U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Dietary Nutrients Intake Assessments
2.3. Blood Pressure and Covariates Measurements
2.4. Study Outcome
2.5. Statistical Analysis
3. Results
3.1. Study Population and Characteristics
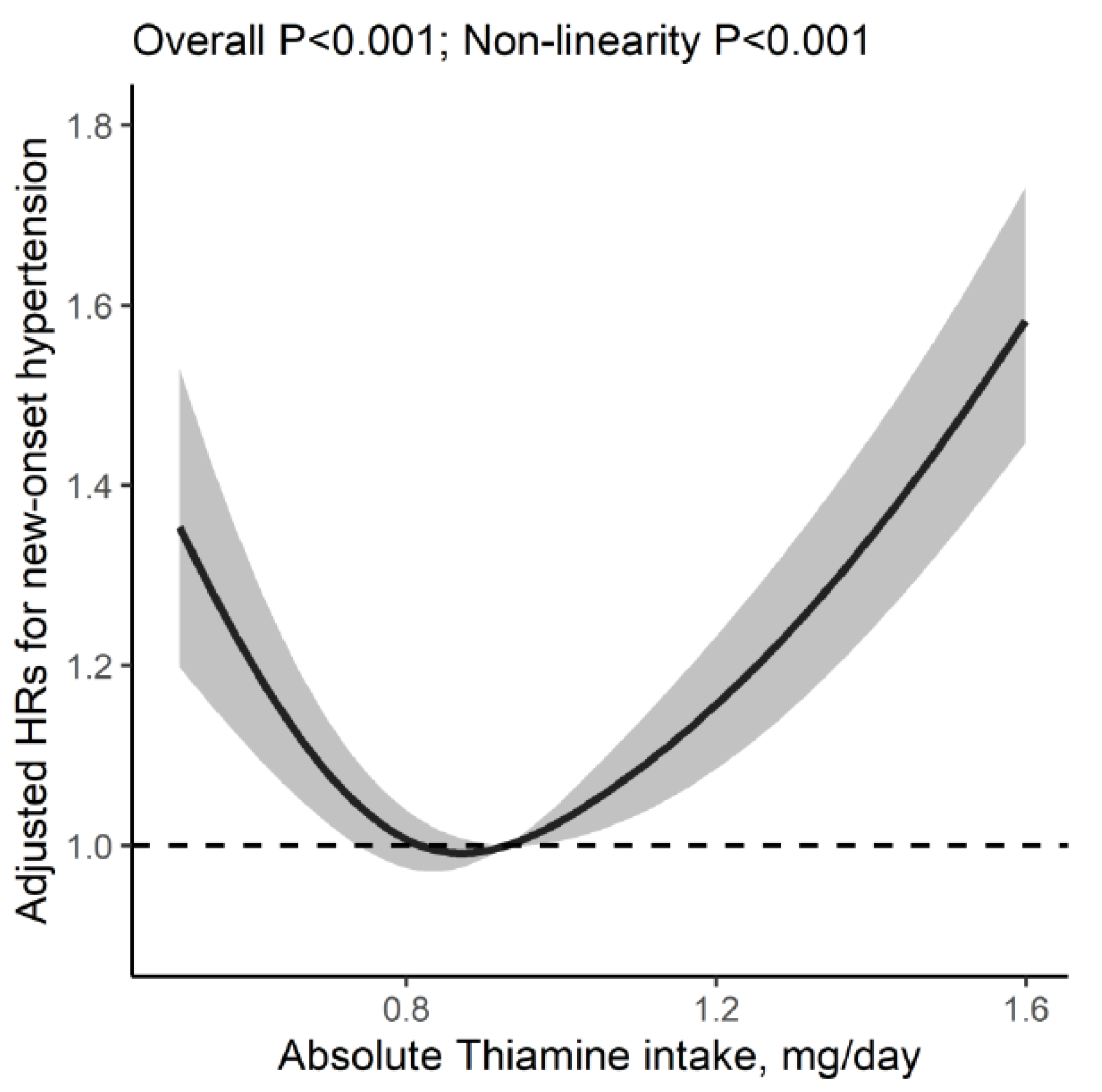
3.2. Association of Dietary Thiamine Intake with New-Onset Hypertension
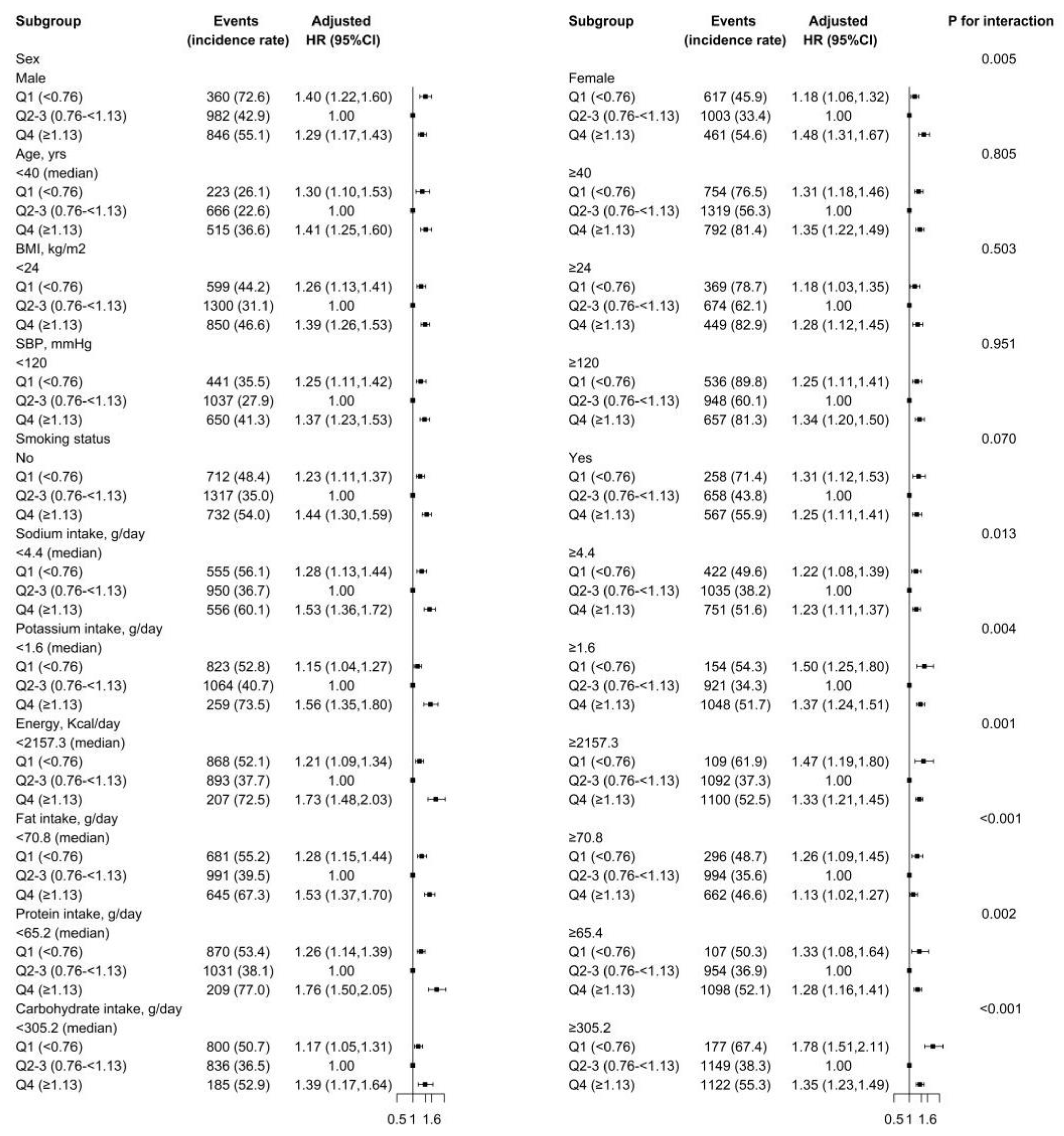
3.3. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Z.; Chen, J.; Xiao, C.; Chen, W. A Global View on Prevalence of Hypertension and Human Develop Index. Ann. Glob. Health 2020, 86, 67. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S.; et al. American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef]
- Carpenter, K.J. The discovery of thiamin. Ann. Nutr. Metab. 2012, 61, 219–223. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Thiamine and Cardiovascular Disease: A Literature Review. Prog. Cardiovasc. Dis. 2018, 61, 27–32. [Google Scholar] [CrossRef]
- Tanaka, T.; Sohmiya, K.; Kono, T.; Terasaki, F.; Horie, R.; Ohkaru, Y.; Muramatsu, M.; Takai, S.; Miyazaki, M.; Kitaura, Y. Thiamine attenuates the hypertension and metabolic abnormalities in CD36-defective SHR: Uncoupling of glucose oxidation from cellular entry accompanied with enhanced protein O-GlcNAcylation in CD36 deficiency. Mol. Cell Biochem. 2007, 299, 23–35. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China health and nutrition survey, 1989–2011. Obes Rev. 2014, 15 (Suppl. 1), 2–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhou, C.; Zhang, Z.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Qin, X. Inverse Association Between Riboflavin Intake and New-Onset Hypertension: A Nationwide Cohort Study in China. Hypertension 2020, 76, 1709–1716. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Zhang, S.; Li, R.; Zhang, Y.; He, P.; Zhang, Z.; Liu, M.; Zhou, C.; Ye, Z.; et al. Dietary Carbohydrate Intake and New-Onset Hypertension: A Nationwide Cohort Study in China. Hypertension 2021, 78, 422–430. