Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. UPLC-MS
2.3. Metabolite Enrichment Analysis
2.4. Metabolic Activity Ex Vivo
2.5. Ex Vivo Absorption of Fluorescently Labeled Metabolites
2.6. Immunofluorescence In Situ
2.6.1. CD31/COLIV
2.6.2. VEGF/CD31
2.6.3. TSP-1/CD31
2.7. Quantitative (Immuno-)Histomorphometry
2.8. Statistics
3. Results
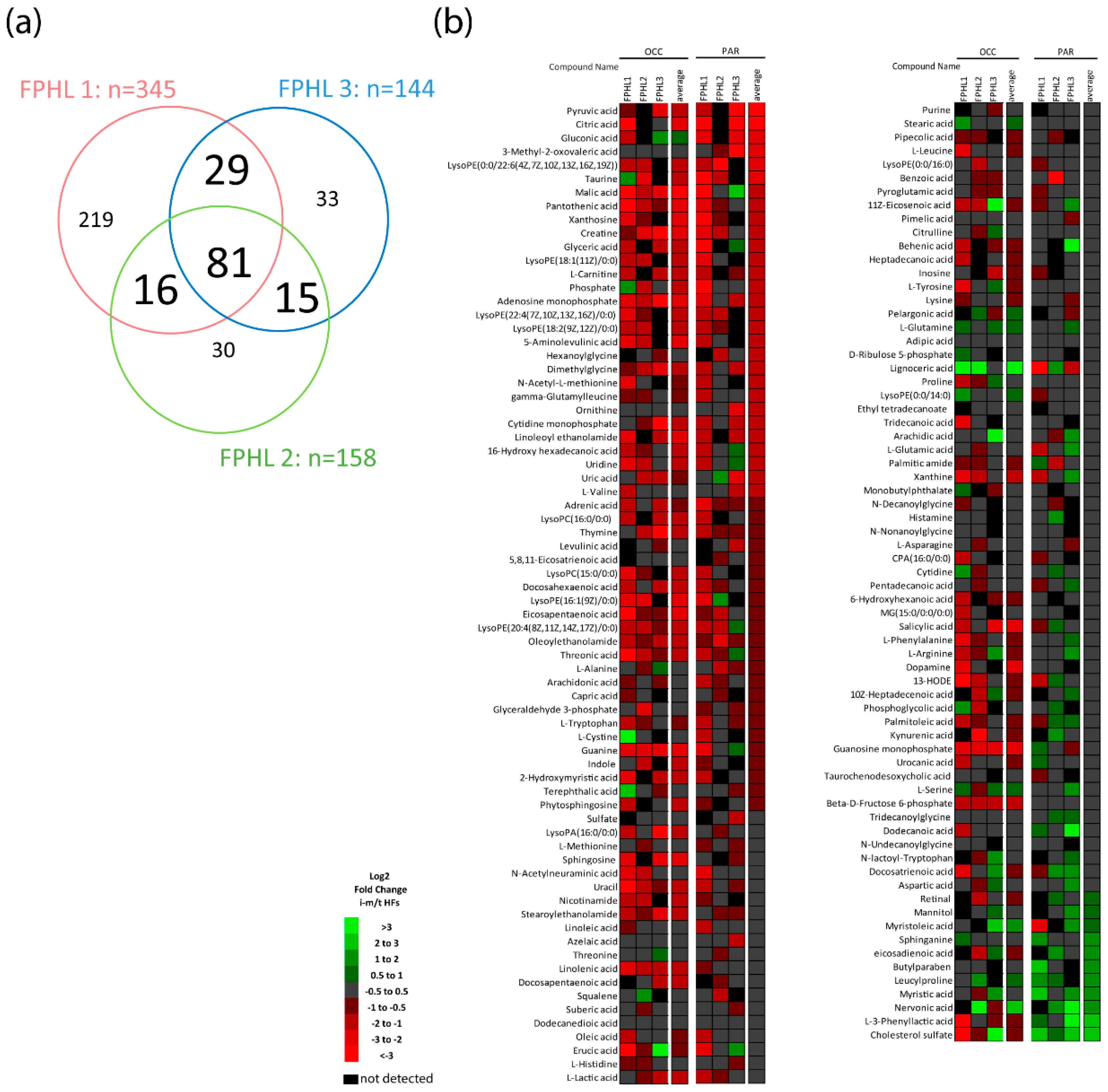
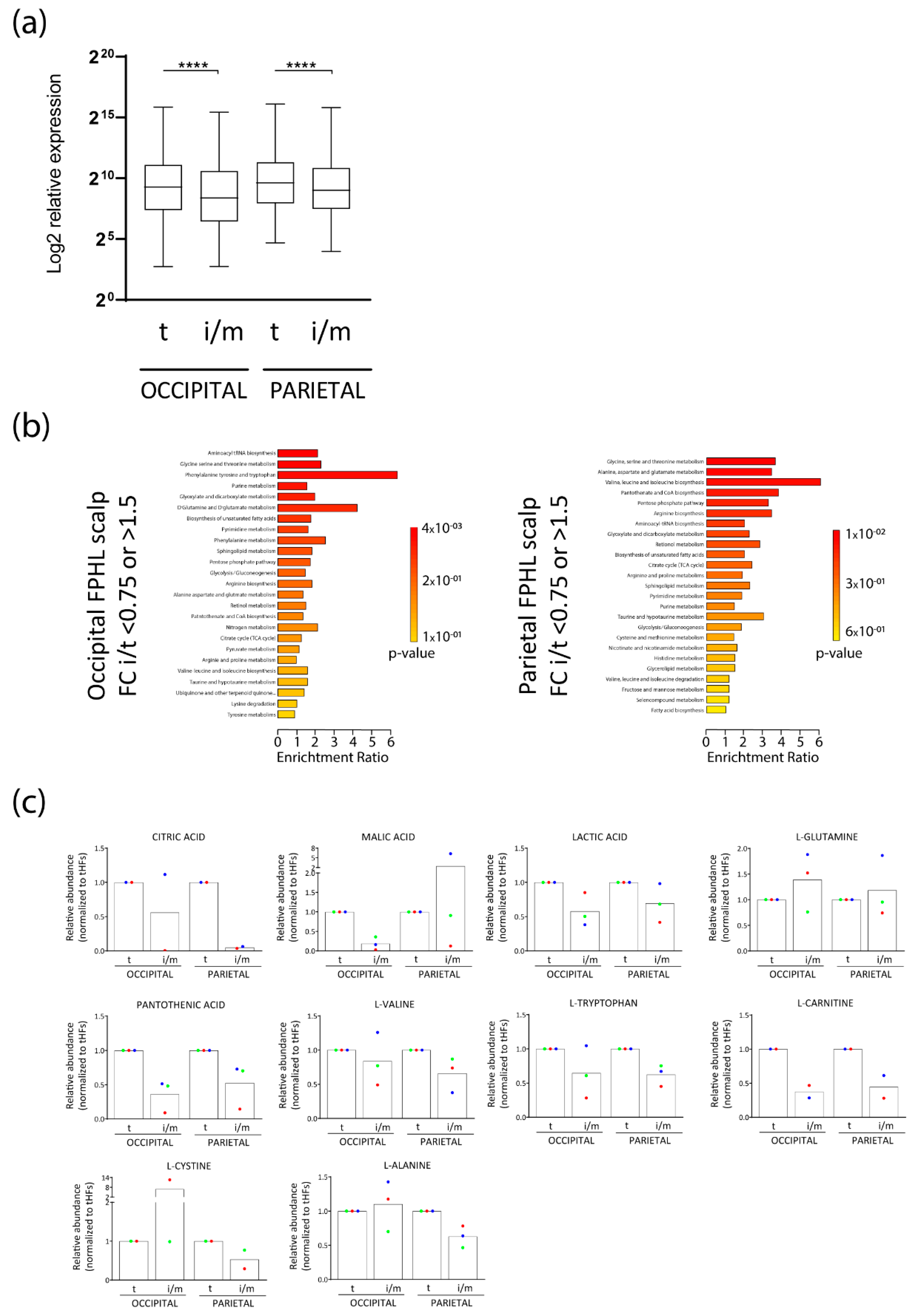
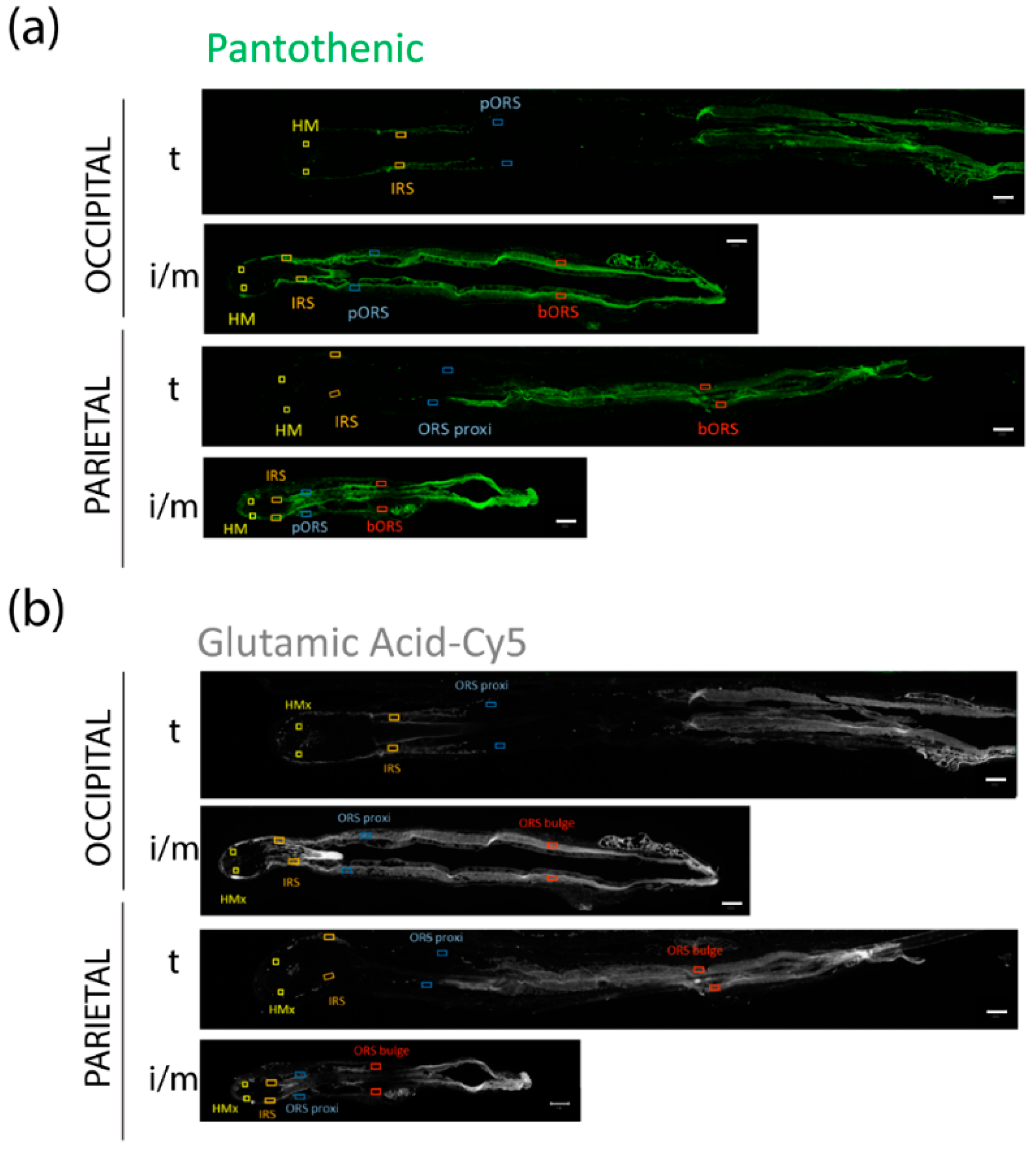
3.1. Intermediate HFs from FPHL Patients Show Nutrient and Metabolite Deficiency
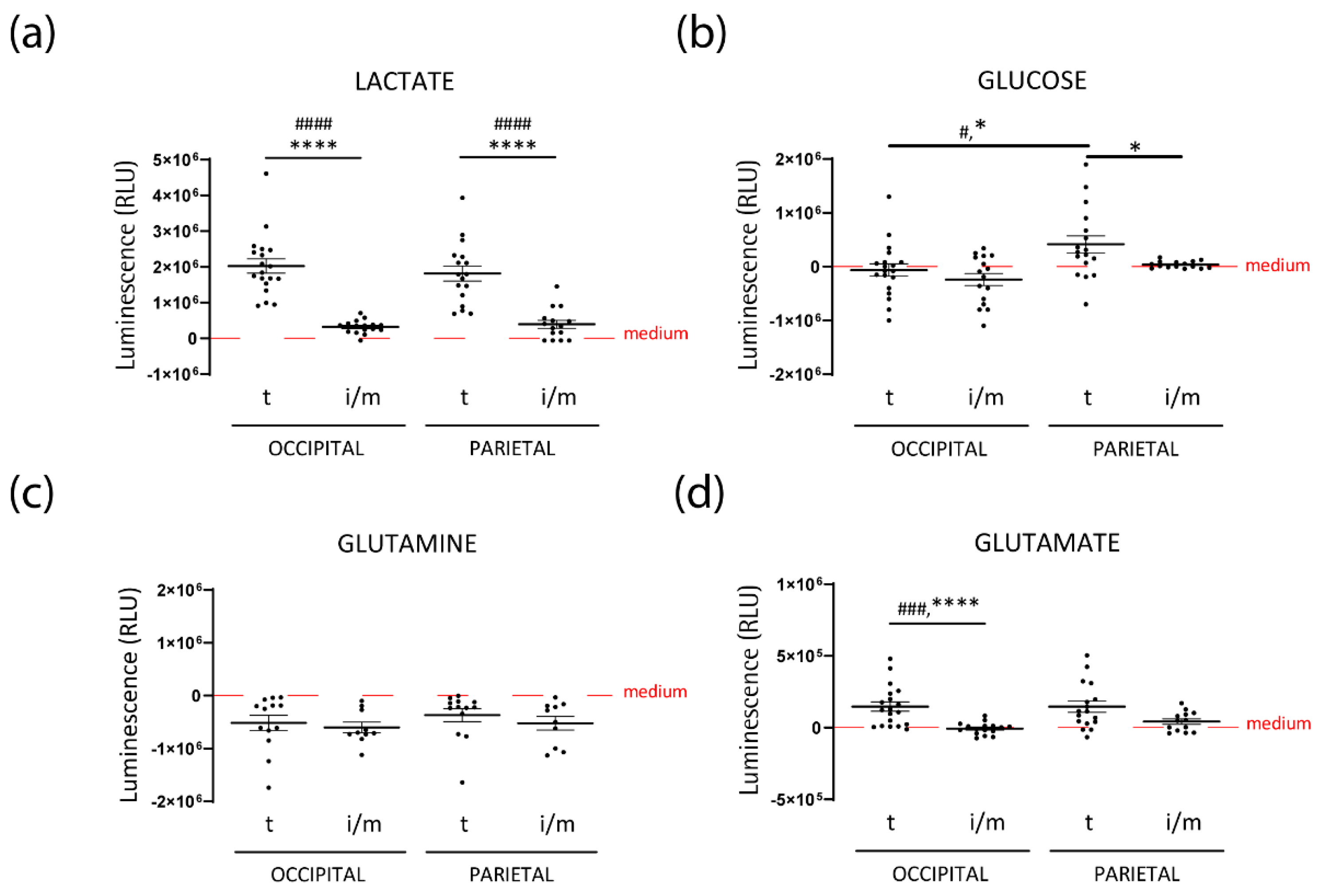
3.2. Intermediate HFs from FPHL Are Characterized by Dormant Metabolism
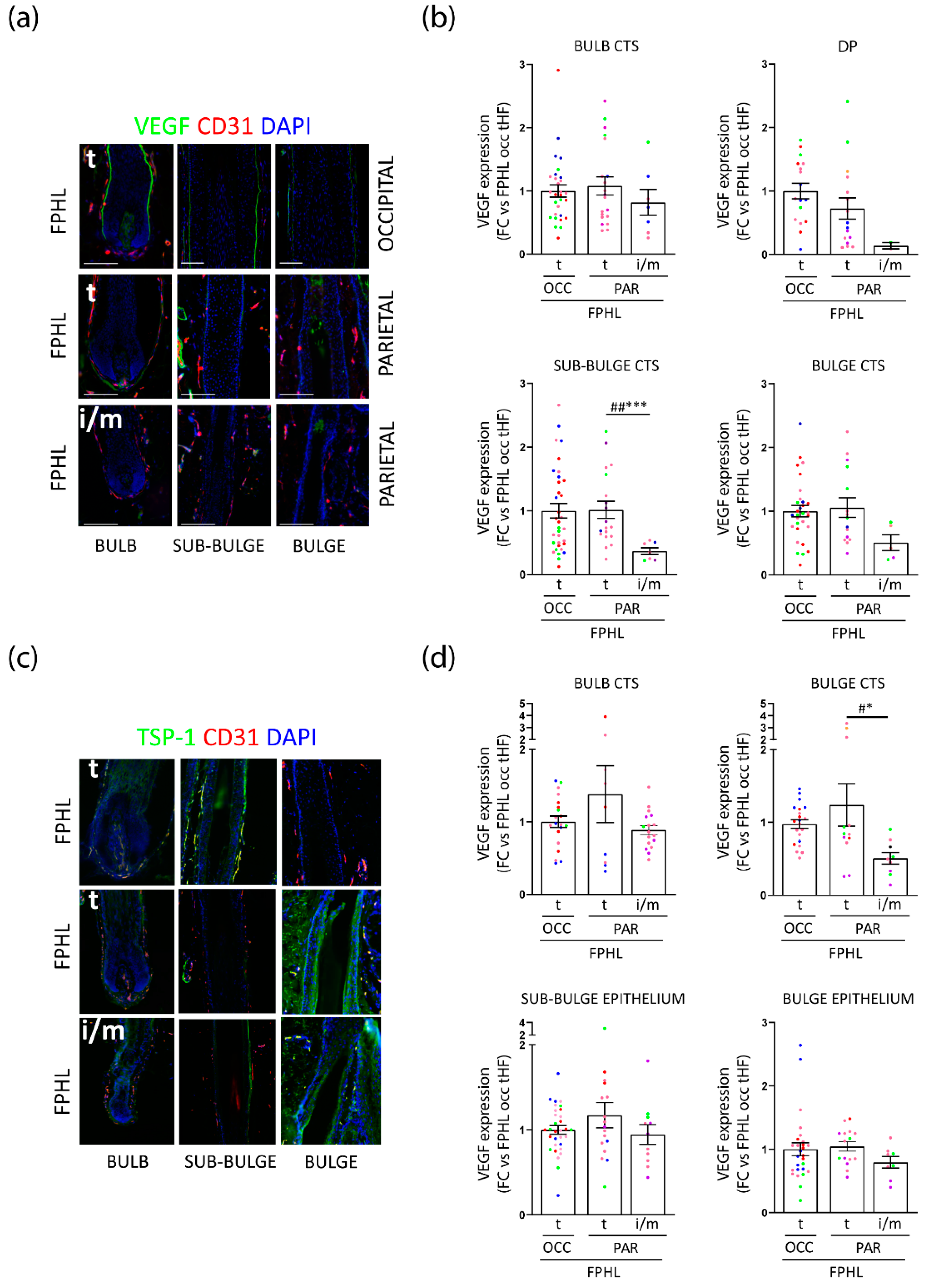
3.3. Intermediate Parietal HFs Display Lower VEGF and TSP-1 Expression, but TSP-1 Expression Is Significantly Increased in FPHL versus Healthy Terminal HFs
3.4. Perifollicular Vascularization Is Relatively Unchanged in Parietal Intermediate HFs, but Is Significantly Decreased in Parietal versus Occipital Terminal FPHL HFs
3.5. FPHL HFs Can Retrieve Exogenously Supplied Nutrients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabbrocini, G.; Cantelli, M.; Masarà, A.; Annunziata, M.C.; Marasca, C.; Cacciapuoti, S. Female Pattern Hair Loss: A Clinical, Pathophysiologic, and Therapeutic Review. Int. J. Womens Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Tosti, A.; Bergfeld, W.; Blume-Peytavi, U.; Shapiro, J.; Lutz, G.; Messenger, A.; Sinclair, R.; Paus, R. Towards a Consensus on How to Diagnose and Quantify Female Pattern Hair Loss-The “Female Pattern Hair Loss Severity Index (FPHL-SI)”. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.A.; Paus, R. Female Pattern Hair Loss: Beyond an Androgenic Aetiology? Br. J. Dermatol. 2010, 163, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G. Hair through the Female Life Cycle. Br. J. Dermatol. 2011, 165 (Suppl. S3), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Redler, S.; Messenger, A.G.; Betz, R.C. Genetics and Other Factors in the Aetiology of Female Pattern Hair Loss. Exp. Dermatol. 2017, 26, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.F.; Price, V.H.; Savin, R.C. Psychological Effects of Androgenetic Alopecia on Women: Comparisons with Balding Men and with Female Control Subjects. J. Am. Acad. Dermatol. 1993, 29, 568–575. [Google Scholar] [CrossRef]
- Davis, D.S.; Callender, V.D. Review of Quality of Life Studies in Women with Alopecia. Int. J. Womens Dermatol. 2018, 4, 18–22. [Google Scholar] [CrossRef]
- Cash, T.F. The Psychosocial Consequences of Androgenetic Alopecia: A Review of the Research Literature. Br. J. Dermatol. 1999, 141, 398–405. [Google Scholar] [CrossRef]
- Van Der Donk, J.; Hunfeld, J.A.; Passchier, J.; Knegt-Junk, K.J.; Nieboer, C. Quality of Life and Maladjustment Associated with Hair Loss in Women with Alopecia Androgenetica. Soc. Sci. Med. 1994, 38, 159–163. [Google Scholar] [CrossRef]
