Factors Determining Effective Probiotic Activity: Evaluation of Survival and Antibacterial Activity of Selected Probiotic Products Using an “In Vitro” Study
Abstract
:1. Introduction
2. The Aim of the Study
3. Material and Methods
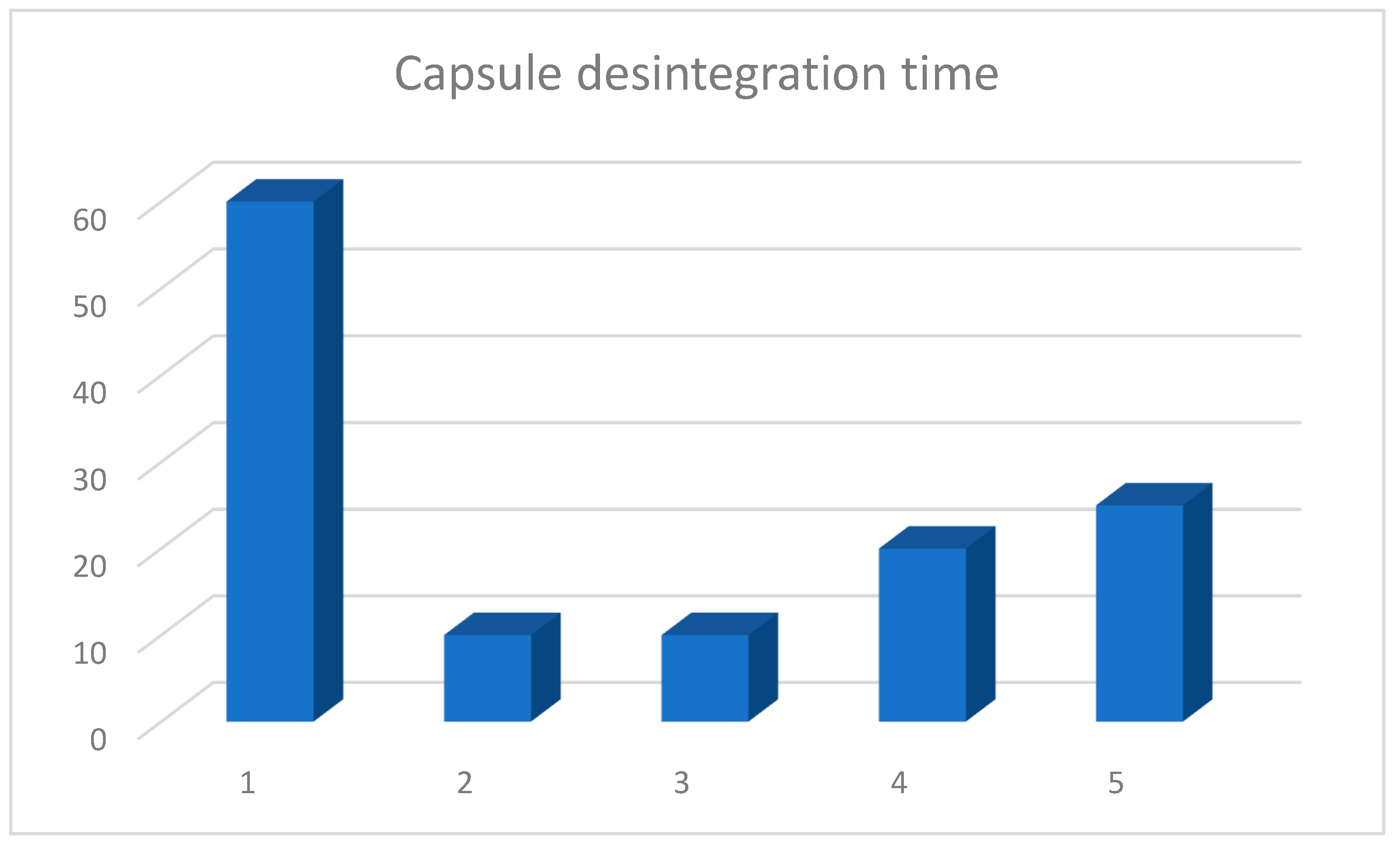
3.1. Capsule Disintegration Study