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Zhou, C.; He, P.; Zhang, Y.; Li, H.; Li, Q.; Liu, C.; Qin, X. Evaluation of Dietary Niacin and New-Onset Hypertension Among Chinese Adults. JAMA Netw. Open. 2021, 4, e2031669. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, C.; Zhang, Z.; He, P.; Li, Q.; Liu, C.; Qin, X. Inverse association between dietary vitamin A intake and new-onset hypertension. Clin Nutr. 2021, 40, 2868–2875. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, C.; Zhang, Z.; Liu, M.; Zhang, Y.; Li, H.; He, P.; Li, Q.; Qin, X. Variety and quantity of dietary protein intake from different sources and risk of new-onset diabetes: A Nationwide Cohort Study in China. BMC Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes. Rev. 2014, 15 (Suppl. 1), 16–26. [Google Scholar] [CrossRef] [Green Version]
- Zhai, F.; Guo, X.; Popkin, B.M.; Ma, L.; Wang, Q.; Shuigao, W.Y.; Ge, J.A.K. Evaluation of the 24-hour individual recall method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Li, H.; Liu, C.; Liu, M.; Zhang, Z.; Zhang, Y.; Zhou, C.; Li, Q.; Ye, Z.; Wu, Q.; et al. U-shaped association between dietary copper intake and new-onset hypertension. Clin. Nutr. 2022, 41, 536–542. [Google Scholar] [CrossRef]
- Alaei-Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: A randomised, double-blind cross-over trial. Diabetes Metab. Syndr. 2015, 9, 213–217. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Hanger, H.C.; Elmslie, J.; George, P.M.; Sainsbury, R. The response to treatment of subclinical thiamine deficiency in the elderly. Am. J. Clin. Nutr. 1997, 66, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [Green Version]
- Ascher, E.; Gade, P.V.; Hingorani, A.; Puthukkeril, S.; Kallakuri, S.; Scheinman, M.; Jacob, T. Thiamine reverses hyperglycemia-induced dysfunction in cultured endothelial cells. Surgery 2001, 130, 851–858. [Google Scholar] [CrossRef]
- Verma, S.; Reddy, K.; Balakumar, P. The defensive effect of benfotiamine in sodium arsenite-induced experimental vascular endothelial dysfunction. Biol. Trace Elem. Res. 2010, 137, 96–109. [Google Scholar] [CrossRef]
