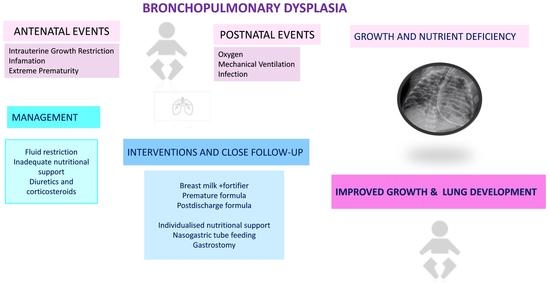
Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit
Abstract
1. Introduction
2. Growth of Neonates with Bronchopulmonary Dysplasia
3. Nutritional Management in Infants with Established BPD, either in the Hospital or after Discharge
4. Human Breast Milk
5. Premature Formulas and Caloric Supplementation
6. Feeding Issues
7. Alternatives to Oral Feeding
8. Monitoring of Growth and Nutritional Status
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonadies, L.; Zaramella, P.; Porzionato, A.; Perilongo, G.; Muraca, M.; Baraldi, E. Present and Future of Bronchopulmonary Dysplasia. J. Clin. Med. 2020, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease: Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 27, 6357–6368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Rehan, V.K. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung 2018, 196, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef]
- Geetha, O.; Rajadurai, V.S.; Anand, A.J.; Dela Puerta, R.; Huey Quek, B.; Khoo, P.C.; Chua, M.C.; Agarwal, P. New BPD-prevalence and risk factors for bronchopulmonary dysplasia/mortality in extremely low gestational age infants 28 weeks. J. Perinatol. 2021, 41, 1943–1950. [Google Scholar] [CrossRef]
- Cardoen, F.; Vermeulen, F.; Proesmans, M.; Moens, M.; De Boeck, K. Lung function evolution in children with old and new type bronchopulmonary dysplasia: A retrospective cohort analysis. Eur. J. Pediatr. 2019, 178, 1859–1866. [Google Scholar] [CrossRef]
- Arigliani, M.; Spinelli, A.M.; Liguoro, I.; Cogo, P. Nutrition and Lung Growth. Nutrients 2018, 10, 919. [Google Scholar] [CrossRef]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M. European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Doyle, L.W. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin. Perinatol. 2018, 42, 478–484. [Google Scholar] [CrossRef]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef]
- Van Marter, L.J. Epidemiology of bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2009, 14, 358–366. [Google Scholar] [CrossRef]
- Principi, N.; Di Pietro, M.G.; Esposito, S. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 2018, 16, 36. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Gortner, L.; Misselwitz, B.; Milligan, D.; Zeitlin, J.; Kollee, L.; Boerch, K.; Agostino, R.; Van Reempts, P.; Chabernaud, J.L.; Bréart, G.; et al. Members of the MOSAIC Research Group. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: Results from the MOSAIC cohort. Neonatology 2011, 99, 112–117. [Google Scholar] [CrossRef]
- Oh, W.; Poindexter, B.B.; Perritt, R.; Lemons, J.A.; Bauer, C.R.; Ehrenkranz, R.A.; Toll, B.J.; Poole, K.; Wright, L.L. Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 2005, 147, 786–790. [Google Scholar] [CrossRef]
- Hartnoll, G.; Bétrémieux, P.; Modi, N. Randomised controlled trial of postnatal sodium supplementation on oxygen dependency and body weight in 25–30 week gestational age infants. Arch. Dis. Child Fetal Neonatal Ed. 2000, 82, F19–F23. [Google Scholar] [CrossRef]
- Stewart, A.; Brion, L.P. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2011, 2011, CD001453. [Google Scholar] [CrossRef]
- Uberos, J.; Jimenez-Montilla, S.; Molina-Oya, M.; García-Serrano, J.L. Early energy restriction in premature infants and bronchopulmonary dysplasia: A cohort study. Br. J. Nutr. 2020, 123, 1024–1031. [Google Scholar] [CrossRef]