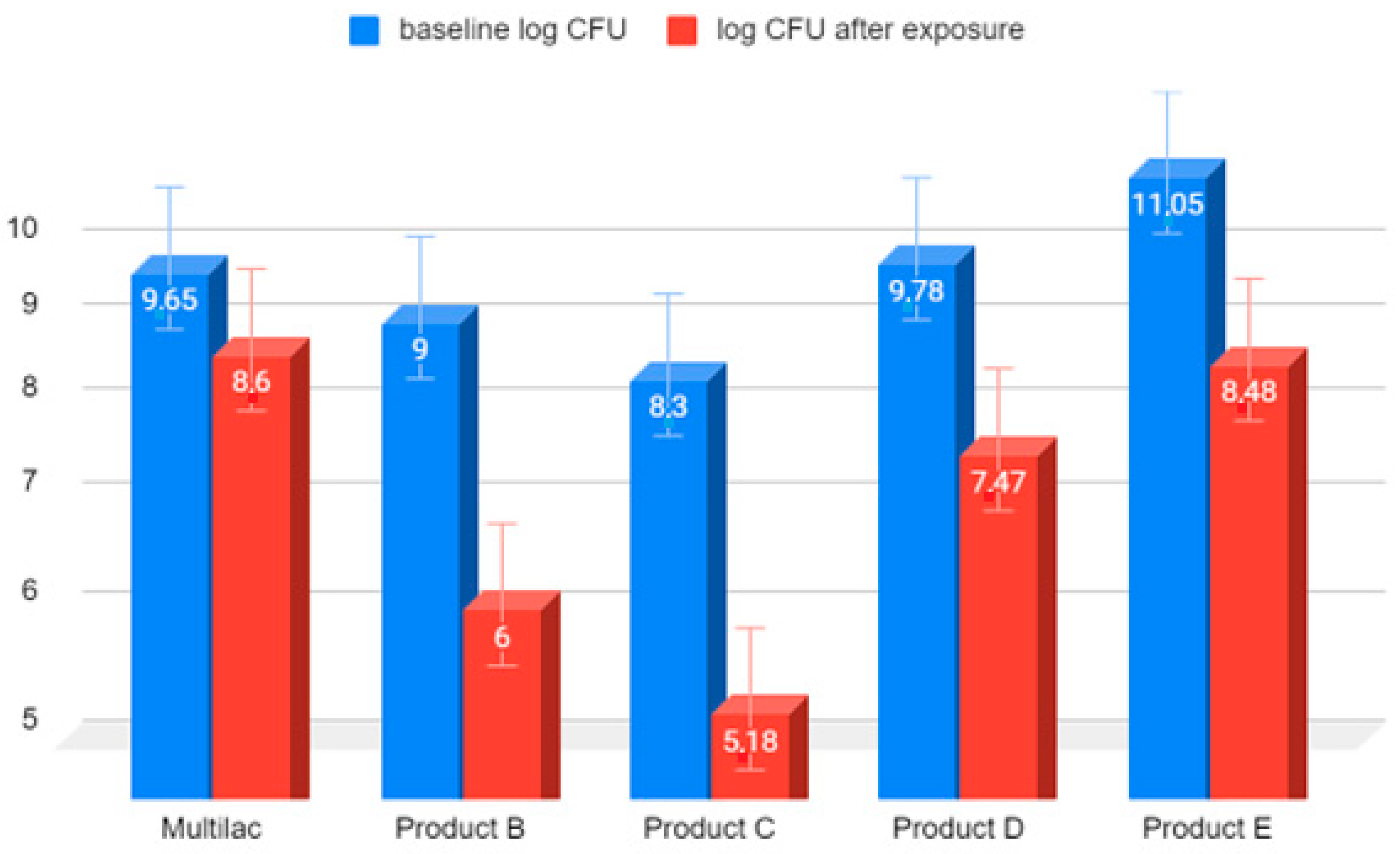
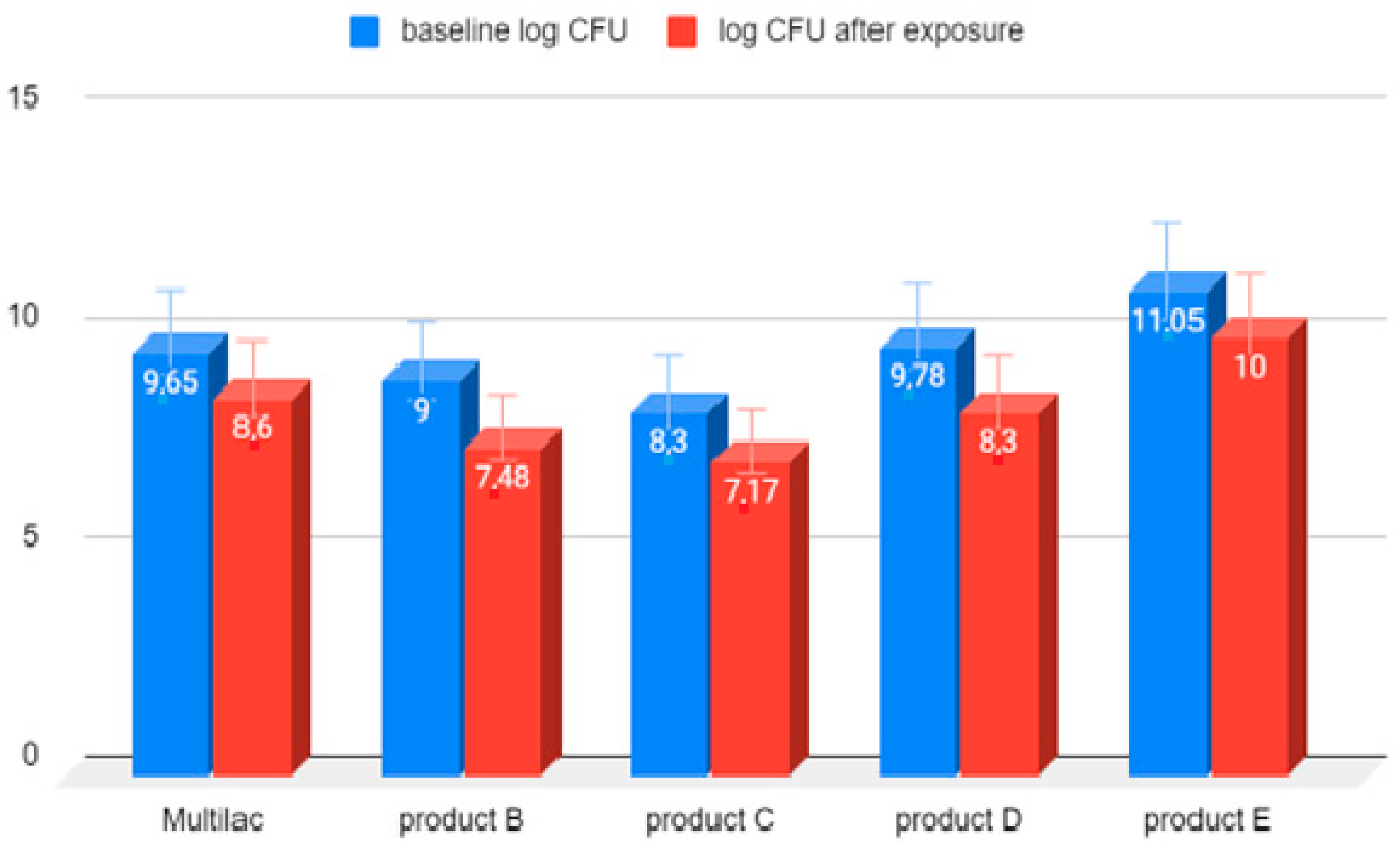
3.2. Microbial Survival Study
3.3. Examination of the Amount of LAB Bacteria