- Yadav, U.C.; Kalariya, N.M.; Srivastava, S.K.; Ramana, K.V. Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic. Biol. Med. 2010, 48, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Martel, J.L.; Kerndt, C.C.; Doshi, H.L.; Franklin, D.S. Vitamin B1 (Thiamine); StatPearls Publishing: Treasure Island, FL, USA, 2022; Bookshelf ID: NBK482360. [Google Scholar]
- Shokri-Mashhadi, N.; Aliyari, A.; Hajhashemy, Z.; Saadat, S.; Rouhani, M.H. Is it time to reconsider the administration of thiamine alone or in combination with vitamin C in critically ill patients? A meta-analysis of clinical trial studies. J. Intensive Care 2022, 10, 8. [Google Scholar] [CrossRef]
- Comín-Anduix, B.; Boren, J.; Martinez, S.; Moro, C.; Centelles, J.J.; Trebukhina, R.; Petushok, N.; Lee, W.N.; Boros, L.G.; Cascante, M. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur. J. Biochem. 2001, 268, 4177–4182. [Google Scholar] [CrossRef]
- Smidt, L.J.; Cremin, F.M.; Grivetti, L.E.; Clifford, A.J. Influence of thiamin supplementation on the health and general well-being of an elderly Irish population with marginal thiamin deficiency. J. Gerontol. 1991, 46, M16–M22. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary reference values for thiamin. EFSA J. 2016, 14, 4653. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Sauberlich, H.E.; Herman, Y.F.; Stevens, C.O.; Herman, R.H. Thiamin requirement of the adult human. Am. J. Clin. Nutr. 1979, 32, 2237–2248. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
| Variables | Thiamine Intake by Quartiles, mg/day | p Value | |||
|---|---|---|---|---|---|
| Q1 (<0.76) | Q2 (0.76–<0.93) | Q3 (0.93–<1.13) | Q4 (≥1.13) | ||
| N | 3044 | 3044 | 3044 | 3045 | |
| Male, No. (%) | 948 (31.1) | 1258 (41.3) | 1531 (50.3) | 1961 (64.4) | <0.001 |
| Age, years | 44.7 ± 16.0 | 41.1 ± 13.7 | 39.5 ± 13.1 | 39.5 ± 13.2 | <0.001 |
| Body mass index, kg/m2 | 22.5 ± 3.3 | 22.3 ± 3.0 | 22.3 ± 3.0 | 22.4 ± 2.9 | 0.029 |
| Systolic blood pressure, mmHg | 114.6 ± 11.9 | 113.0 ± 11.5 | 113.3 ± 11.3 | 114.6 ± 10.9 | <0.001 |
