Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. In Vivo Treatments
2.3. Hematological Parameter Test
2.4. HSC Pool Analysis and Sorting
2.5. Cytoplasmic Protein Expression Analysis
2.6. Nuclear Protein Expression Analysis
2.7. HSC Apoptosis Analysis
2.8. Mitochondrial Membrane Potential (MMP) Analysis
2.9. Pi Determination
2.10. Gene Set Enrichment Analysis (GSEA)
2.11. Quantitative Polymerase Chain Reaction (qPCR)
2.12. Transcription Factor Binding Profile Analysis
2.13. Chromatin Immunoprecipitation (ChIP)
2.14. Statistical Analysis
3. Results
3.1. Irradiation-Induced Myelosuppression Accompanies Pi Loss in BM Niche and Pi Metabolic Inhibition in HSCs
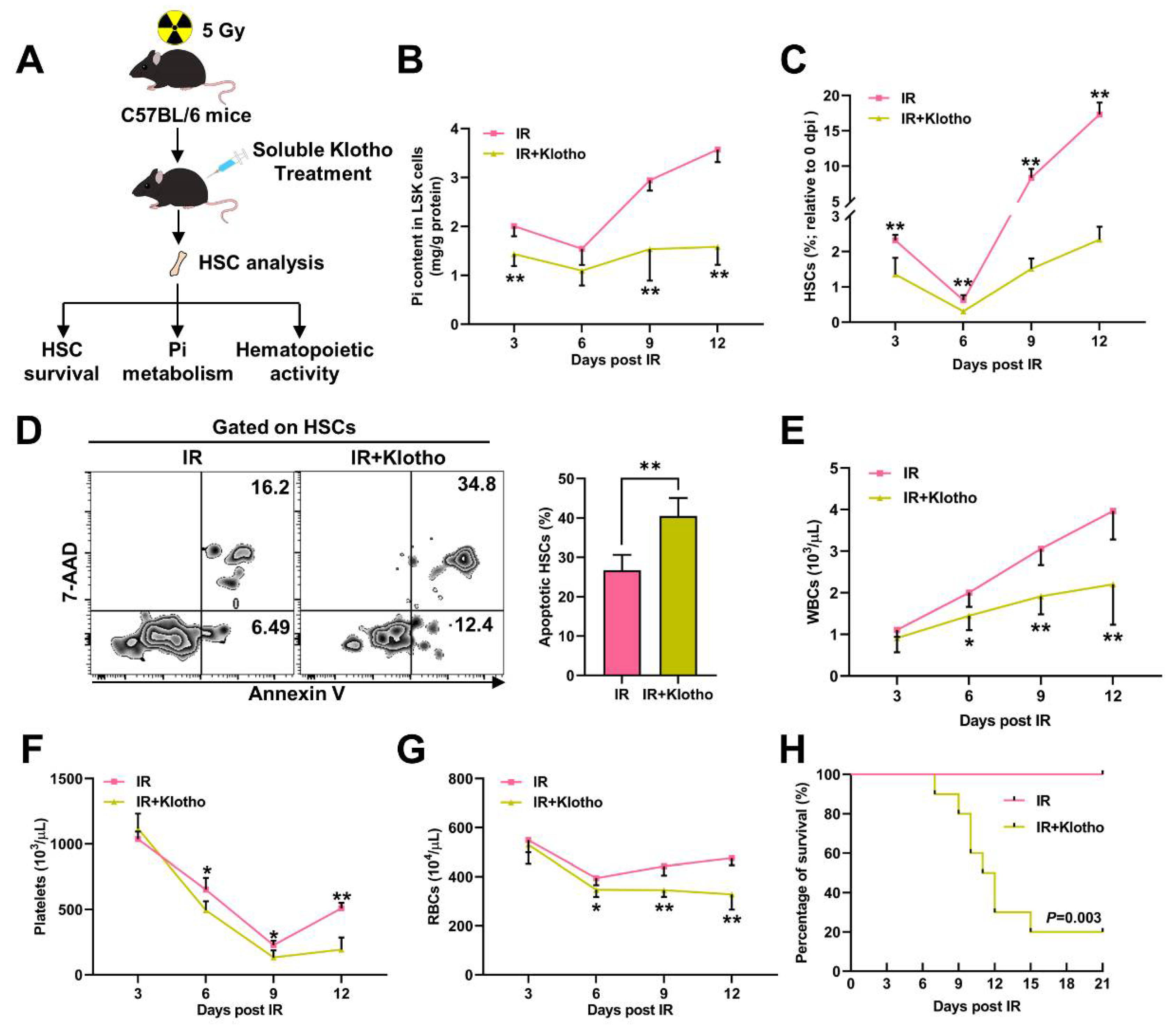
3.2. Pi Metabolic Inhibition Dampens HSC Survival Post IR
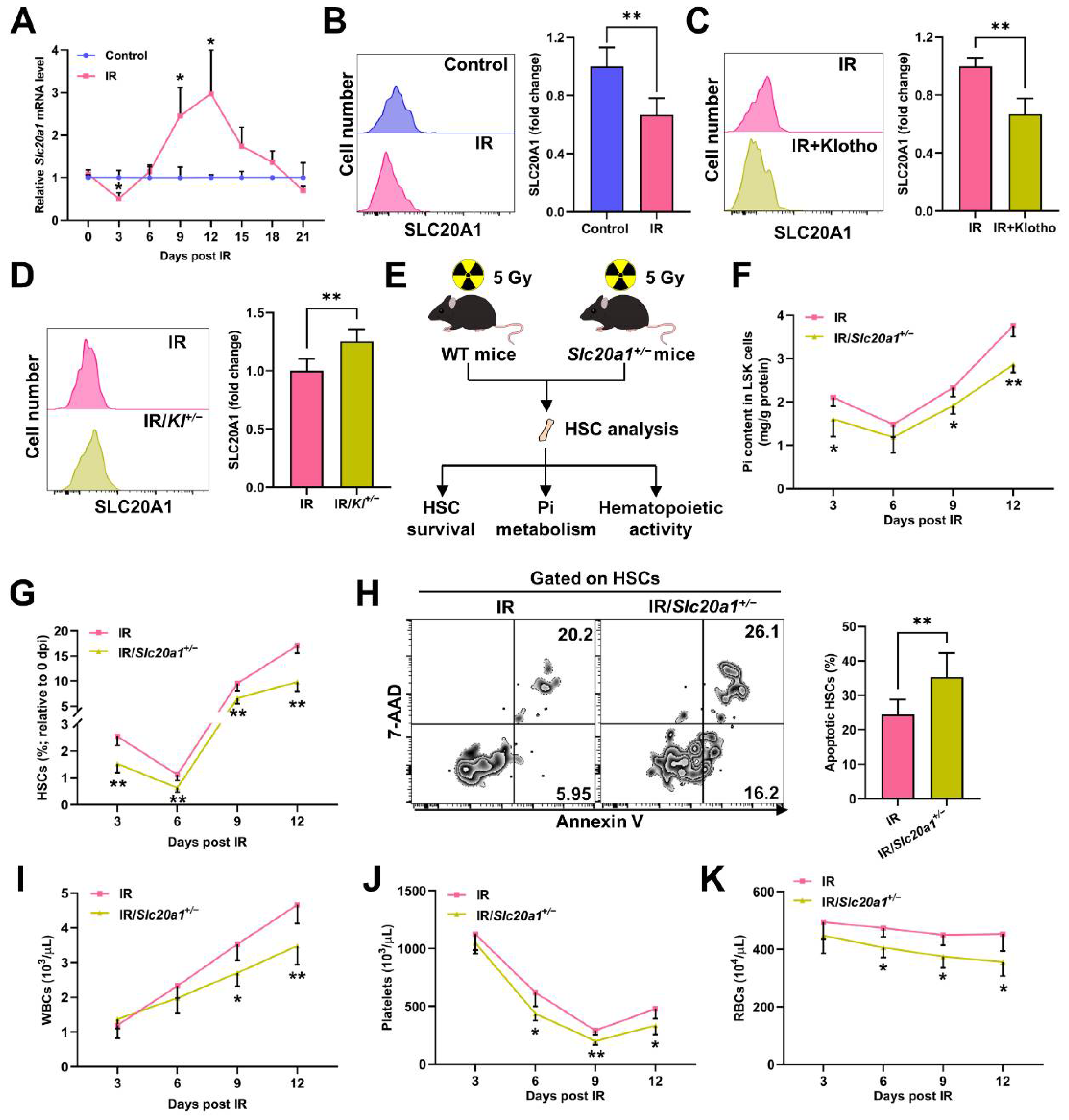
3.3. SLC20A1 Is Indispensable for Pi Metabolism and Survival of HSCs Post IR
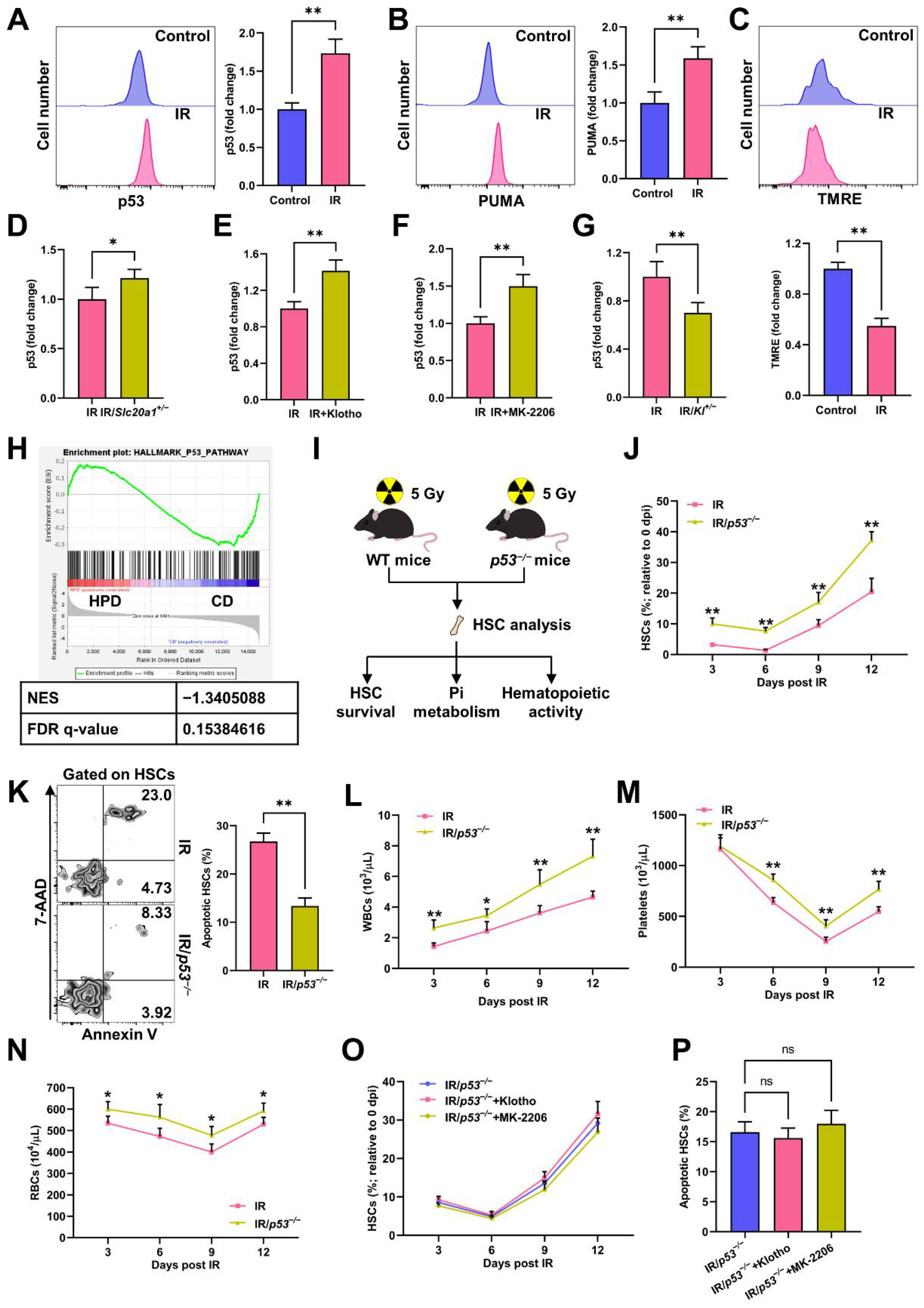
3.4. Akt Counteracts Pi Metabolic Inhibition and Apoptosis of HSCs Post IR
3.5. p53 Promotes Pi Metabolic Inhibition and Apoptosis of HSCs Post IR
3.6. Pi Supplementation Promotes HSC Survival and Protects against Irradiation-Induced Myelosuppression
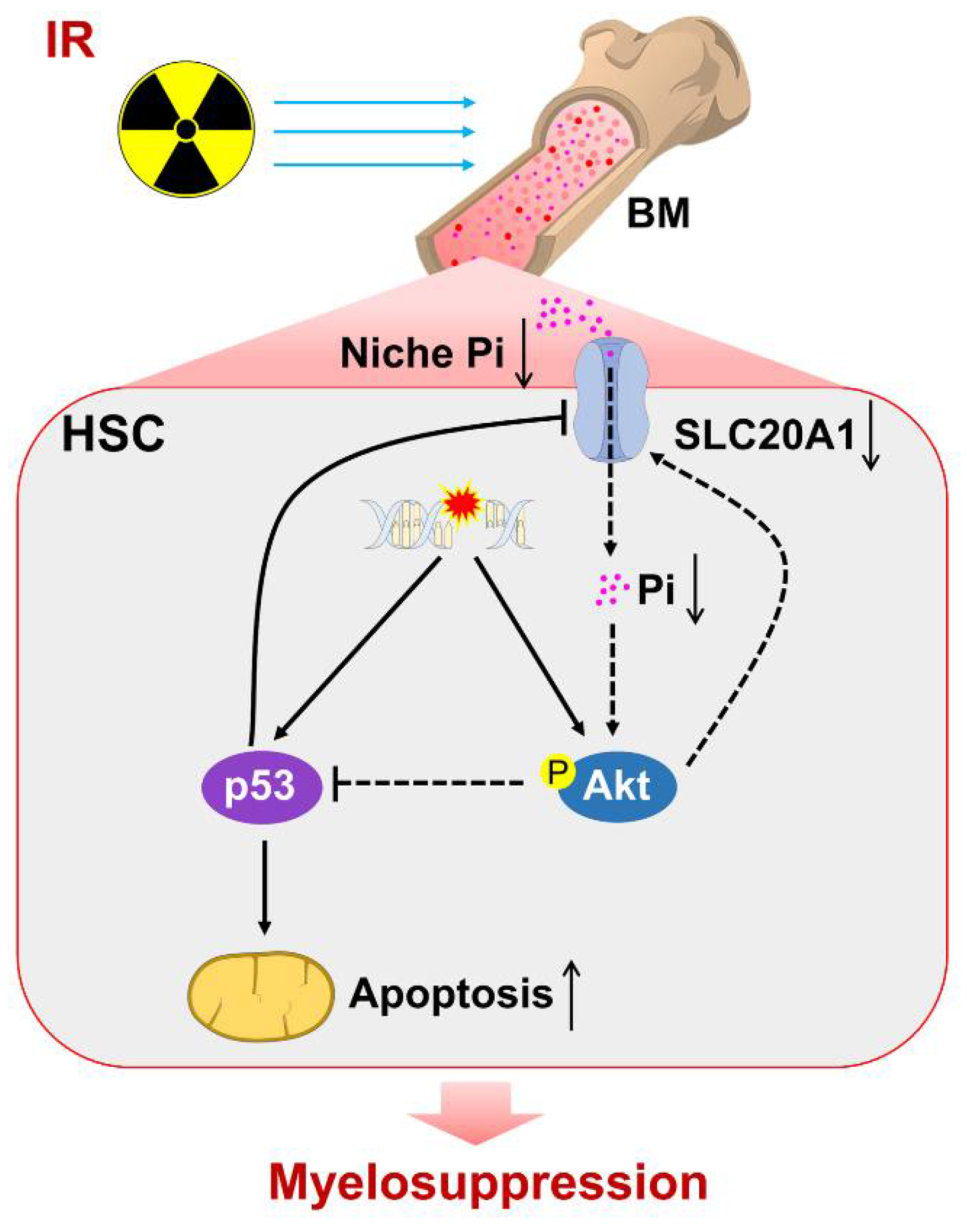
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar] [PubMed]
- Brigle, K.; Pierre, A.; Finley-Oliver, E.; Faiman, B.; Tariman, J.D.; Miceli, T. Myelosuppression, Bone Disease, and Acute Renal Failure: Evidence-Based Recommendations for Oncologic Emergencies. Clin. J. Oncol. Nurs. 2017, 21, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Du, C.; Wang, J. The cGAS-STING Pathway in Hematopoiesis and Its Physiopathological Significance. Front. Immunol. 