- Price, V.H. Androgenetic Alopecia in Women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Saqib, N.-U.; Latif, I.; Hassan, I. Female Pattern Hair Loss—An Update. Indian Dermatol. Online J. 2020, 11, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; Sinclair, R. Follicular Miniaturization in Female Pattern Hair Loss: Clinicopathological Correlations. Br. J. Dermatol. 2006, 155, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Tamashunas, N.L.; Bergfeld, W.F. Male and Female Pattern Hair Loss: Treatable and Worth Treating. Cleve. Clin. J. Med. 2021, 88, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, D. Exhaustive Analysis of Scalp Hair Regression: Subjective and Objective Perception from Initial Hair Loss to Severe Miniaturisation and Drug-Induced Regrowth. Plast. Aesthet. Res. 2021, 8, 16. [Google Scholar] [CrossRef]
- Miranda, B.H.; Tobin, D.J.; Sharpe, D.T.; Randall, V.A. Intermediate Hair Follicles: A New More Clinically Relevant Model for Hair Growth Investigations. Br. J. Dermatol. 2010, 163, 287–295. [Google Scholar] [CrossRef]
- Olsen, E.A. Female Pattern Hair Loss. J. Am. Acad. Dermatol. 2001, 45, S70–S80. [Google Scholar] [CrossRef]
- Herskovitz, I.; Tosti, A. Female Pattern Hair Loss. Int. J. Endocrinol. Metab. 2013, 11, e9860. [Google Scholar] [CrossRef]
- Dinh, Q.Q.; Sinclair, R. Female Pattern Hair Loss: Current Treatment Concepts. Clin. Interv. Aging 2007, 2, 189–199. [Google Scholar]
- Jimenez, F.; Alam, M.; Vogel, J.E.; Avram, M. Hair Transplantation: Basic Overview. J. Am. Acad. Dermatol. 2021, 85, 803–814. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Hillmann, K.; Dietz, E.; Canfield, D.; Garcia Bartels, N. A Randomized, Single-Blind Trial of 5% Minoxidil Foam Once Daily versus 2% Minoxidil Solution Twice Daily in the Treatment of Androgenetic Alopecia in Women. J. Am. Acad. Dermatol. 2011, 65, 1126–1134. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review: The Platelet-Rich Plasma Use in Female Androgenetic Alopecia as Effective Autologous Treatment of Regenerative Plastic Surgery. J. Plast. Reconstr. Aesthet. Surg. 2021, 75, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Cousen, P.; Messenger, A. Female Pattern Hair Loss in Complete Androgen Insensitivity Syndrome. Br. J. Dermatol. 2010, 162, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Orme, S.; Cullen, D.R.; Messenger, A.G. Diffuse Female Hair Loss: Are Androgens Necessary? Br. J. Dermatol. 1999, 141, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Sadick, N.; Arruda, S. Understanding Causes of Hair Loss in Women. Dermatol. Clin. 2021, 39, 371–374. [Google Scholar] [CrossRef]
