Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PE
2.2. Sample Preparation for HPLC Analysis
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Animals and Diet
2.5. Determination of Serum Metabolic Parameters
2.6. Hepatic and Fecal Lipid Extraction
2.7. Fecal BA Analysis
2.8. Histological Analysis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. AMP-Activated Protein Kinase (AMPK) Activity
2.11. Statistical Analysis
3. Results
3.1. Phenolic Compound Contents of PE
3.2. Body Weight, Food Intake, and Serum AST and ALT Activities
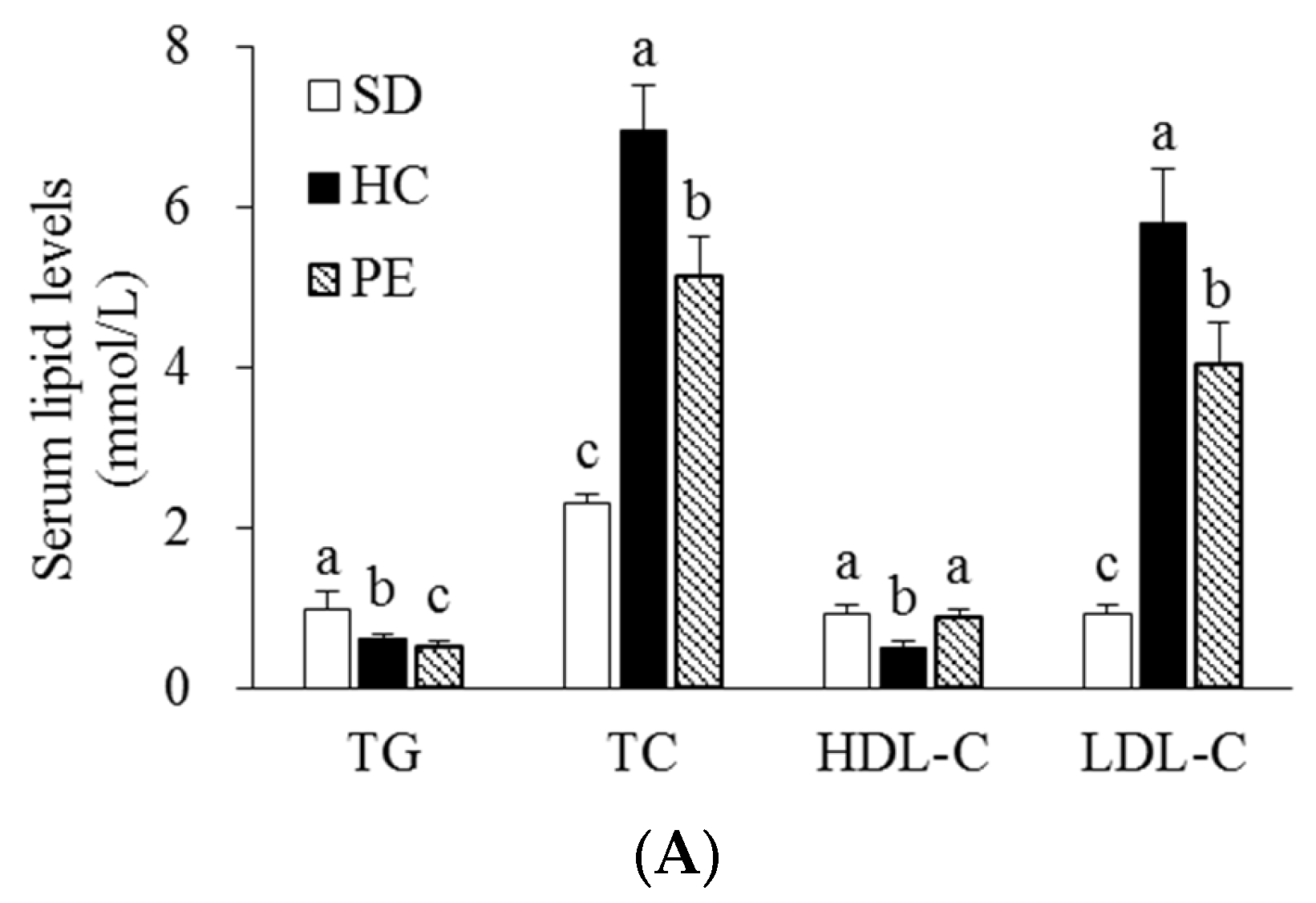
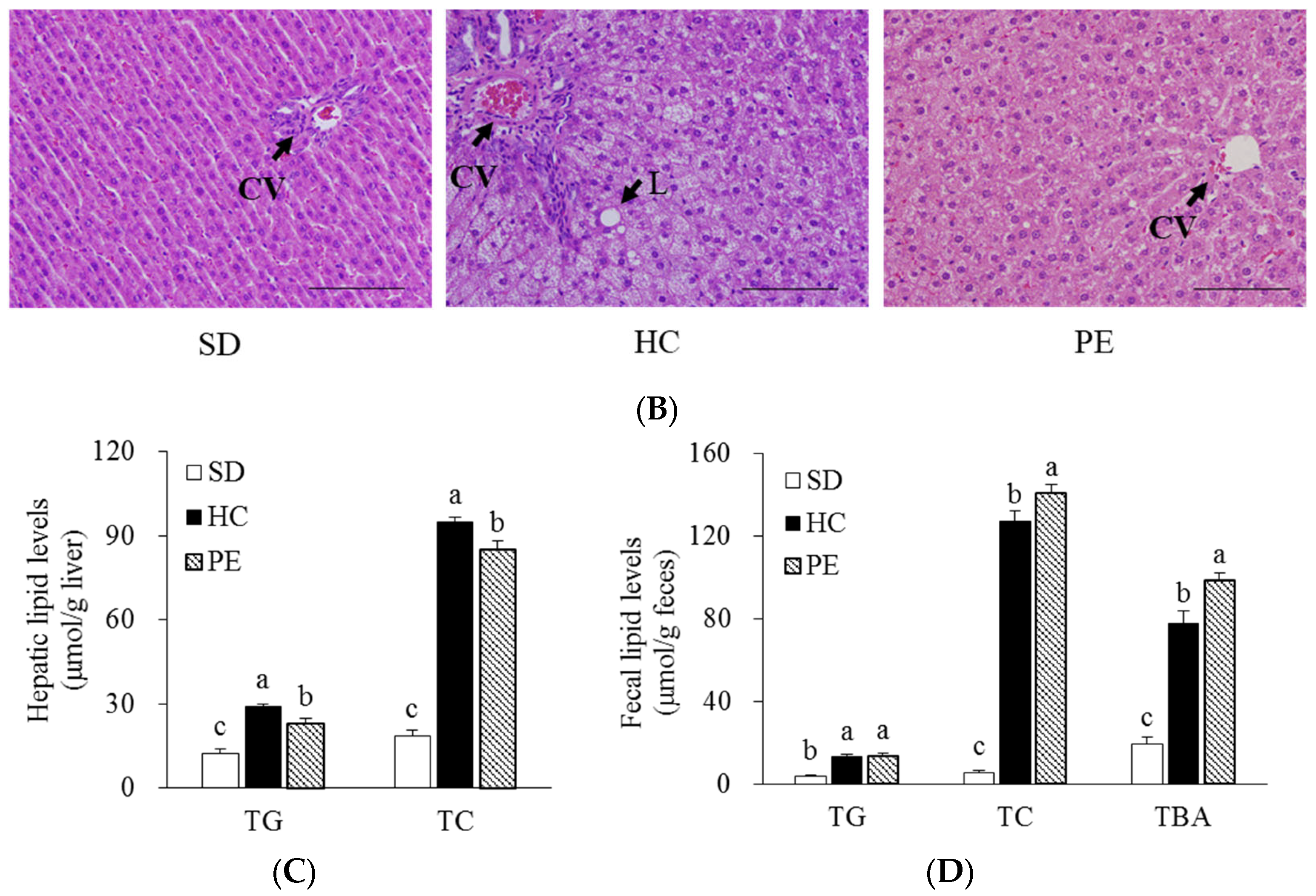
3.3. Effects of PE on Serum, Liver, and Fecal Lipid Profiles
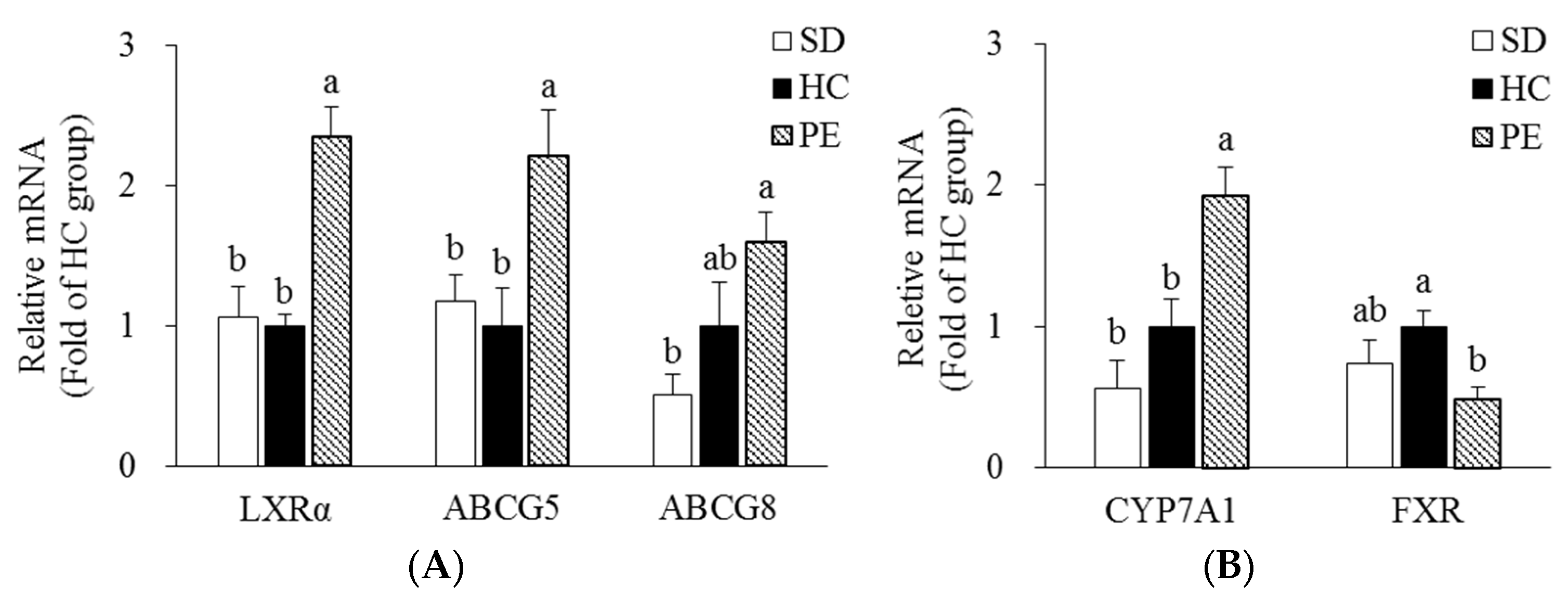
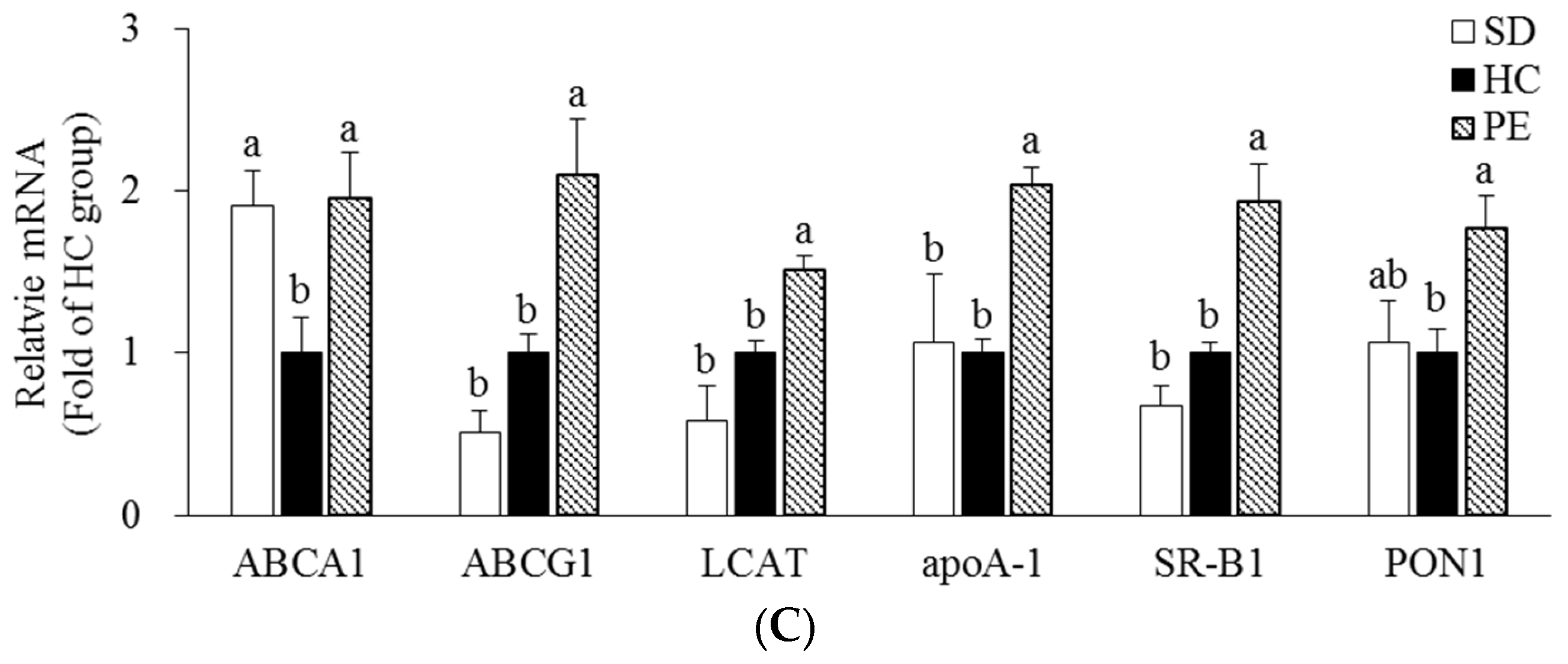
3.4. Effect of PE on Expression of Genes Related to Cholesterol Metabolism
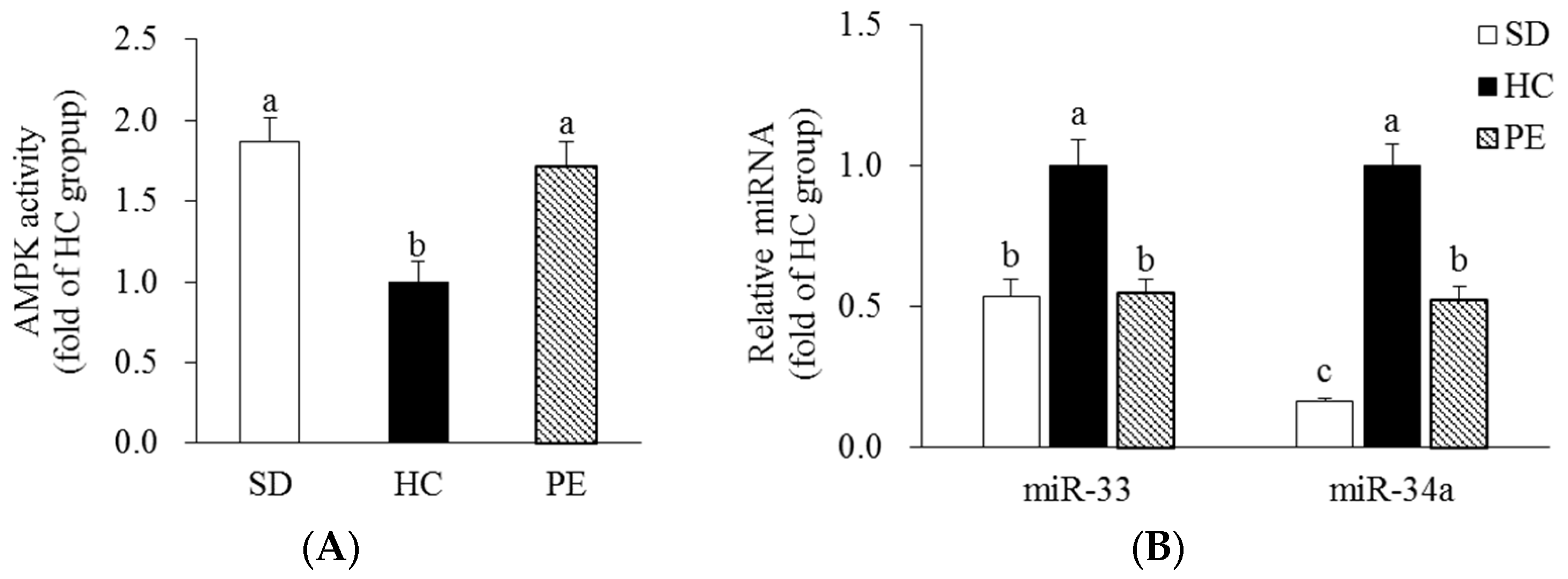
3.5. Effects of PE on AMPK Activity and miR-33/34a Expression
4. Discussion
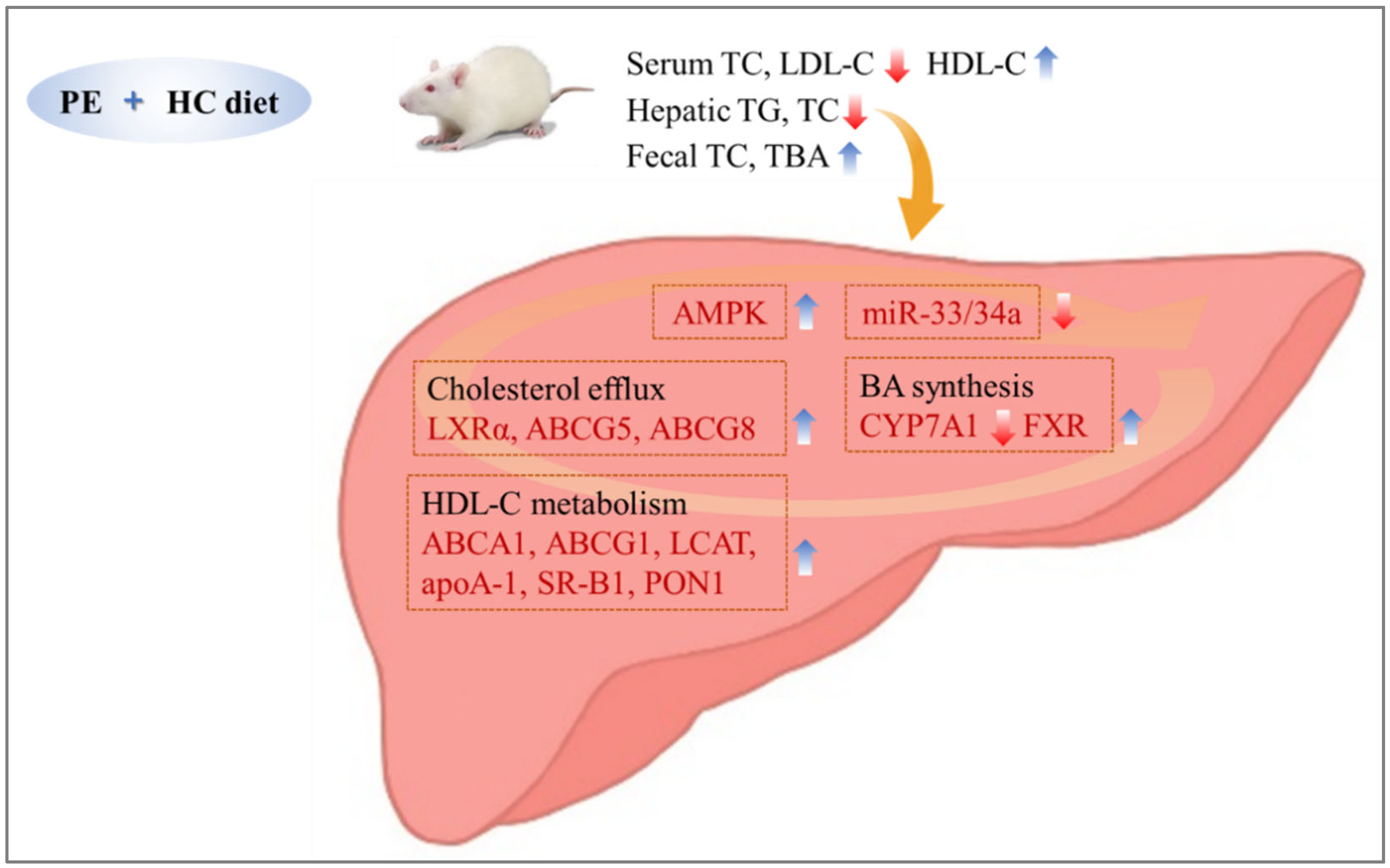
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef]
- Plösch, T.; Kruit, J.K.; Bloks, V.W.; Huijkman, N.C.; Havinga, R.; Duchateau, G.S.; Lin, Y.; Kuipers, F. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J. Nutr. 2006, 136, 2135–2140. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Lin, C.S.; Goedeke, L.; Suárez, Y.; Fernández-Hernando, C. Micro-RNAs and High-Density Lipoprotein Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1076–1084. [Google Scholar] [CrossRef]