2020, 11, 573915. [Google Scholar] [CrossRef]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic Stem Cell Injury Induced by Ionizing Radiation. Antioxid. Redox Signal. 2013, 20, 1447–1462. [Google Scholar] [CrossRef]
- Henry, E.; Arcangeli, M.-L. How Hematopoietic Stem Cells Respond to Irradiation: Similarities and Differences between Low and High Doses of Ionizing Radiations. Exp. Hematol. 2021, 94, 11–19. [Google Scholar] [CrossRef]
- Pestina, T.I.; Cleveland, J.L.; Yang, C.; Zambetti, G.P.; Jackson, C.W. Mpl ligand prevents lethal myelosuppression by inhibiting p53-dependent apoptosis. Blood 2001, 98, 2084–2090. [Google Scholar] [CrossRef]
- Garrison, S.P.; Phillips, D.C.; Jeffers, J.R.; Chipuk, J.E.; Parsons, M.J.; Rehg, J.E.; Opferman, J.T.; Green, D.R.; Zambetti, G.P. Genetically defining the mechanism of Puma- and Bim-induced apoptosis. Cell Death Differ. 2012, 19, 642–649. [Google Scholar] [CrossRef]
- Asai, T.; Liu, Y.; Di Giandomenico, S.; Bae, N.; Ndiaye-Lobry, D.; Deblasio, A.; Menendez, S.; Antipin, Y.; Reva, B.; Wevrick, R.; et al. Necdin, a p53 target gene, regulates the quiescence and response to genotoxic stress of hematopoietic stem/progenitor cells. Blood 2012, 120, 1601–1612. [Google Scholar] [CrossRef]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Batsivari, A.; Haltalli, M.L.R.; Passaro, D.; Pospori, C.; Lo Celso, C.; Bonnet, D. Dynamic responses of the haematopoietic stem cell niche to diverse stresses. Nat. Cell Biol. 2020, 22, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef]