| Diastolic blood pressure, mmHg | 74.1 ± 8.0 | 73.8 ± 7.9 | 74.1 ± 7.8 | 74.7 ± 7.6 | <0.001 |
| Physical activity, MET-hours/week | 125.0 ± 91.6 | 144.2 ± 92.9 | 149.1 ± 91.4 | 151.9 ± 95.8 | <0.001 |
| Smoking, No. (%) | 657 (21.7) | 808 (26.6) | 980 (32.4) | 1243 (41.0) | <0.001 |
| Alcohol drinking, No. (%) | 760 (25.1) | 908 (30.2) | 1086 (36.2) | 1384 (45.9) | <0.001 |
| Urban residence, No. (%) | 1389 (45.6) | 1105 (36.3) | 1010 (33.2) | 901 (29.6) | <0.001 |
| Regions, No. (%) | <0.001 | ||||
| Central | 1373 (45.1) | 1189 (39.1) | 1249 (41.0) | 1771 (58.2) | |
| North | 870 (28.6) | 655 (21.5) | 545 (17.9) | 414 (13.6) | |
| South | 801 (26.3) | 1200 (39.4) | 1250 (41.1) | 860 (28.2) | |
| Occupation, No. (%) | <0.001 | ||||
| Farmer | 702 (23.3) | 1105 (36.7) | 1192 (39.6) | 1349 (44.8) | |
| Worker | 315 (10.4) | 386 (12.8) | 373 (12.4) | 383 (12.7) | |
| Retire | 1069 (35.4) | 753 (25.0) | 644 (21.4) | 571 (19.0) | |
| Other | 932 (30.9) | 764 (25.4) | 803 (26.7) | 706 (23.5) | |
| Education, No. (%) | <0.001 | ||||
| Illiteracy | 640 (21.4) | 559 (18.8) | 501 (16.7) | 505 (16.9) | |
| Primary school | 499 (16.7) | 600 (20.2) | 638 (21.3) | 590 (19.8) | |
| Middle school | 859 (28.7) | 973 (32.7) | 1067 (35.6) | 1092 (36.6) | |
| High school or above | 995 (33.2) | 840 (28.3) | 793 (26.4) | 793 (26.6) | |
| Dietary intake | |||||
| Thiamine, mg/day | 0.6 ± 0.1 | 0.8 ± 0.0 | 1.0 ± 0.1 | 1.4 ± 0.3 | <0.001 |
| Energy, Kcal/day | 1684.1 ± 374.9 | 2082.6 ± 330.1 | 2315.9 ± 354.1 | 2629.7 ± 468.7 | <0.001 |
| Fat, g/day | 65.0 ± 26.9 | 72.5 ± 25.7 | 78.8 ± 27.6 | 80.9 ± 34.3 | <0.001 |
| Protein, g/day | 50.7 ± 13.7 | 62.7 ± 12.6 | 70.6 ± 13.0 | 83.3 ± 18.1 | <0.001 |
| Carbohydrate, g/day | 224.0 ± 70.9 | 294.8 ± 67.3 | 331.0 ± 72.9 | 392.1 ± 99.6 | <0.001 |
| Sodium, g/day | 4.8 ± 3.1 | 4.8 ± 2.8 | 5.1 ± 3.0 | 5.4 ± 3.1 | <0.001 |
| Potassium, g/day | 1.3 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.5 | 2.1 ± 0.7 | <0.001 |