- Lenahan, C.; Huang, L.; Travis, Z.D.; Zhang, J.H. Scavenger Receptor Class B type 1 (SR-B1) and the modifiable risk factors of stroke. Chin. Neurosurg. J. 2019, 5, 30. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef]
- Han, R.; Lai, R.; Ding, Q.; Wang, Z.; Luo, X.; Zhang, Y.; Cui, G.; He, J.; Liu, W.; Chen, Y. Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia 2007, 50, 1960–1968. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Liang, B.; Shirwany, N.; Zhu, Y.; Zou, M.H. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: The role of uncoupling protein 2. PLoS ONE 2011, 6, e25436. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Ford, R.J.; McGregor, C.P.; LeBlond, N.D.; Snider, S.A.; Stypa, S.A.; Day, E.A.; Lhoták, Š.; Schertzer, J.D.; Austin, R.C.; et al. Salicylate improves macrophage cholesterol homeostasis via activation of Ampk. J. Lipid Res. 2015, 56, 1025–1033. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Anti-Oxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [Google Scholar] [CrossRef]
- Zheng, G.; Mo, F.; Ling, C.; Peng, H.; Gu, W.; Li, M.; Chen, Z. Portulaca oleracea L. alleviates liver injury in streptozotocin-induced diabetic mice. Drug Des. Dev. Ther. 2018, 12, 47–55. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E.; Duke, J.A. Common purslane: A source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr. 1992, 11, 374–382. [Google Scholar] [CrossRef]
- Jung, J.H.; Hwang, S.B.; Park, H.J.; Jin, G.R.; Lee, B.H. Antiobesity and Antidiabetic Effects of Portulaca oleracea Powder Intake in High-Fat Diet-Induced Obese C57BL/6 Mice. Evid. Based Complement. Alternat. Med. 2021, 2021, 5587848. [Google Scholar] [CrossRef] [PubMed]
- Zidan, Y.; Bouderbala, S.; Djellouli, F.; Lacaille-Dubois, M.A.; Bouchenak, M. Portulaca oleracea reduces triglyceridemia, cholesterolemia, and improves lecithin: Cholesterol acyltransferase activity in rats fed enriched-cholesterol diet. Phytomedicine 2014, 21, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Bieri, J.G. AIN-76 diet. J. Nutr. 1979, 109, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, M.S.; Chang, E.; Lee, Y.; Lee, J.; Kim, J.; Kim, C.T.; Kim, I.H.; Kim, Y. Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients 2020, 12, 1499. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-K.; Kim, C.-J.; Kim, C.-T. Extrusion of Apple Pomace Facilitates Pectin Extraction. J. Food Sci. 1998, 63, 841–844. [Google Scholar] [CrossRef]