- Oburoglu, L.; Tardito, S.; Fritz, V.; de Barros Stéphanie, C.; Merida, P.; Craveiro, M.; Mamede, J.; Cretenet, G.; Mongellaz, C.; An, X.; et al. Glucose and Glutamine Metabolism Regulate Human Hematopoietic Stem Cell Lineage Specification. Cell Stem Cell 2014, 15, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Taya, Y.; Ota, Y.; Wilkinson Adam, C.; Kanazawa, A.; Watarai, H.; Kasai, M.; Nakauchi, H.; Yamazaki, S. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science 2016, 354, 1152–1155. [Google Scholar] [CrossRef]
- Luchsinger, L.L.; Strikoudis, A.; Danzl, N.M.; Bush, E.C.; Finlayson, M.O.; Satwani, P.; Sykes, M.; Yazawa, M.; Snoeck, H.-W. Harnessing Hematopoietic Stem Cell Low Intracellular Calcium Improves Their Maintenance In Vitro. Cell Stem Cell 2019, 25, 225–240.e227. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Li, H.; Borger, D.K.; Wei, Q.; Yang, E.; Xu, C.; Pinho, S.; Frenette, P.S. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 2022, 29, 232–247.e237. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Wu, Y.; Liao, W.; Xiong, J.; Zhu, Y.; Liu, C.; Han, W.; Wang, Y.; Han, S.; et al. Renal Klotho and inorganic phosphate are extrinsic factors that antagonistically regulate hematopoietic stem cell maintenance. Cell Rep. 2022, 38, 110392. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Ito, K.; Suda, T. Hematopoietic Stem Cell Metabolism during Development and Aging. Dev. Cell 2020, 54, 239–255. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Yamazaki, S. The hematopoietic stem cell diet. Int. J. Hematol. 2018, 107, 634–641. [Google Scholar] [CrossRef]