before and after Exposure to Hydrochloric Acid
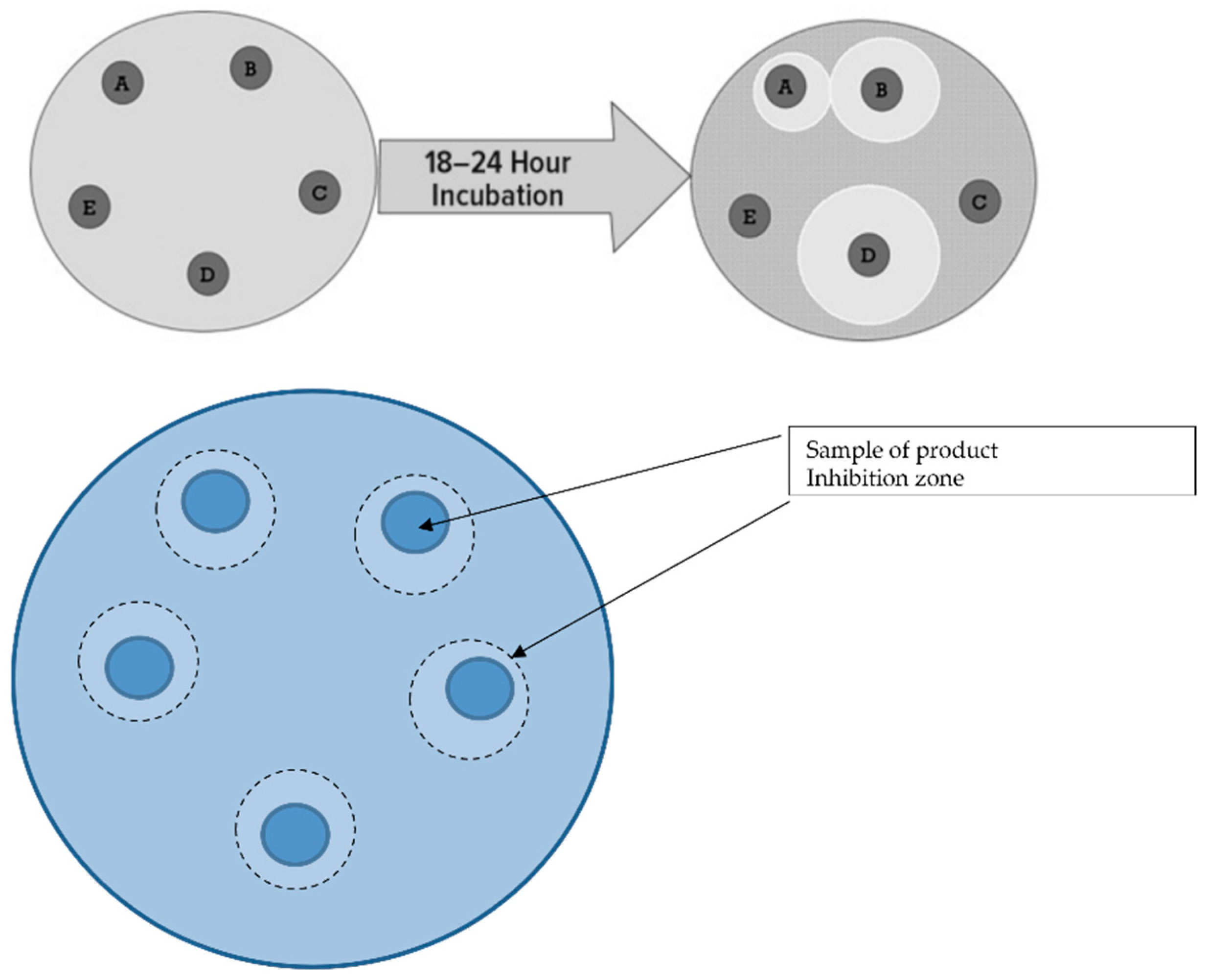
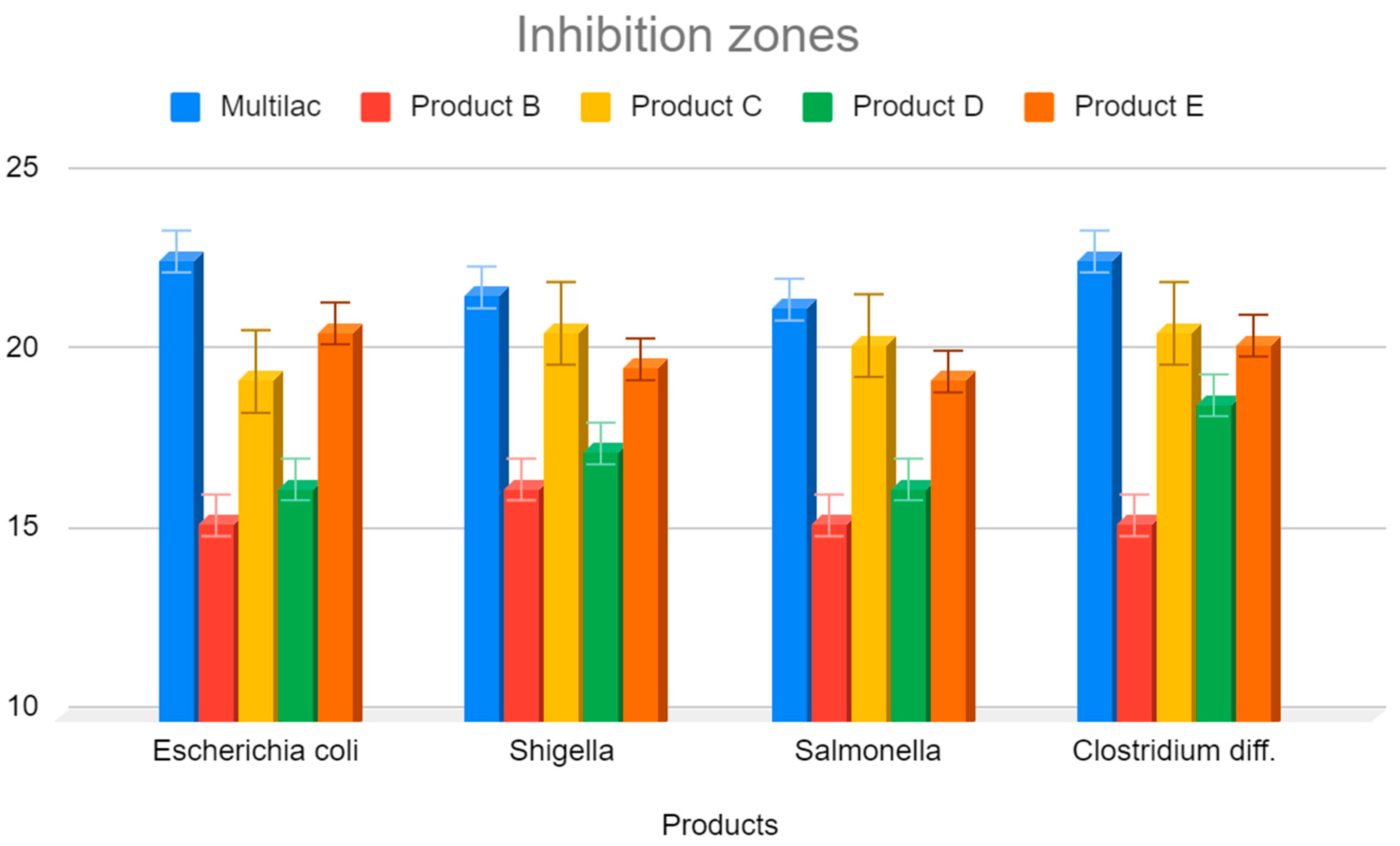
3.4. Investigation of the Inhibition Zones of Marketed Probiotic against Pathological Bacteria
- Eschericha coli;
- Shigella;
- Salmonella spp.;
- Clostridioides difficile [27].
