Abstract
Nonalcoholic steatohepatitis (NASH) is a subtype of nonalcoholic fatty liver disease (NAFLD). Either Lycium barbarum polysaccharide (LBP) or aerobic exercise (AE) has been reported to be beneficial to hepatic lipid metabolism. However, whether the combination of LBP with AE improves lipid accumulation of NASH remains unknown. Our study investigated the influence of 10 weeks of treatment of LBP, AE, and the combination (LBP plus AE) on high-fat-induced NASH in Sprague–Dawley rats. The results showed that LBP or AE reduced the severity of the NASH. LBP plus AE treatment more effectively ameliorated liver damage and lowered levels of serum lipid and inflammation. In addition, the combination can also regulate genes involved in hepatic fatty acid synthesis and oxidation. LBP plus AE activated AMPK, thereby increasing the expression of PPARα which controls hepatic fatty acid oxidation and its coactivator PGC-1α. Our study demonstrated the improvement of LBP plus AE on NASH via enhancing fatty acid oxidation (FAO) which was dependent on AMPK/PPARα/PGC-1α pathway.
1. Introduction
At present, nonalcoholic fatty liver disease (NAFLD) has been one of the most common causes of chronic liver diseases associated with type 2 diabetes as well as metabolic syndrome. The disease spectrum of NAFLD involves a spectrum of conditions ranging from nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC). Nowadays, as a more aggressive form of the disease, NASH can lead to higher risk of cirrhosis, HCC, and the increased liver-related mortality in individuals with NAFLD [1]. It has been reported that NASH has been nowadays recognized as a serious health issue, affecting 3–5% of the world’s population [2,3,4]. It is estimated that 6.65 million American adults had NASH in 2017 and $222.6 billion would be spent by those people over their life span [5]. Based on a meta-analysis of paired-biopsy studies, liver fibrosis progresses more rapidly in patients with NASH than nonalcoholic fatty liver [6]. In addition, NASH is found to be the fastest-increasing indication for liver transplant in the United States in 2019 [7]. Lipid acquisition exceeds disposal, resulting in lipid deposition in the liver. There are two ways of intrahepatic lipid disposal: oxidation (in the mitochondria, peroxisomes, and cytochromes) and export of lipids in very low-density lipoproteins [8]. Basal hepatic fat oxidation was found to be reduced in overweight patients with NAFLD, indicating that enhancement of fat oxidation may be a therapeutic strategy for NAFLD [9].
As a heteropolysaccharide, Lycium barbarum polysaccharide (LBP), one of the main active components of Lycium barbarum, exhibits a range of effects of promoting health, such as lowering blood glucose [10], lowering blood lipids [10], regulating immunological activities [11], reducing oxidative stress [12] and so on. There have been some studies demonstrating that LBP is beneficial to the liver. LBP markedly decreases the accumulation of triglyceride in the liver by deacetylation of SIRT1 [13]. Using a methionine-choline deficient mouse as a NASH model, LBP was found to have hepatoprotective effects by suppressing NLRP3/6 pathway and NF-κB activation [14]. Moreover, oxidative stress, lipid accumulation, and inflammatory response in the liver were reported to be improved by LBP based on the NASH rats and cellular steatosis model [15].
Lifestyle changes (balanced diet and proper exercise) are proposed to be the primary intervention of NASH [16], among which the importance of exercise is self-evident. Based on a randomized controlled trial of 47 diabetic obese people with NAFLD, Walid Kamal Abdelbasset et al. found that intrahepatic triglycerides and visceral lipids were reduced in people doing high-intensity interval or moderate-intensity continuous aerobic exercise (AE) [17]. In addition, patients with NAFLD improved their intrahepatic triglycerides and decreased body mass index after AE training [18]. As LBP or aerobic exercise possess these protective roles against NAFLD, respectively, whether the combination of LBP and AE could play a further synergetic role in preventing NASH needs further investigation.
The AMP-activated protein kinase (AMPK), a serine/threonine protein kinase, was demonstrated to play an important part in hepatocyte fatty acid metabolism [19]. By murine and simian models, activation of AMPK was demonstrated to ameliorate the progression of NASH [20]. As ligand-activated transcription factors, peroxisome proliferator-activated receptors (PPARs) regulate genes which play vital roles in cell differentiation and various metabolic processes [21]. One of the isoforms is PPARα, which is mainly expressed in fatty tissues [22]. Hepatic PPARα was reported to be required for protection in steatohepatitis [23]. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) performs a critical regulation in the expression of related genes involved in lipid metabolism [24]. It is reported that PGC-1α and PPARα can cooperate to regulate the expression of genes involved in mitochondrial fatty acid oxidation [25].