- Le Floc’h, C.; Cheniti, A.; Connétable, S.; Piccardi, N.; Vincenzi, C.; Tosti, A. Effect of a Nutritional Supplement on Hair Loss in Women. J. Cosmet. Dermatol. 2015, 14, 76–82. [Google Scholar] [CrossRef]
- Ring, C.; Heitmiller, K.; Correia, E.; Gabriel, Z.; Saedi, N. Nutraceuticals for Androgenetic Alopecia. J. Clin. Aesthet. Dermatol. 2022, 15, 26–29. [Google Scholar]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2018, 9, 51–70. [Google Scholar] [CrossRef]
- Burns, L.J.; Senna, M.M. Supplement Use among Women Experiencing Hair Loss. Int. J. Womens Derm. 2020, 6, 211. [Google Scholar] [CrossRef]
- Cheung, E.J.; Sink, J.R.; English Iii, J.C. Vitamin and Mineral Deficiencies in Patients With Telogen Effluvium: A Retrospective Cross-Sectional Study. J. Drugs Dermatol. 2016, 15, 1235–1237. [Google Scholar]
- Gowda, D.; Premalatha, V.; Imtiyaz, D. Prevalence of Nutritional Deficiencies in Hair Loss among Indian Participants: Results of a Cross-Sectional Study. Int. J. Trichol. 2017, 9, 101–104. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and Hair Loss: Effects of Nutrient Deficiency and Supplement Use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Serum Biotin Levels in Women Complaining of Hair Loss. Int. J. Trichol. 2016, 8, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Conic, R.R.Z.; Piliang, M.; Bergfeld, W.; Atanaskova-Mesinkovska, N. Vitamin D Status in Scarring and Nonscarring Alopecia. J. Am. Acad. Dermatol. 2021, 85, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.D.; Rushton, D.H. Reduced Serum Vitamin B12 Levels during Oral Cyproterone-Acetate and Ethinyl-Oestradiol Therapy in Women with Diffuse Androgen-Dependent Alopecia. Clin. Exp. Dermatol. 1990, 15, 277–281. [Google Scholar] [CrossRef]
- AlGhamdi, A.A.; Mohammed, M.R.S.; Zamzami, M.A.; Al-Malki, A.L.; Qari, M.H.; Khan, M.I.; Choudhry, H. Untargeted Metabolomics Identifies Key Metabolic Pathways Altered by Thymoquinone in Leukemic Cancer Cells. Nutrients 2020, 12, 1792. [Google Scholar] [CrossRef]
- Purba, T.S.; Brunken, L.; Peake, M.; Shahmalak, A.; Chaves, A.; Poblet, E.; Ceballos, L.; Gandarillas, A.; Paus, R. Characterisation of Cell Cycle Arrest and Terminal Differentiation in a Maximally Proliferative Human Epithelial Tissue: Lessons from the Human Hair Follicle Matrix. Eur. J. Cell Biol. 2017, 96, 632–641. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic Regulation of Cell Growth and Proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Agathocleous, M.; Harris, W.A. Metabolism in Physiological Cell Proliferation and Differentiation. Trends Cell Biol. 2013, 23, 484–492. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Figlak, K.; Williams, G.; Bertolini, M.; Paus, R.; Philpott, M.P. Human Hair Follicles Operate an Internal Cori Cycle and Modulate Their Growth via Glycogen Phosphorylase. Sci. Rep. 2021, 11, 20761. [Google Scholar] [CrossRef]