- Yi, L.; Song, C.; Hu, Z.; Yang, L.; Xiao, L.; Yi, B.; Jiang, W.; Cao, Y.; Sun, L. A metabolic discrimination model for nasopharyngeal carcinoma and its potential role in the therapeutic evaluation of radiotherapy. Metabolomics 2014, 10, 697–708. [Google Scholar] [CrossRef]
- Du, C.; Xu, Y.; Yang, K.; Chen, S.; Wang, X.; Wang, S.; Wang, C.; Shen, M.; Chen, F.; Chen, M.; et al. Estrogen promotes megakaryocyte polyploidization via estrogen receptor beta-mediated transcription of GATA1. Leukemia 2017, 31, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Liao, Y.; Xia, W.; Zou, Y.; Spohn, B.; Hung, M.-C. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 2001, 3, 973–982. [Google Scholar] [CrossRef]
- Fang, S.; Chen, S.; Nurmi, H.; Leppänen, V.-M.; Jeltsch, M.; Scadden, D.; Silberstein, L.; Mikkola, H.; Alitalo, K. VEGF-C protects the integrity of the bone marrow perivascular niche in mice. Blood 2020, 136, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Termini, C.M.; Pang, A.; Fang, T.; Roos, M.; Chang, V.Y.; Zhang, Y.; Setiawan, N.J.; Signaevskaia, L.; Li, M.; Kim, M.M.; et al. Neuropilin 1 regulates bone marrow vascular regeneration and hematopoietic reconstitution. Nat. Commun. 2021, 12, 6990. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Jeong, H.-W.; Stehling, M.; Dinh, V.V.; Zhou, B.; Adams, R.H. Apelin+ Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury. Cell Stem Cell 2019, 25, 768–783.e766. [Google Scholar] [CrossRef]