In this study, we aim to explore the effects of LBP, AE, and LBP plus AE on nonalcoholic steatohepatitis in high-fat-induced Sprague–Dawley rats and the underlying mechanism. Our results indicate that all three treatments had benefits and LBP plus AE showed the greatest benefit. LBP plus AE alleviated fat accumulation in rats with NASH by AMPK/PPARα/PGC-1α pathway.
2. Materials and Methods
2.1. Experimental Protocol
Eight-week-old male Sprague–Dawley rats (180–220 g) were obtained from the Experimental Animal Center of Ningxia Medical University (license number: SCXK Ning. 2015-0001) and maintained on a 12-h light/12-h dark cycle. All rats had free access to food and drink. Rats were randomly distributed into two groups: the normal-fat diet group (NFD) consuming the normal diet (Beijing Keao Xieli Feeds Co., Ltd., Beijing, China, n = 12), and the high-fat fed diet group (HFD) (60 kcal% from fat, MD12033, Medicience Ltd., Jiangsu, China, n = 33). Twenty-eight weeks later, three NFD and HFD rats were sacrificed, and their liver tissues were histopathologically sectioned to check if NASH models were successfully established according to Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases [26]. Nonalcoholic fatty liver activity score (NAS) was used for evaluating liver damage. Then rats in HFD group were randomly assigned into four groups: HFD (high-fat diet) (n = 8), HFD plus LBP (high-fat diet with LBP supplement, 100 mg/kg) (n = 7), HFD plus AE (high-fat diet and aerobic exercise) (n = 8), HFD plus LBP plus AE (high-fat diet with supplementation of LBP combined with aerobic exercise) (n = 7) for 10 weeks (Figure 1).
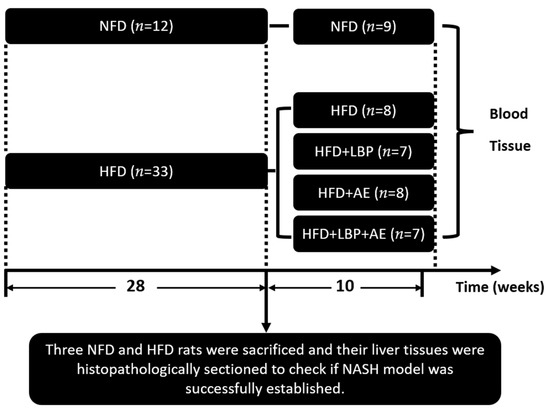
Figure 1.
Experimental design. NFD = normal-fed diet, HFD = high-fat fed diet, LBP = Lycium barbarum polysaccharide, AE = aerobic exercise.
2.2. Aerobic Exercise and the Source of LBP
The HFD plus AE and HFD plus LBP plus AE groups were adapted to a treadmill (Panlab/Harvard apparatus small animal treadmills, LE8710RTS, RWD Life science Co., Ltd., Shenzhen, China) for one week (speed from 5 m/min to 20 m/min, time from 10 min/day to 50 min/day). From the second week, rats did aerobic exercise at the speed of 20 m/min, 60 min/day, 5 days/week. LBP (B20460) was from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). The characterization of LBP was performed in previous study [27].
2.3. Oil Red O Staining
Collected liver tissues stored in liquid nitrogen were embedded in optimal cutting temperature compound. Sections (4 μm thick) were stained with oil red (Nanjing Jiancheng Institute of Bio Engineering, Inc., Nanjing, China) and visualized under the microscope to determine the degree of fat accumulation.
2.4. Transmission Electron Microscopy (TEM)
Collected liver tissues were cut into no more than 1 mm3. Then they were immediately placed in 2.5% glutaraldehyde after washing in pre-cooled saline and fixed in the refrigerator at 4 °C for 4 h. The sections were fixed with osmium acid after washing with PBS three times. The washing steps were repeated, followed by ethanol gradient dehydration and epoxy resin embedding. The ultrastructure of the liver tissues was observed by TEM (HT7800, Hitachi, Tokyo, Japan) after special staining.
2.5. Biochemical Analyses and Enzyme-Linked Immunosorbent Assay (ELISA)
Blood samples were collected from the abdominal aorta. According to the manufacturer’s instructions, aspartate aminotransferase (AST) serum alanine aminotransferase (ALT), triglycerides (TG), and total cholesterol (TC) were measured by assay kits (Nanjing Jiancheng Institute of Bio Engineering, Inc., Nanjing, China). The levels of monocyte chemotactic protein-1 (MCP-1), Interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were determined by the commercial ELISA kits (Elabscience Biotechnology Co., Ltd., Wuhan, China).