- Zieliński, H.; Michalska, A.; Piskuła, M.K.; Kozłowska, H. Antioxidants in thermally treated buckwheat groats. Mol. Nutr. Food Res. 2006, 50, 824–832. [Google Scholar] [CrossRef]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Akila, G.; Djamil, K.; Saadia, B. Portulaca oleracea extract increases lecithin:cholesterol acyltransferase and paraoxonase 1 activities and enhances reverse cholesterol transport in streptozotocin-induced diabetic rat. Pharmacogn. J. 2014, 6, 1–9. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Venkatakrishna, K.; Patel, D.; Shyamprasad, K. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complementary Altern. Med. 2016, 16, 274. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.S.; Quintao, E.C.R.; Moore, K.J.; Lottenberg, A.M. Molecular Pathways Underlying Cholesterol Homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef]
- Tanaka, S.; Couret, D.; Tran-Dinh, A.; Duranteau, J.; Montravers, P.; Schwendeman, A.; Meilhac, O. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 2020, 24, 134. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-X.; Wang, L.-K. Effect of ferulic acid on cholesterol efflux in macrophage foam cell formation and potential mechanism. Zhongguo Zhong Yao Za Zhi 2015, 40, 533–537. [Google Scholar]
- Liang, N.; Li, Y.M.; He, Z.; Hao, W.; Zhao, Y.; Liu, J.; Zhu, H.; Kwek, E.; Ma, K.Y.; He, W.S.; et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules 2021, 26, 3766. [Google Scholar] [CrossRef]