- Himburg, H.A.; Termini, C.M.; Schlussel, L.; Kan, J.; Li, M.; Zhao, L.; Fang, T.; Sasine, J.P.; Chang, V.Y.; Chute, J.P. Distinct Bone Marrow Sources of Pleiotrophin Control Hematopoietic Stem Cell Maintenance and Regeneration. Cell Stem Cell 2018, 23, 370–381.e375. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yu, H.; Yue, R.; Zhao, Z.; Rios, J.J.; Naveiras, O.; Morrison, S.J. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 2017, 19, 891–903. [Google Scholar] [CrossRef]
- Green, D.E.; Rubin, C.T. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone 2014, 63, 87–94. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt Signaling in a Mammalian Model of Accelerated Aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Floratou, K.; Giannopoulou, E.; Antonacopoulou, A.; Karakantza, M.; Adonakis, G.; Kardamakis, D.; Matsouka, P. Oxidative stress due to radiation in CD34+ Hematopoietic progenitor cells: Protection by IGF-1. J. Radiat. Res. 2012, 53, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Lento, W.; Ito, T.; Zhao, C.; Harris, J.R.; Huang, W.; Jiang, C.; Owzar, K.; Piryani, S.; Racioppi, L.; Chao, N.; et al. Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 2014, 28, 995–1004. [Google Scholar] [CrossRef]
- Pietras, E.M.; Reynaud, D.; Kang, Y.-A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Göttgens, B.; Passegué, E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 2015, 17, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Viniegra, J.G.; Martínez, N.; Modirassari, P.; Losa, J.H.; Cobo, C.P.; Lobo, V.J.S.