- Bauer, S.E.; Vanderpool, C.P.B.; Ren, C.; Cristea, A.I. Nutrition and growth in infants with established bronchopulmonary dysplasia. Pediatr. Pulmonol. 2021, 56, 3557–3562. [Google Scholar] [CrossRef]
- Rocha, G.; Guimarães, H.; Pereira-da-Silva, L. Τhe Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. Int. J. Environ. Res. Public Health 2021, 18, 6245. [Google Scholar] [CrossRef]
- Milanesi, B.G.; Lima, P.A.T.; Villela, L.D.; Martins, A.S.; Gomes-Junior, S.C.S.; Moreira, M.E.L.; Baker Méio, M.D.B. Assessment of early nutritional intake in preterm infants with bronchopulmonary dysplasia: A cohort study. Eur. J. Pediatr. 2021, 180, 1423–1430. [Google Scholar] [CrossRef]
- Jiang, H.; Lv, Y.; Hou, W.; Xu, X.; Zhu, L.L.; Zhang, H.; Shu, G. Association between neonatal malnutrition and bronchopulmonary dysplasia in very low-birth-weight infants: A propensity score-matched analysis. Nutr. Clin. Pract. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Malikiwi, A.I.; Lee, Y.M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28 weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef]
- Al-Jebawi, Y.; Agarwal, N.; Groh Wargo, S.; Shekhawat, P.; Mhanna, M.J. Low caloric intake and high fluid intake during the first week of life are associated with the severity of bronchopulmonary dysplasia in extremely low birth weight infants. J. Neonatal Perinatal Med. 2020, 13, 207–214. [Google Scholar] [CrossRef]
- Uberos, J.; Lardón-Fernández, M.; Machado-Casas, I.; Molina-Oya, M.; Narbona-López, E. Nutrition in extremely low birth weight infants: Impact on bronchopulmonary dysplasia. Minerva Pediatr. 2016, 68, 419–426. [Google Scholar]
- Huysman, W.A.; de Ridder, M.; de Bruin, N.C.; van Helmond, G.; Terpstra, N.; Van Goudoever, J.B.; Sauer, P.J. Growth and body composition in preterm infants with bronchopulmonary dysplasia. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F46–F51. [Google Scholar] [CrossRef]
- Spiegler, J.; Preuss, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Gopel, W. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 2016, 169, 76–80. [Google Scholar] [CrossRef]
- Giannì, M.L.; Roggero, P.; Colnaghi, M.R.; Piemontese, P.; Amato, O.; Orsi, A.; Morlacchi, L.; Mosca, F. The role of nutrition in promoting growth in pre-term infants with bronchopulmonary dysplasia: A prospective non-randomised interventional cohort study. BMC Pediatr. 2014, 14, 235. [Google Scholar] [CrossRef]
- Yeh, T.F.; Mc Clenan, D.A.; Ajayi, O.A.; Pildes, R.S. Metabolic rate and energy balance in infants with bronchopulmonary dysplasia. J. Pediatr. 1989, 114, 448–451. [Google Scholar] [CrossRef]
- Baner, S.E.; Huff, K.A.; Vanderpool, C.P.B.; Rose, R.S.; Cristea, A.I. Growth and nutrition in children with established bronchopulmonary dysplasia: A review of the literature. Nutr. Clin. Pract. 2022, 37, 282–298. [Google Scholar] [CrossRef]
- De Meer, K.; Westerterp, K.R.; Houwen, R.H.J.; Brouwers, H.A.A.; Berger, R.; Okken, A. Total energy expenditure in infants with bronchopulmonary dysplasia is associated with respiratory status. Eur. J. Pediatr. 1997, 156, 299–304. [Google Scholar] [CrossRef]
- Lauro, A.; Lacaille, F. Short bowel syndrome in children and adults: From rehabilitation to transplantation. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 55–70. [Google Scholar] [CrossRef]
- Bell, E.F.; Acarregui, M.J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2014, 2014, CD000503. [Google Scholar] [CrossRef]