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, B.R.; Flint, J.H.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ljungh, A.; Wadström, T. Lactic acid bacteria as probiotics. Curr. Issues Intest. Microbiol. 2006, 7, 73–89. [Google Scholar] [PubMed]
- Heczko, P.B.; Strus, M.; Kochan, P. Projektowanie probiotyków do zastosowań medycznych. Post. Mikrobiol. 2008, 47, 431–434. [Google Scholar]
- Klewicka, E.; Śleżewska, K.; Nowak, A. Ocena przeżywalności bakterii Lactobacillus zawartych w preparacie probiotycznym podczas pasażu w stymulowanym przewodzie pokarmowym. Żywność. Nauka. Technologia. Jakość. 2014, 6, 170–181. [Google Scholar]
- Del Piano, M.; Carmagnola, S.; Andorno, S.; Pagliarulo, M.; Tari, R.; Mogna, L.; Strozzi, G.P.; Sforza, F.; Capurso, L. Evaluation of the intestinal colonization by microencapsulated probiotic bacteria in comparison with the same uncoated strains. J. Clinic. Gastroenterol. 2010, 44, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Carmagnola, S.; Ballarè, M.; Sartori, M.; Orsello, M.; Balzarini, M.; Pagliarulo, M.; Tari, R.; Anderloni, A.; Strozzi, G.P.; et al. Is microencapsulation the future of probiotic preparations? The increased efficacy of gastro-protected probiotics. Gut Microbes 2011, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lillie, A.; Johansen, A.K. Disintegration and in vitro acid stability testes on nine lactic acid bacteria preparations. In Proceedings of the XXIII, Nordic Gastroenterology Meeting, Reykjavik, Iceland, 14–16 June 1990. [Google Scholar]
- Corneliussen, L.; Skov, R.; Espersen, F. Effect of low pH on survival of lactic producing bacteria in oral formulation. In Proceedings of the 9th European Congress of Clinical Microbiology and infectious Diseases, Berlin, Germany, 21–24 May 1999. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; Lebeer, S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Strozzi, P.; Barba, M.; Allesina, S.; Deidda, F.; Lorenzini, P.; Lorenzo, M.; Stefania, C.; Pagliarulo, M.; Balzarini, M.; et al. In vitro sensitivity of probiotics to human pancreatic juice. J. Clinic. Gastroenterol. 2008, 42, S170–S173. [Google Scholar] [CrossRef] [PubMed]
- Mahbubani, K.T.; Slater, N.K.; Edwards, A.D. Protection of dried probiotic bacteria from bile using bile adsorbent resins. N. Biotechnol. 2014, 31, 69–72. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.; de Valdez, G.F.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gómez, J.G.; López-Bonilla, A.; Trejo-Tapia, G.; Ávila-Reyes, S.V.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. In Vitro Bile Salt Hydrolase (BSH) Activity Screening of Different Probiotic Microorganisms. Foods 2021, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Krauss, H.; Ciechelska-Rybarczyk, A.; Bernatek, M.; Wojtyla-Buciora, P.; Sommermeyer, H. In-Vitro Growth Inhibition of Bacterial Pathogens by Probiotics and a Synbiotic: Product Composition Matters. Int. J. Environ. Res. Public Health 2020, 17, 3332. [Google Scholar] [CrossRef]
- Schwendicke, F.; Korte, F.; Dörfer, C.E.; Kneist, S.; Fawzy, K.; Paris, S. Inhibition of Streptococcus mutans Growth and Biofilm Formation by Probiotics in vitro. Caries Res. 2017, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Igor Łoniewski, I. NSAIDs and PPI induced enteropathy— Important and neglected clinical problem. Gastroenterologia Kliniczna. 2014, 6, 24–33. [Google Scholar]
- Bryrup, T.; Thomsen, C.W.; Kern, T.; Allin, K.H.; Brandslun, I.; Jørgensen, N.R.; Vestergaard, H.; Hansen, T.; Hansen, T.H.; Pedersen, O.; et al. Metformin-induced changes of the gut microbiota in healthy young men: Results of a non-blinded, one-armed intervention study. Diabetologia 2019, 62, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Frassl, G.A.; Finlay, B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2006, 85, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Sommermeyer, H.; Bernatek, M.; Ciechelska-Rybarczyk, A.; Oleskow, B.; Sommer Mikkelsen, L.; Barken, K.B. Persistent infection by Salmonella enterica servovar Typhimurium: Are synbiotics a therapeutic option?–A case report. Benef. Microbes 2019, 10, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Strus, M.; Pakosz, K.; Gościniak, H.; Przondo-Mordarska, A.; Rozynek, E.; Pituch, H.; Meisel-Mikołajczyk, F.; Heczko, P.B. Antagonistic activity of Lactobacillus bacteria strains against anaerobic gastrointestinal tract pathogens (Helicobacter pylori, Campylobacter coli, Campylobacter jejuni, Clostridium difficile). Med. Doswiad. Mikrob. 2001, 53, 133–142. [Google Scholar]
- Sørensen, K. Enumeration of probiotics microorganisms exposed to acidic condition. Report 2010.