- Hao, S.; Xiao, Y.; Lin, Y.; Mo, Z.; Chen, Y.; Peng, X.; Xiang, C.; Li, Y.; Li, W. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm. Biol. 2016, 54, 251–259. [Google Scholar] [CrossRef]
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Cooney, O.D.; Lin, X.; Nadarajah, S.; Dragoljevic, D.; Huynh, K.; Onda, D.A.; Galic, S.; Meikle, P.J.; Edlund, T.; et al. Defective AMPK regulation of cholesterol metabolism accelerates atherosclerosis by promoting HSPC mobilization and myelopoiesis. Mol. Metab. 2022, 61, 101514. [Google Scholar] [CrossRef]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE(-/-) mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; Assanga, S.I.; Lujan, L.L.; O’Keefe, J.H. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart 2022, 9, e001801. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharm. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, M.S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.H.; Kim, Y. Rutin Increases Muscle Mitochondrial Biogenesis with AMPK Activation in High-Fat Diet-Induced Obese Rats. Nutrients 2015, 7, 8152–8169. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, N.; Tousoulis, D.; Androulakis, E.; Siasos, G.; Briasoulis, A.; Vogiatzi, G.; Kampoli, A.M.; Tsiamis, E.; Tentolouris, C.; Stefanadis, C. The role of microRNAs in cardiovascular disease. Curr. Med. Chem. 2012, 19, 2605–2610. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Zhu, Y.; Sun, H.; Juguilon, C.; Li, F.; Fan, D.; Yin, L.; Zhang, Y. Macrophage miR-34a Is a Key Regulator of Cholesterol Efflux and Atherosclerosis. Mol. Ther. 2020, 28, 202–216. [Google Scholar] [CrossRef]
- He, Z.; Hu, C.; Jia, W. miRNAs in non-alcoholic fatty liver disease. Front. Med. 2016, 10, 389–396. [Google Scholar] [CrossRef]
- Akbari, G.; Savari, F.; Mard, S.A.; Rezaie, A.; Moradi, M. Gallic acid protects the liver in rats against injuries induced by transient ischemia-reperfusion through regulating microRNAs expressions. Iran. J. Basic Med. Sci. 2019, 22, 439–444. [Google Scholar] [CrossRef]
- Deng, N.; He, Z.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. Highland Barley Whole Grain (Hordeum vulgare L.) Ameliorates Hyperlipidemia by Modulating Cecal Microbiota, miRNAs, and AMPK Pathways in Leptin Receptor-Deficient db/db Mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef]
| Component | SD | HC | PE |
|---|---|---|---|
| Corn starch | 150 | 150 | 150 |
| Casein | 200 | 200 | 200 |
| Sucrose | 500 | 485 | 477 |
| Corn oil | 50 | 50 | 50 |
| Cellulose | 50 | 50 | 50 |
| Mineral mix (AIN-76) | 35 | 35 | 35 |
| Vitamin mix (AIN-76) | 10 | 10 | 10 |
| DL-Methionine | 3 | 3 | 3 |