2.6. Total RNA Isolation and Real-Time Polymerase Chain Reaction (qRT-PCR)
The messenger RNA (mRNA) expressions of toll-like receptor 4 (TLR4), mitogen-activated protein kinases 38 (p38 MAPK), and nuclear transcription factor kappa B1 (NF-ΚB 1) were determined by qRT-PCR. Total RNA extraction was prepared from liver tissue by Trizol reagent (Ambion, Austin, DX, USA) and the cDNAs were generated by reverse transcription kit (Takara, Kusatsu, Japan). A qRT-PCR was performed using a standard protocol in the real-time PCR system (Bio-Rad, Hercules, CA, USA). The primer sequences for qPCR are listed in Table 1. The expression levels of target genes were normalized to β-actin mRNA.

Table 1.
Primers of qRT-PCR.
2.7. Western Blot
Three samples of mice in each group were used, from which 100 mg of liver tissue was homogenized using lysis buffer for total protein extraction and the concentration of protein was assessed by BCA protein assay kit. After being separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), protein samples were transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% (w/v) skim milk for 1.5 h and then were incubated with the primary antibodies overnight at 4 °C. TLR4 (1:1000, Cell Signaling Technology, Danvers, MA, USA) and AMPK (1:1000, Cell Signaling Technology, USA), p-AMPK (1:1000, Cell Signaling Technology, USA), PPARα (1:1000, Santa Cruz Biotechnology, Dallas, DX, USA), PGC-1α (1:1000, Abcam, Cambridge, UK), and β-actin (1:5000, Abcam, UK). After 3 × 10 min wash in PBST, the secondary antibody was added and incubated for 1 h. A chemiluminescence detection was responsible for protein chemiluminescence and a bioanalytical imaging system for signal capture. Western blot was repeated three times.
2.8. Statistical Analysis
Using SPSS23.0 software (Chicago, IL, USA), the results were analyzed by one-way analysis of variance (ANOVA) among multiple groups and least significant difference (LSD) test for further pairwise comparison. Data are presented as mean ± standard deviation (SD) and considered significantly different as values of p < 0.05.
3. Results
3.1. Supplementation of LBP with AE Alleviated Hepatocyte Injury in NASH Rats
Compared with the NFD group, HFD successfully induced severe NASH (Figure 2C). Nonalcoholic fatty liver activity score (NAS) was reduced in each treatment group to varying degrees, with the most significant reduction in HFD plus LBP plus AE group (Figure 2C). As visualized in Oil Red O staining, there were a large number of lipid vacuoles in the cytoplasm of hepatocytes in the model group (Figure 2A). Different treatments obviously reduced the degree of lipid deposition in the hepatocyte. In addition, compared with the NFD group, we found more lipid droplets, karyopyknosis, swollen mitochondria, dilation of endoplasmic reticulum, and reduction of rough endoplasmic reticulum in the HFD group (Figure 2B). However, the morphological structures of the nucleus and mitochondria were improved by all treatments. Thirty-eight weeks of high-fat diet led to an increase in ALT and AST levels by 70.23% and 23.01% compared with the NFD group, however, LBP, AE, and combination therapy groups obviously decreased the levels (p < 0.05). LBP plus AE was the most significant way to reduce the level of ALT statistically and AST in trend (Figure 2D,E). As NASH is reported to be an inflammatory subtype of NAFLD [2], the levels of liver inflammatory factors in HFD group were higher than that in NFD group. (Figure 2F–H). The levels of TLR4 and NF-κB1 were reduced after 10 weeks of AE with or without LBP supplementation (Figure 2F,H). Notably, AE combined with LBP was the most effective way to reduce the mRNA expression of TLR4 and p38 MAPK (Figure 2F,G).
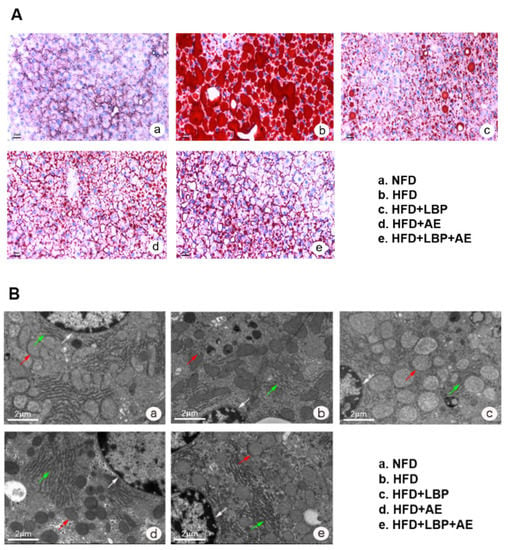
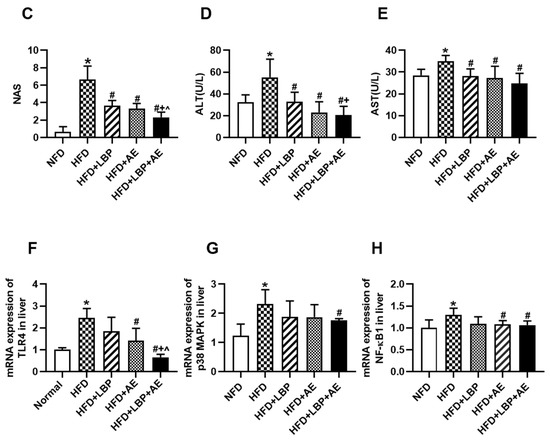
Figure 2.