| Thiamine Intake, mg/day | Crude Model | Thiamine Intake, mg/day | Adjusted Model * | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| <0.89 | 0.57 (0.50,0.64) | <0.001 | <0.93 | 0.62 (0.53,0.72) | <0.001 |
| ≥0.89 | 1.31 (1.27,1.36) | <0.001 | ≥0.93 | 1.38 (1.32,1.44) | <0.001 |
| Thiamine Intake, mg/day | N | Cases (Incidence Rate †) | Crude Model | Adjusted Model * | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Quartiles | ||||||
| Q1 (<0.76) | 3044 | 977 (53.1) | ref | ref | ||
| Q2 (0.76–<0.93) | 3044 | 981 (38.3) | 0.70 (0.64, 0.77) | <0.001 | 0.82 (0.74, 0.90) | <0.001 |
| Q3 (0.93–<1.13) | 3044 | 1004 (36.8) | 0.67 (0.62, 0.74) | <0.001 | 0.78 (0.70, 0.87) | <0.001 |
| Q4 (≥1.13) | 3045 | 1307 (54.9) | 1.02 (0.94, 1.10) | 0.702 | 1.08 (0.95, 1.22) | 0.229 |
| Categories | ||||||
| Q1 (<0.76) | 3044 | 977 (53.1) | 1.45 (1.35, 1.57) | <0.001 | 1.25 (1.14, 1.37) | <0.001 |
| Q2–3 (0.76–<1.13) | 6088 | 1985 (37.5) | ref | ref | ||
| Q4 (≥1.13) | 3045 | 1307 (54.9) | 1.48 (1.38, 1.58) | <0.001 | 1.36 (1.25, 1.47) | <0.001 |
| Thiamine Intake, mg/day | N | Cases (Incidence Rate †) | Crude Model | Adjusted Model * | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Physician-diagnosed hypertension | ||||||
| Quartiles | ||||||
| Q1 (<0.76) | 3024 | 200 (10.9) | ref | ref | ||
| Q2 (0.76–<0.93) | 3024 | 201 (7.9) | 0.73 (0.60, 0.89) | 0.002 | 0.98 (0.78, 1.22) | 0.852 |
| Q3 (0.93–<1.13) | 3024 | 187 (6.9) | 0.64 (0.52, 0.78) | <0.001 | 0.95 (0.74, 1.21) | 0.669 |
| Q4 (≥1.13) | 3024 | 238 (10.1) | 0.93 (0.77, 1.12) | 0.459 | 1.30 (0.98, 1.71) | 0.067 |
| Categories | ||||||
| Q1 (<0.76) | 3024 | 200 (10.9) | 1.47 (1.24, 174) | <0.001 | 1.03 (0.84, 1.27) | 0.751 |
| Q2–3 (0.76–<1.13) | 6048 | 388 (7.4) | ref | ref | ||
| Q4 (≥1.13) | 3024 | 238 (10.1) | 1.37 (1.16, 1.61) | <0.001 | 1.35 (1.11, 1.63) | 0.002 |
| Antihypertensive treatment during follow-up | ||||||
| Quartiles | ||||||
| Q1 (<0.76) | 3026 | 132 (7.2) | ref | ref | ||
| Q2 (0.76–<0.93) | 3025 | 123 (4.8) | 0.69 (0.54, 0.88) | 0.003 | 1.02 (0.77, 1.35) | 0.879 |
| Q3 (0.93–<1.13) | 3025 | 116 (4.3) | 0.61 (0.47, 0.78) | <0.001 | 1.07 (0.79, 1.44) | 0.685 |