- Barrington, K.J.; Fortin-Pellerin, E.; Pennaforte, T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database Syst. Rev. 2017, 2, CD005389. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, X.Z.; Chang, Y.M.; Liu, X.H.; Tong, X.M.; Ding, G.F. Nutritional Committee of Neonatology Branch of Chinese Medical Doctor Association. Editorial Committee of Chinese Journal of Contemporary Pediatrics Expert consensus on nutritional management of preterm infants with bronchopulmonary dysplasia. Chin. J. Contemp. Paediatr. 2020, 22, 805–814. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary FROM the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, B.; Cui, Q.; Chen, C. Omega-3 Long-chain Polyunsaturated Fatty Acids for Bronchopulmonary Dysplasia: A Meta-analysis. J. Pediatr. 2019, 144, e20190181. [Google Scholar] [CrossRef]
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F560–F566. [Google Scholar] [CrossRef]
- Abrams, S.A.; Bhatia, J.J.S.; Corkins, M.R.; De Ferranti, S.D.; Golden, N.H.; Silverstein, J. The Committee on Nutrition. Calcium and Vitamin D Requirements of Enterally Fed Preterm Infants. Am. Acad. Pediatr. 2013, 131, e1676–e1683. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef]
- Pereira-Da-Silva, L.; Virella, D.; Fusch, C. Nutritional Assessment in Preterm Infants: A Practical Approach in the NICU. Nutrients 2019, 11, 1999. [Google Scholar] [CrossRef]
- Tindell, R.; Tipple, T. Selenium: Implications for outcomes in extremely preterm infants. J. Perinatol. 2018, 38, 197–202. [Google Scholar] [CrossRef]
- Darlow, B.A.; Inder, T.E.; Graham, P.J.; Sluis, K.B.; Malpas, T.J.; Taylor, B.J.; Winterbourn, C.C. The relationship of selenium status to respiratory outcome in the very low birth weight infant. Pediatrics 1995, 96, 314–319. [Google Scholar] [CrossRef]
- Darlow, B.A.; Austin, N.C. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003, 4, CD003312. [Google Scholar] [CrossRef]
- Terrin, G.; Canani, R.B.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–11044. [Google Scholar] [CrossRef]
- Wu, W.; Bromberg, P.A.; Samet, J.M. Zinc ions as effectors of environmental oxidative lung injury. Free Radic. Biol. Med. 2013, 65, 57–69. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef]
- Dicky, O.; Ehlinger, V.; Montjaux, N.; Gremmo-Féger, G.; Sizun, J.; Rozé, J.C.; Arnaud, C.; Casper, C. EPIPAGE 2 Nutrition Study Group, EPINUTRI Study Group. Policy of feeding very preterm infants with their mother’s own fresh expressed milk was associated with a reduced risk of bronchopulmonary dysplasia. Acta Paediatr. 2017, 106, 755–762. [Google Scholar] [CrossRef]
- Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Kim, L.Y.; McGrath-Morrow, S.A.; Collaco, J.M. Impact of breast milk on respiratory outcomes in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2019, 54, 313–318. [Google Scholar] [CrossRef]
- Gilfillan, M.; Bhandari, A.; Bhandari, V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 2021, 375, n1974. [Google Scholar] [CrossRef] [PubMed]
- Montealegre-Pomar, A.D.P.; Charpak, N. Anemia, nutrition, and ambulatory oxygen weaning in a cohort of oxygen-dependent premature infants. Pediatr. Pulmonol. 2021, 56, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Aslanoglu, S.; Boquien, C.Y.; King, K.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Aslanoglu, S.; Moro, G.E.; Ziegler, E.E. Adjustable fortification of human milk fed to preterm infants: Does it make a difference? J. Perinatol. 2006, 26, 614–621. [Google Scholar] [CrossRef]
