenlee, H.; Oppeneer, S.J.; Robien, K. The association between postdiagnosis dietary supplement use and total mortality differs by diet quality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2014, 23, 865–875. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Pierce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013, 139, 529–537. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wang, J.; Huang, X.; Cheng, Y. Longitudinal, observational study on associations between postoperative nutritional vitamin D supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci. Rep. 2016, 6, 38962. [Google Scholar] [CrossRef]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N.; Liu, Q.; Markward, N.J.; Montero, A.J.; Glück, S.; Silva, O.; Ahn, E.R. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Du, M.; Luo, H.; Blumberg, J.B.; Rogers, G.; Chen, F.; Ruan, M.; Shan, Z.; Biever, E.; Zhang, F.F. Dietary Supplement Use among Adult Cancer Survivors in the United States. J. Nutr. 2020, 150, 1499–1508. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Lips, P.; Eekhoff, M.; van Schoor, N.; Oosterwerff, M.; de Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef]
- Scragg, R. Emerging Evidence of Thresholds for Beneficial Effects from Vitamin D Supplementation. Nutrients 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.; Li, J.; Chu, R.; Xie, D.; Wang, H. Review: The impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Z.; Shi, H.; Wang, C. Prognostic role of vitamin D receptor in breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 1051. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Feldman, D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 2012, 77, 1107–1112. [Google Scholar] [CrossRef]
- Chakraborti, C.K. Vitamin D as a promising anticancer agent. Indian J. Pharmacol. 2011, 43, 113–120. [Google Scholar] [CrossRef]
- Mazess, R.B.; Bischoff-Ferrari, H.A.; Dawson-Hughes, B. Vitamin D: Bolus Is Bogus-A Narrative Review. JBMR Plus 2021, 5, e10567. [Google Scholar] [CrossRef]
- Ataide, F.L.; Bastos, L.M.C.; Matias, M.F.V.; Skare, T.L.; de Carvalho, J.F. Safety and effectiveness of vitamin D mega-dose: A systematic review. Clin. Nutr. ESPEN 2021, 46, 115–120. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-Y.; Kim, S.; Lee, B.; Jeong, G.; Lee, D.H.; Keum, N.; Manson, J.E.; Giovannucci, E.L. Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients 2022, 14, 3418. https://doi.org/10.3390/nu14163418
Chen Q-Y, Kim S, Lee B, Jeong G, Lee DH, Keum N, Manson JE, Giovannucci EL. Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients. 2022; 14(16):3418. https://doi.org/10.3390/nu14163418
Chicago/Turabian StyleChen, Qiao-Yi, Sohyun Kim, Bohyoon Lee, Gyeongin Jeong, Dong Hoon Lee, NaNa Keum, JoAnn E. Manson, and Edward L. Giovannucci. 2022. "Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis" Nutrients 14, no. 16: 3418. https://doi.org/10.3390/nu14163418
APA StyleChen, Q.-Y., Kim, S., Lee, B., Jeong, G., Lee, D. H., Keum, N., Manson, J. E., & Giovannucci, E. L. (2022). Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients, 14(16), 3418. https://doi.org/10.3390/nu14163418