- Kealey, T.; Williams, R.; Philpott, M.P. The Human Hair Follicle Engages in Glutaminolysis and Aerobic Glycolysis: Implications for Skin, Splanchnic and Neoplastic Metabolism. Skin Pharmacol. 1994, 7, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Philpott, M.P.; Kealey, T. Metabolism of Freshly Isolated Human Hair Follicles Capable of Hair Elongation: A Glutaminolytic, Aerobic Glycolytic Tissue. J. Investig. Dermatol. 1993, 100, 834–840. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial Aerobic Respiration Is Activated during Hair Follicle Stem Cell Differentiation, and Its Dysfunction Retards Hair Regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.; Pohl, S.; Moll, I.; Paus, R. Active Hair Growth (Anagen) Is Associated with Angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Zilio, F.; Rossi, A.; Kleditzsch, P.; Emelianov, V.E.; Gilhar, A.; Keren, A.; Meyer, K.C.; Wang, E.; Funk, W.; et al. Abnormal Interactions between Perifollicular Mast Cells and CD8+ T-Cells May Contribute to the Pathogenesis of Alopecia Areata. PLoS ONE 2014, 9, e94260. [Google Scholar] [CrossRef]
- Edelkamp, J.; Gherardini, J.; Bertolini, M. Methods to Study Human Hair Follicle Growth Ex Vivo: Human Microdissected Hair Follicle and Human Full Thickness Skin Organ Culture. In Molecular Dermatology; Botchkareva, N.V., Westgate, G.E., Eds.; Springer: New York, NY, USA, 2020; Volume 2154, pp. 105–119. [Google Scholar]
- Roux, A.; Lison, D.; Junot, C.; Heilier, J.-F. Applications of Liquid Chromatography Coupled to Mass Spectrometry-Based Metabolomics in Clinical Chemistry and Toxicology: A Review. Clin. Biochem. 2011, 44, 119–135. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Langan, E.A.; Philpott, M.P.; Kloepper, J.E.; Paus, R. Human Hair Follicle Organ Culture: Theory, Application and Perspectives. Exp. Dermatol. 2015, 24, 903–911. [Google Scholar] [CrossRef]
- Piccini, I.; Brunken, L.; Chéret, J.; Ghatak, S.; Ramot, Y.; Alam, M.; Purba, T.S.; Hardman, J.; Erdmann, H.; Jimenez, F.; et al. Peroxisome Proliferator-activated Receptor-γ Signalling Protects Hair Follicle Stem Cells from Chemotherapy-induced Apoptosis and Epithelial–Mesenchymal Transition Br. J. Dermatol. 2022, 186, 129–141. [Google Scholar] [CrossRef]
- Keren, A.; Bertolini, M.; Keren, Y.; Ullmann, Y.; Paus, R.; Gilhar, A. Human Organ Rejuvenation by VEGF-A: Lessons from the Skin. Sci. Adv. 2022, 8, eabm6756. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.; Gloor, M. Use of the Phototrichogram to Assess the Stimulation of Hair Growth-An in Vivo Study of Women with Androgenetic Alopecia Das Phototrichogramm Als Verfahren Zur Beurteilung Haarwachstumsfördernder Präparate Am Beispiel Einer Kombination von Hirsefruchtextrakt, L-Cystin Und Calcium Panthothenat-Ergebnisse Einer in Vivo Untersuchung Bei Frauen Mit Androgenetischem Haarausfall. H+G Z. Fur Hautkrankh. 2000, 75, 419–423. [Google Scholar]