- PN-ISO 15214:2002; Mikrobiologia żywności I pasz. Horyzontalna metoda oznaczania liczby mezofilnych bakterii fermentacji mlekowej. Metoda płytkowa w temperaturze 30 °C. Polish Committee for Standardization: Warsaw, Poland, 2002; ISBN 832368331X.
- Lancet Infectious Diseases. C difficile—A rose by any other name. Lancet Infect. Dis. 2018, 19, 449.
- Strus, M. A new mthod for evaluation of the antagonistic action of bacterial lactic acid (LAB) on selected pathogenic indicator bacteria. Med. Doswiad. Mikrobiol. 1998, 50, 123–130. [Google Scholar]
- Rostas, J.W.; Mai, T.T.; Richards, W.O. Gastric motility physiology and surgical intervention. Surg. Clin. North Am. 2011, 91, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Camilleri, M.; Crowe, S.E.; El-Omar, E.M.; Fox, J.G.; Kuipers, E.J.; Malfertheiner, P.; McColl, K.E.; Pritchard, D.M.; Rugge, M.; et al. The stomach in health and disease. Gut 2015, 64, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.J.; Kelly, K.A. Gastric motor physiology and pathophysiology. Surg. Clin. North Am. 1993, 73, 1145–1160. [Google Scholar] [CrossRef]
- Finegold, S.M.; Sutter, V.L.; Mathisen, G.E. Normal indigenous intestinal flora. In Human Intestinal Microflora in Health and Disease; Academic Press: London, UK, 1983; pp. 3–31. [Google Scholar]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Truszkowska, K.; Gomesa, A.M.; Pintadoa, M.E.; Malcataa, F.X. Sur-vival of probiotic bacteria in a whey cheese vector submitted to environmental conditions prevailing in the gastrointestinal tract. Int. Dairy. J. 2005, 15, 921–927. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Contreras, C.A. Enteropathogenic E. coli (EPEC) infection in children. Curr. Opin. Infect. Dis. 2011, 24, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Fijan, S.; Sulc, D.; Steyer, A. Study of the In Vitro Antagonistic Activity of Various Mono-Strain and Multi-Strain Probiotics against Escherichia coli. Int. J. Environ. Res. Public Health 2018, 15, 1539. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Kunicka-Styczynska, A.; Rygala, A. Probiotic Activity of Saccharomyces cerevisiae var. boulardii Against Human Pathogens. Food Technol. Biotechnol. 2012, 50, 230–236. [Google Scholar]
- Hütt, P.; Shchepetova, J.; Lõivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Jahromi, M.F.; Ho, Y.W. Probiotic Potential of Lactobacillus Strains with Antimicrobial Activity against Some Human Pathogenic Strains. BioMed Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.N.; Rosenfeldt Nielsen, V.; Hayford, A.E.; Møller, L.; Michaelsen, K.F.; Pærregaard, A.; Sandström, B.; Tvede, M.; Jakobsen, M. Screening of Probiotic Activities of Forty-Seven Strains of Lactobacillus spp. by In Vitro Techniques and Evaluation of the Colonization Ability of Five Selected Strains in Humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Bernatek, M.; Sommermeyer, H.; Pituch, H.; Wultańska, D.; Kopczyński, Z.; Piątek, J.; Wojtyła-Buciora, P. Clindamycin-resistant Clostridioides difficile: A challenge in dentistry. J. Health Inequal. 