| Choline bitartrate | 2 | 2 | 2 |
| Cholesterol | - | 10 | 10 |
| Cholic acid | - | 5 | 5 |
| PO extract | - | - | 8 |
| Total | 1000 | 1000 | 1000 |
| Name | Genebank No. | Primer Sequence (5′–3′) |
|---|---|---|
| ABCA1 | NM_178095.3 | F: GCT ACA CTG GTC GTT ATC AT R: GAC CAC CCA TAT AGC AAG AG |
| ABCG1 | NM_053502.2 | F: GCT CTG TGG AGG TAG TTA ATG R: CTC CTT CCA GAC TTC CTT TC |
| ABCG5 | NM_053754 | F: ATG AGT GAG CTG CCC TTT CT R: CGC TGA AGG ACA CAT TCA GG |
| ABCG8 | NM_130414.2 | F: CAC CCT AGA CTC TAA CTC CA R: GGA GCA CTG GAT AGT ATT GG |
| apoA-1 | NM_012738 | F: GTG GGT TCA ACT GTT GGT CG R: GGG CTG CAT CTT CTG TTT CA |
| CYP7A1 | NM_012942.2 | F: GCC TTC CTA TTC ACT TGT TC R: GTG GAG AGC GTG TCA TTG |
| FXR | NM_021745 | F: TTC ACT GTC TGA TCC GCA TG R: CGC CGT GTA CAA GTG TAA GA |
| GAPDH | NM_017008.4 | F: GAT GAC ATC AAG AAG GTG GT R: GCA TCA AAG GTG GAA GAA TG |
| LCAT | NM_017024.2 | F: TAA CAA TGG GTA TGT GCG GG R: GCC AAG GCT GTG TCC AAT AA |
| LXRα | NM_031627 | F: GAC TTC GAG TCA CGC CTT GG R: GTC CTC CCT GCT CAG CTG TA |
| PON1 | BC_091403.1 | F: GT GGT AAT CCA CCC AGA CTC R: AA GCT CTC AGG TCC AAC AGC |
| SR-B1 | NM_031541 | F: GG CAA ATT TGG CCT GTT CGT R: GC CGT TCC ACT TAT CCA CCA |
| Compound | Content (mg/g) |
|---|---|
| Gallic acid | 1.13 ± 0.02 |
| Chlorogenic acid | 2.52 ± 0.16 |