- Yano, K.; Brown, L.F.; Detmar, M. Control of Hair Growth and Follicle Size by VEGF-Mediated Angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Fernandez, L.A.; Tirrell, S.J. The Angiogenic Properties of the Rat Vibrissa Hair Follicle Associate with the Bulb. J. Investig. Dermatol. 1988, 90, 409–411. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular Permeability Factor/Vascular Endothelial Growth Factor, Microvascular Hyperpermeability, and Angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Ludwig, E. Classification of the Types of Androgenetic Alopecia (Common Baldness) Occurring in the Female Sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Sinclair, R.; Jolley, D.; Mallari, R.; Magee, J. The Reliability of Horizontally Sectioned Scalp Biopsies in the Diagnosis of Chronic Diffuse Telogen Hair Loss in Women. J. Am. Acad. Dermatol. 2004, 51, 189–199. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 Inhibits Inflammatory Lymphangiogenesis by CD36 Ligation on Monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Greenaway, J.; Lawler, J.; Moorehead, R.; Bornstein, P.; Lamarre, J.; Petrik, J. Thrombospondin-1 Inhibits VEGF Levels in the Ovary Directly by Binding and Internalization Via the Low Density Lipoprotein Receptor-Related Protein-1 (LRP-1). J. Cell Physiol. 2007, 210, 807–818. [Google Scholar] [CrossRef]
- Zhang, X.; Lawler, J. Thrombospondin-Based Antiangiogenic Therapy. Microvasc. Res. 2007, 74, 90–99. [Google Scholar] [CrossRef]
- Palm, W.; Thompson, C.B. Nutrient Acquisition Strategies of Mammalian Cells. Nature 2017, 546, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Budde, J.; Tronnier, H.; Rahlfs, V.W.; Frei-Kleiner, S. Systemic therapy of diffuse effluvium and hair structure damage. Hautarzt 1993, 44, 380–384. [Google Scholar] [PubMed]
- Hengl, T.; Herfert, J.; Soliman, A.; Schlinzig, K.; Trüeb, R.M.; Abts, H.F. Cystine-Thiamin-Containing Hair-Growth Formulation Modulates the Response to UV Radiation in an in Vitro Model for Growth-Limiting Conditions of Human Keratinocytes. J. Photochem. Photobiol. B 2018, 189, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.P.; de Andrade Berti, B.; Mendes, N.F.; Engel, D.F.; Zanesco, A.M.; Souza, G.F.; Velloso, L.A.; Araujo, E.P. Glutamic Acid Promotes Hair Growth in Mice. Sci. Rep. 2021, 11, 15453. [Google Scholar] [CrossRef] [PubMed]
- Riegel, K.; Hengl, T.; Krischok, S.; Schlinzig, K.; Abts, H.F. L-Cystine-Containing Hair-Growth Formulation Supports Protection, Viability, and Proliferation of Keratinocytes. Clin. Cosmet. Investig. Dermatol. 2020, 13, 499–510. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, J.; Choi, Y.-H.; Kang, N.-G.; Lee, S. Dexpanthenol Promotes Cell Growth by Preventing Cell Senescence and Apoptosis in Cultured Human Hair Follicle Cells. Curr. Issues Mol. Biol. 2021, 43, 1361–1373. [Google Scholar] [CrossRef]