2022, 8, 82–88. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Elbere, I.; Kalnina, I.; Silamikelis, I.; Konrade, I.; Zaharenko, L.; Sekace, K.; Radovica-Spalvina, I.; Fridmanis, D.; Gudra, D.; Pirags, V.; et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS ONE 2018, 13, e0204317. [Google Scholar] [CrossRef] [PubMed]
| Product | Bacterial Composition of the Product | Capsule Type |
|---|---|---|
| Product A (Multilac®) 4.5 × 109 bacteria in the capsule | Lactococcus lactis Ll-23 13 × 108 CFU, Lactobacillus plantarum LP-115 2.5 × 108 CFU, Lactobacillus rhamnosus BI-FOLAC™ GG 1.5 × 108 CFU, Streptococcus thermophilus ST-21 1.1 × 108 CFU, Bifidobacterium breve BB-03 1 × 108 CFU, Lactobacillus casei Lc-11 0.4 × 108 CFU, Bifidobacterium bifidum Bb-02 1 × 108 CFU, Bifidobacterium animalis ssp. lacti BIFOLAC™ 12 21.5 × 108 CFU, Lactobacillus acidophilus LA-14 2 × 108 CFU | Gastro-resistant capsules |
| Product B 1 × 109 bacteria in the capsule | Lactobacillus plantarum | Regular capsules |
| Product C 2 × 108 bacteria in the capsule | 4 Lactobacillis 2 Bifidobacterium Lactococcus lactis | Regular capsules |
| Product D 6 × 109 bacteria in the capsule | Lactobacillus rhamnosus | Regular capsules |
| Product E 1.12 × 1011 bacteria in the capsule | 4 Lactobacillis 3 BifidobacteriumStreptococcus thermophilus | Regular capsules |
| Multilac® | Product B | Product C | Product D | Product E |
|---|---|---|---|---|
| 60 | 10 | 10 | 20 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatek, M.; Żukiewicz-Sobczak, W.; Lachowicz-Wiśniewska, S.; Piątek, J. Factors Determining Effective Probiotic Activity: Evaluation of Survival and Antibacterial Activity of Selected Probiotic Products Using an “In Vitro” Study. Nutrients 2022, 14, 3323. https://doi.org/10.3390/nu14163323
Bernatek M, Żukiewicz-Sobczak W, Lachowicz-Wiśniewska S, Piątek J. Factors Determining Effective Probiotic Activity: Evaluation of Survival and Antibacterial Activity of Selected Probiotic Products Using an “In Vitro” Study. Nutrients. 2022; 14(16):3323. https://doi.org/10.3390/nu14163323
Chicago/Turabian StyleBernatek, Malgorzata, Wioletta Żukiewicz-Sobczak, Sabina Lachowicz-Wiśniewska, and Jacek Piątek. 2022. "Factors Determining Effective Probiotic Activity: Evaluation of Survival and Antibacterial Activity of Selected Probiotic Products Using an “In Vitro” Study" Nutrients 14, no. 16: 3323. https://doi.org/10.3390/nu14163323
APA StyleBernatek, M., Żukiewicz-Sobczak, W., Lachowicz-Wiśniewska, S., & Piątek, J. (2022). Factors Determining Effective Probiotic Activity: Evaluation of Survival and Antibacterial Activity of Selected Probiotic Products Using an “In Vitro” Study. Nutrients, 14(16), 3323. https://doi.org/10.3390/nu14163323