| Ferulic acid | 0.41 ± 0.01 |
| Rutin | 1.37 ± 0.02 |
| Variables | SD | HC | PE |
|---|---|---|---|
| Initial body weight (g) | 191.25 ± 1.25 | 191.65 ± 2.00 | 190.64 ± 1.68 |
| Final body weight (g) | 404.40 ± 6.95 | 402.25 ± 2.62 | 403.19 ± 6.12 |
| Body weight gain (g/4 weeks) | 213.14 ± 6.90 | 210.59 ± 3.24 | 212.55 ± 5.60 |
| Food intake (g/day) | 24.93 ± 0.52 | 24.60 ± 0.82 | 23.72 ± 0.45 |
| Liver weight (g/100 g body weight) | 3.15 ± 0.06 | 4.75 ± 0.17 | 4.63 ± 0.14 |
| Serum ALT (IU/L) | 5.82 ± 0.50 | 7.03 ± 0.86 | 6.37 ± 0.58 |
| Serum AST (IU/L) | 48.02 ± 1.86 | 45.69 ± 3.07 | 44.04 ± 2.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Lee, M.-S.; Kang, S.-A.; Kim, C.-T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330. https://doi.org/10.3390/nu14163330
Jang S, Lee M-S, Kang S-A, Kim C-T, Kim Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients. 2022; 14(16):3330. https://doi.org/10.3390/nu14163330
Chicago/Turabian StyleJang, Sojeong, Mak-Soon Lee, Sun-A Kang, Chong-Tai Kim, and Yangha Kim. 2022. "Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet" Nutrients 14, no. 16: 3330. https://doi.org/10.3390/nu14163330
APA StyleJang, S., Lee, M.-S., Kang, S.-A., Kim, C.-T., & Kim, Y. (2022). Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients, 14(16), 3330. https://doi.org/10.3390/nu14163330