- Philpott, M.P.; Kealey, T. Metabolic Studies on Isolated Hair Follicles: Hair Follicles Engage in Aerobic Glycolysis and Do Not Demonstrate the Glucose Fatty Acid Cycle. J. Investig. Dermatol. 1991, 96, 875–879. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]
- Coller, H.A. The Paradox of Metabolism in Quiescent Stem Cells. FEBS Lett. 2019, 593, 2817–2839. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Roosterman, D.; Cottrell, G.S. Rethinking the Citric Acid Cycle: Connecting Pyruvate Carboxylase and Citrate Synthase to the Flow of Energy and Material. Int. J. Mol. Sci. 2021, 22, 604. [Google Scholar] [CrossRef] [PubMed]
- Goos, C.M.; Beaumont, A.H.; Vermeesch-Markslag, A.M.; van der Stappen, J.W.; Sultan, C.; Vermorken, A.J. Determination of Glycogen and Enzymes of Glycogen Metabolism in Human Hair Follicles. Mol. Biol. Rep. 1987, 12, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Shipman, M.; Chase, H.B.; Montagna, W. Glycogen in Skin of the Mouse During Cycles of Hair Growth. Proc. Exp. Biol. Med. 1955, 88, 449–451. [Google Scholar] [CrossRef]
- Kim, C.S.; Ding, X.; Allmeroth, K.; Biggs, L.C.; Kolenc, O.I.; L’Hoest, N.; Chacón-Martínez, C.A.; Edlich-Muth, C.; Giavalisco, P.; Quinn, K.P.; et al. Glutamine Metabolism Controls Stem Cell Fate Reversibility and Long-Term Maintenance in the Hair Follicle. Cell Metab. 2020, 32, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Di Paola, F.J.; Campos-Sandoval, J.A.; Mazurek, S.; Márquez, J. Therapeutic Targeting of Glutaminolysis as an Essential Strategy to Combat Cancer. Semin. Cell Dev. Biol. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/XCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Miniaci, M.C.; Irace, C.; Capuozzo, A.; Piccolo, M.; Di Pascale, A.; Russo, A.; Lippiello, P.; Lepre, F.; Russo, G.; Santamaria, R. Cysteine Prevents the Reduction in Keratin Synthesis Induced by Iron Deficiency in Human Keratinocytes. J. Cell Biochem. 2016, 117, 402–412. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kusama, M.; Onda, M.; Nakahata, N. The Effect of Pantothenic Acid Deficiency on Keratinocyte Proliferation and the Synthesis of Keratinocyte Growth Factor and Collagen in Fibroblasts. J. Pharm. Sci. 2011, 115, 230–234. [Google Scholar] [CrossRef]
- Lacroix, B.; Didier, E.; Grenier, J.F. Effects of Pantothenic Acid on Fibroblastic Cell Cultures. Res. Exp. Med. 1988, 188, 391–396. [Google Scholar] [CrossRef]
- Wang, Z.; Nan, W.; Si, H.; Wang, S.; Zhang, H.; Li, G. Pantothenic Acid Promotes Dermal Papilla Cell Proliferation in Hair Follicles of American Minks via Inhibitor of DNA Binding 3/Notch Signaling Pathway. Life Sci. 2020, 252, 117667. [Google Scholar] [CrossRef]