-A.; Luquero, C.I.A.; Álvarez-Vallina, L.; Ramón y Cajal, S.; Rojas, J.M.; et al. Full Activation of PKB/Akt in Response to Insulin or Ionizing Radiation Is Mediated through ATM. J. Biol. Chem. 2005, 280, 4029–4036. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Monteiro, R.d.Q.; Rumjanek, F.D.; Meyer-Fernandes, J.R. Inorganic phosphate transporters in cancer: Functions, molecular mechanisms and possible clinical applications. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2018, 1870, 291–298. [Google Scholar] [CrossRef]
- Bergwitz, C.; Jüppner, H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- Edmonston, D.; Wolf, M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat. Rev. Nephrol. 2020, 16, 7–19. [Google Scholar] [CrossRef]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Heather, A.H.; Joshua, S.; Xiao, Y.; Jenny, K.; Holly, D.; John, P.C. A Molecular Profile of the Endothelial Cell Response to Ionizing Radiation. Radiat. Res. 2016, 186, 141–152. [Google Scholar]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.-C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Schwartz, A.; Kalickman, I.; Nisman, B.; Gurman, G.; Shoenfeld, Y. Prevalence of hypophosphatemia in sepsis and infection: The role of cytokines. Am. J. Med. 1998, 104, 40–47. [Google Scholar] [CrossRef]
- Raanani, P.; Levi, I.; Holzman, F.; Grotto, I.; Brok-Simoni, F.; Avigdor, A.; Davidson, J.; Shpilberg, O.; Ben-Bassat, I. Engraftment-associated hypophosphatemia--the role of cytokine release and steep leukocyte rise post stem cell transplantation. Bone Marrow Transplant. 