- Seshadri, S.; Kim, L.Y.; McGrath-Morrow, S.A.; Collaco, J.M. Human Milk Cessation in the NICU in Infants with 55. Bronchopulmonary Dysplasia. Am. J. Perinatol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Hay, W.W.; Hendrickson, K.C. Preterm formula use in the preterm very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 15–22. [Google Scholar] [CrossRef]
- Brunton, J.A.; Saigal, S.; Atkinson, S.A. Growth and body composition in infants with bronchopulmonary dysplasia up to 3 months corrected age: A randomized trial of a high-energy nutrient-enriched formula fed after hospital discharge. J. Pediatr. 1998, 133, 340–345. [Google Scholar] [CrossRef]
- Theile, A.R.; Radmacher, P.G.; Anschutz, T.W.; Davis, D.W.; Adamkin, D.H. Nutritional strategies and growth in extremely low birth weight infants with bronchopulmonary dysplasia over the past 10 years. J. Perinatol. 2012, 32, 117–122. [Google Scholar] [CrossRef]
- Puangco, M.A.; Schanler, R.J. Clinical experience in enteral nutrition support for premature infants with bronchopulmonary dysplasia. J. Perinatol. 2000, 20, 87–91. [Google Scholar] [CrossRef][Green Version]
- Wang, L.J.; Hu, Y.; Wang, W.; Zhang, C.Y.; Bai, Y.Z.; Zhang, S.C. Gastroesophageal Reflux Poses a Potential Risk for Late Complications of Bronchopulmonary Dysplasia: A Prospective Cohort Study. Chest 2020, 158, 1596–1605. [Google Scholar] [CrossRef]
- Misuno, K.; Nishida, Y.; Taki, M.; Hibino, S.; Murase, M.; Sakurai, M.; Itabashi, K. Infants with bronchopulmonary dysplasia suckle with week pressures to maintain breathing during feeding. Pediatrics 2007, 120, e1035–e1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Luo, H.J.; Hsieh, W.S.; Hsu, C.H.; Chen, P.S.; Chiu, N.C.; Lee, W.T.; Jeng, S.F. Severity of bronchopulmonary dysplasia and increased risk of feeding desaturation and growth delay in very low birth weight preterm infants. Pediatr. Pulmonol. 2010, 45, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Matharu, P.; Cristea, A.I.; Slaven, J.E.; Becker, S.; Niehaus, J.Z. Feeding Outcomes for Infants with Bronchopulmonary Dysplasia Discharged on Nasogastric Feeds. Am. J. Perinatol. 2021, 38, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.G.; Do, B.; Das, A.; Smith, P.B.; Adams-Chapman, I.; Jadcherla, S.; Jensen, E.A.; Goldstein, R.F.; Goldberg, R.N.; Cotten, C.M.; et al. Gastrostomy Tube Feeding in Extremely Low Birthweight Infants: Frequency, Associated Comorbidities, and Long-term Outcomes. J. Pediatr. 2019, 214, 41–46. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatza, A.A.; Gkentzi, D.; Varvarigou, A. Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit. Nutrients 2022, 14, 3311. https://doi.org/10.3390/nu14163311
Karatza AA, Gkentzi D, Varvarigou A. Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit. Nutrients. 2022; 14(16):3311. https://doi.org/10.3390/nu14163311
Chicago/Turabian StyleKaratza, Ageliki A., Despoina Gkentzi, and Anastasia Varvarigou. 2022. "Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit" Nutrients 14, no. 16: 3311. https://doi.org/10.3390/nu14163311
APA StyleKaratza, A. A., Gkentzi, D., & Varvarigou, A. (2022). Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit. Nutrients, 14(16), 3311. https://doi.org/10.3390/nu14163311