- Woolley, D.W. Relationship of Pantothenic Acid and Inositol to Alopecia in Mice. Proc. Exp. Biol. Med. 1941, 46, 565–569. [Google Scholar] [CrossRef]
- Stein, E.D.; Diamond, J.M. Do Dietary Levels of Pantothenic Acid Regulate Its Intestinal Uptake in Mice? J. Nutr. 1989, 119, 1973–1983. [Google Scholar] [CrossRef]
- Glynis, A. A Double-Blind, Placebo-Controlled Study Evaluating the Efficacy of an Oral Supplement in Women with Self-Perceived Thinning Hair. J. Clin. Aesthet. Dermatol. 2012, 5, 28–34. [Google Scholar]
- Siavash, M.; Tavakoli, F.; Mokhtari, F. Comparing the Effects of Zinc Sulfate, Calcium Pantothenate, Their Combination and Minoxidil Solution Regimens on Controlling Hair Loss in Women: A Randomized Controlled Trial. J. Res. Pharm. Pract. 2017, 6, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Detmar, M.; Brown, L.F.; Lawler, J.; Miyakawa, T. Thrombospondin-1 Plays a Critical Role in the Induction of Hair Follicle Involution and Vascular Regression During the Catagen Phase. J. Investig. Dermatol. 2003, 120, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cormia, F.E.; Ernyey, A. Circulatory Changes in Alopecia: Preliminary Report, with a Summary of the Cutaneous Circulation of the Normal Scalp. Arch. Dermatol. 1961, 84, 772–789. [Google Scholar] [CrossRef]
- Goldman, C.K.; Tsai, J.C.; Soroceanu, L.; Gillespie, G.Y. Loss of Vascular Endothelial Growth Factor in Human Alopecia Hair Follicles. J. Investig. Dermatol. 1995, 104, 18S–20S. [Google Scholar] [CrossRef]
- Piérard-Franchimont, C.; Loussouarn, G.; Panhard, S.; Saint Léger, D.; Mellul, M.; Piérard, G.E. Immunohistochemical Patterns in the Interfollicular Caucasian Scalps: Influences of Age, Gender, and Alopecia. BioMed Res. Int. 2013, 2013, e769489. [Google Scholar] [CrossRef]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro Needling: A Novel Therapeutic Approach for Androgenetic Alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef]
- Ramos, P.M.; Gohad, P.; McCoy, J.; Wambier, C.; Goren, A. Minoxidil Sulfotransferase Enzyme (SULT1A1) Genetic Variants Predicts Response to Oral Minoxidil Treatment for Female Pattern Hair Loss. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e24–e26. [Google Scholar] [CrossRef]
- Steward, E.N.; Patel, H.; Pandya, H.; Dewan, H.; Bhavsar, B.; Shah, U.; Dholakia, K. Efficacy of Platelet-Rich Plasma and Concentrated Growth Factor in Treating Androgenetic Alopecia-A Retrospective Study. Ann. Maxillofac. Surg. 2020, 10, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the Skin of Hair Aging: The Impact of the Hair Follicle Environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, C.; Ferraro, D.A.; Soares, T.C.B.; Moraes, A.M.; Cintra, M.L. Chronic Telogen Effluvium and Female Pattern Hair Loss Are Separate and Distinct Forms of Alopecia: A Histomorphometric and Immunohistochemical Analysis. Clin. Exp. Dermatol. 2014, 39, 868–873. [Google Scholar] [CrossRef] [PubMed]