| Q4 (≥1.13) | 3026 | 155 (6.5) | 0.93 (0.74, 1.18) | 0.564 | 1.56 (1.11, 2.19) | 0.011 |
| Categories | ||||||
| Q1 (<0.76) | 3026 | 132 (7.2) | 1.55 (1.25, 1.92) | <0.001 | 0.96 (0.74, 1.25) | 0.774 |
| Q2–3 (0.76–<1.13) | 6050 | 239 (4.5) | ref | ref | ||
| Q4 (≥1.13) | 3026 | 155 (6.5) | 1.44 (1.18, 1.77) | <0.001 | 1.49 (1.17, 1.89) | 0.001 |
| New-onset SBP ≥140 mmHg or DBP ≥90 mmHg | ||||||
| Quartiles | ||||||
| Q1 (<0.76) | 3044 | 886 (48.1) | ref | ref | ||
| Q2 (0.76–<0.93) | 3044 | 895 (34.9) | 0.71 (0.64, 0.78) | <0.001 | 0.81 (0.73, 0.90) | <0.001 |
| Q3 (0.93–<1.13) | 3044 | 931 (34.1) | 0.69 (0.63, 0.76) | <0.001 | 0.79 (0.70, 0.88) | <0.001 |
| Q4 (≥1.13) | 3045 | 1211 (50.9) | 1.04 (0.95, 1.13) | 0.411 | 1.09 (0.96, 1.24) | 0.201 |
| Categories | ||||||
| Q1 (<0.76) | 3044 | 886 (48.1) | 1.44 (1.32, 1.56) | <0.001 | 1.25 (1.13, 1.37) | <0.001 |
| Q2–3 (0.76–<1.13) | 6088 | 1826 (34.5) | ref | ref | ||
| Q4 (≥1.13) | 3045 | 1211 (50.9) | 1.49 (1.38, 1.60) | <0.001 | 1.36 (1.25, 1.49) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y.; Yang, S.; Ye, Z.; Wu, Q.; Liu, M.; Zhou, C.; He, P.; Jiang, J.; Liang, M.; et al. U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension. Nutrients 2022, 14, 3251. https://doi.org/10.3390/nu14163251
Zhang Y, Zhang Y, Yang S, Ye Z, Wu Q, Liu M, Zhou C, He P, Jiang J, Liang M, et al. U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension. Nutrients. 2022; 14(16):3251. https://doi.org/10.3390/nu14163251
Chicago/Turabian StyleZhang, Yuanyuan, Yanjun Zhang, Sisi Yang, Ziliang Ye, Qimeng Wu, Mengyi Liu, Chun Zhou, Panpan He, Jianping Jiang, Min Liang, and et al. 2022. "U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension" Nutrients 14, no. 16: 3251. https://doi.org/10.3390/nu14163251
APA StyleZhang, Y., Zhang, Y., Yang, S., Ye, Z., Wu, Q., Liu, M., Zhou, C., He, P., Jiang, J., Liang, M., Wang, G., Hou, F., Liu, C., & Qin, X. (2022). U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension. Nutrients, 14(16), 3251. https://doi.org/10.3390/nu14163251