2001, 27, 311–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kollek, M.; Voigt, G.; Molnar, C.; Murad, F.; Bertele, D.; Krombholz, C.F.; Bohler, S.; Labi, V.; Schiller, S.; Kunze, M.; et al. Transient apoptosis inhibition in donor stem cells improves hematopoietic stem cell transplantation. J. Exp. Med. 2017, 214, 2967–2983. [Google Scholar] [CrossRef]
- Baldridge, M.T.; King, K.Y.; Goodell, M.A. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011, 32, 57–65. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liao, W.; Chen, J.; Liu, C.; Zhang, S.; Yu, K.; Wang, X.; Chen, M.; Wang, S.; Ran, X.; et al. Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival. Nutrients 2022, 14, 3395. https://doi.org/10.3390/nu14163395
Wu Y, Liao W, Chen J, Liu C, Zhang S, Yu K, Wang X, Chen M, Wang S, Ran X, et al. Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival. Nutrients. 2022; 14(16):3395. https://doi.org/10.3390/nu14163395
Chicago/Turabian StyleWu, Yiding, Weinian Liao, Jun Chen, Chaonan Liu, Shuzhen Zhang, Kuan Yu, Xinmiao Wang, Mo Chen, Song Wang, Xinze Ran, and et al. 2022. "Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival" Nutrients 14, no. 16: 3395. https://doi.org/10.3390/nu14163395
APA StyleWu, Y., Liao, W., Chen, J., Liu, C., Zhang, S., Yu, K., Wang, X., Chen, M., Wang, S., Ran, X., Su, Y., Cheng, T., Wang, J., & Du, C. (2022). Phosphate Metabolic Inhibition Contributes to Irradiation-Induced Myelosuppression through Dampening Hematopoietic Stem Cell Survival. Nutrients, 14(16), 3395. https://doi.org/10.3390/nu14163395