| Donors | Age | FPHL Grade * | Scalp Region | Source | Experiment | |
|---|---|---|---|---|---|---|
| Healthy donors | 1 | 45 | - | occipital | skin | in situ immunostaining |
| 2 | 24 | - | occipital | skin | in situ immunostaining | |
| 3 | 40 | - | occipital | skin | in situ immunostaining | |
| 4 | 48 | - | occipital | FUEs | metabolic activity | |
| 5 | 54 | - | occipital | FUEs | metabolic activity | |
| 6 | 26 | - | occipital | FUEs | metabolic activity | |
| FPHL patients | 1 | 50 | I | occipital and parietal | skin | in situ immunostaining |
| 2 | 54 | II | occipital and parietal | skin | in situ immunostaining | |
| 3 | 62 | II | occipital and parietal | skin | in situ immunostaining | |
| 4 | 28 | II | parietal | skin | in situ immunostaining | |
| 5 | 60 | I | occipital and parietal | skin | in situ immunostaining | |
| 6 | 35 | II–III | occipital and parietal | FUEs | UPLC-MS | |
| 7 | 53 | II | occipital and parietal | FUEs | UPLC-MS | |
| 8 | 30 | I | occipital and parietal | FUEs | UPLC-MS | |
| 9 | 70 | Ludwig III | occipital and parietal | FUEs | ex vivo absorption | |
| 10 | 43 | Ludwig II | occipital and parietal | FUEs | metabolic activity/ex vivo absorption | |
| 11 | 65 | Sinclair II | occipital and parietal | FUEs | metabolic activity/ex vivo absorption | |
| 12 | 53 | Sinclair II–III | occipital and parietal | FUEs | metabolic activity/ex vivo absorption | |
| 13 | 42 | Sinclair III | occipital and parietal | FUEs | metabolic activity/ex vivo absorption | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccini, I.; Sousa, M.; Altendorf, S.; Jimenez, F.; Rossi, A.; Funk, W.; Bíró, T.; Paus, R.; Seibel, J.; Jakobs, M.; et al. Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients 2022, 14, 3357. https://doi.org/10.3390/nu14163357
Piccini I, Sousa M, Altendorf S, Jimenez F, Rossi A, Funk W, Bíró T, Paus R, Seibel J, Jakobs M, et al. Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients. 2022; 14(16):3357. https://doi.org/10.3390/nu14163357
Chicago/Turabian StylePiccini, Ilaria, Marta Sousa, Sabrina Altendorf, Francisco Jimenez, Alfredo Rossi, Wolfgang Funk, Tamás Bíró, Ralf Paus, Jens Seibel, Mira Jakobs, and et al. 2022. "Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype" Nutrients 14, no. 16: 3357. https://doi.org/10.3390/nu14163357
APA StylePiccini, I., Sousa, M., Altendorf, S., Jimenez, F., Rossi, A., Funk, W., Bíró, T., Paus, R., Seibel, J., Jakobs, M., Yesilkaya, T., Edelkamp, J., & Bertolini, M. (2022). Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients, 14(16), 3357. https://doi.org/10.3390/nu14163357