LBP plus AE ameliorated the hepatic injury. (A) Oil red O staining of the liver tissue was photographed at 200× magnification. (B) Transmission electron microscopy of the liver histology was photographed at 6000× magnification. White arrow: nucleus. Red arrow: mitochondrion. Green arrow: rough endoplasmic reticulum. (C) Hepatic NAS in different groups. (D) Serum levels of ALT. (E) Serum levels of AST. (F) mRNA expression of TLR4 in liver. (G) mRNA expression of p38 MAPK in liver. (H) mRNA expression of NF-kB1 in liver. * p < 0.05 vs. the NFD group. # p < 0.05 vs. the HFD group. + p < 0.05 vs. the HFD plus LBP group. ^ p < 0.05 vs. the HFD plus AE group.
3.2. Supplementation of LBP with AE Reduced Body Weight, Serum Lipid and Inflammation Levels
Using a rat model of NASH, we noticed that HFD feeding for 10 weeks led to obvious increases in body weight compared with standard diet feeding (Figure 3A,B). In terms of final body weight, the HFD group increased by 30.04% compared with the NFD group. In addition, the body weight of the AE group and the LBP plus AE group decreased by 9.72% and 11.25% compared with the model group, respectively (Figure 3B). AE and LBP plus AE reduced TG level by 39.4% and 43.2%, respectively, relative to the HFD group (Figure 3C). All treatments had the capacity to lower the level of TC relative to the HFD group (Figure 3D). Then the effects of LBP, AE, and both on systemic inflammation was examined. Obviously, relative to the NFD group, higher values for inflammatory factors can be found in the HFD group. Compared with the HFD group, all treatments have reduced the level of MCP-1 by 33.6%, 40.6%, and 60.9% (Figure 3E). As shown in Figure 3F, the level of TNF-α of rats significantly decreased by supplementation of LBP (p < 0.05) and the combination therapy (p < 0.01) compared with the HFD group. In addition, all treatments can lower the level of IL-6 in comparison with the HFD group (p < 0.01) and LBP plus AE was more effective than AE alone (Figure 3G).
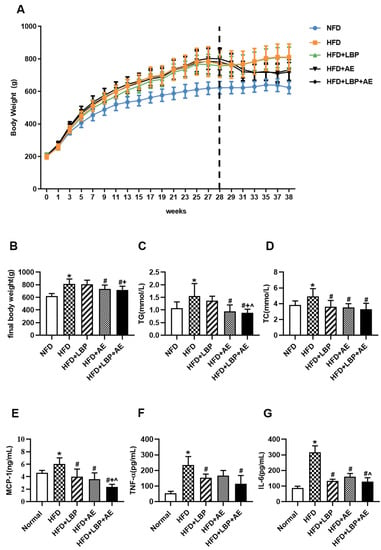
Figure 3.
LBP plus AE reduced body weight, serum lipid as well as inflammation levels. (A) Body weight throughout the 38-week period. (B) Final body weight. (C) Serum levels of TG. (D) Serum levels of TC. (E) Serum levels of MCP-1. (F) Serum levels of TNF-α. (G) Serum levels of IL-6. * p < 0.05 vs. the NFD group. # p < 0.05 vs. the HFD group. + p < 0.05 vs. the HFD plus LBP group. ^ p < 0.05 vs. the HFD plus AE group.
3.3. Supplementation of LBP with AE Regulated the Expression of Genes Involved in Lipid Metabolism
Acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN) and sterol regulatory element-binding protein 1c (SREBP1c) are genes related to de novo lipogenesis (DNL). The expression levels of the three genes were obviously higher in the HFD group compared with the NFD group, suggesting that lipid synthesis in the liver was indeed increased in the presence of NASH, thereby causing fat accumulation. Compared with the HFD group, different treatments reduced the expression levels of the three genes to varying degrees. There were no differences in the expression levels of ACC and FASN between single treatment and combined treatment (Figure 4A,B). LBP and AE tended to decrease SREBP1c expression levels, but only LBP plus AE made a statistical decrease (Figure 4C). As a gene strongly associated with lipid metabolism [24], hepatic expression of PGC-1α was higher through different treatment groups in comparison with the HFD group and the combination therapy group had the most significant effect (Figure 4D). We then examined the expression of two genes of fatty acid oxidation (FAO). It turned out that treatments effectively prevented the decrease of PPARα and carnitine palmitoyltransferase-1A (CPT-1A) (Figure 4D,F).
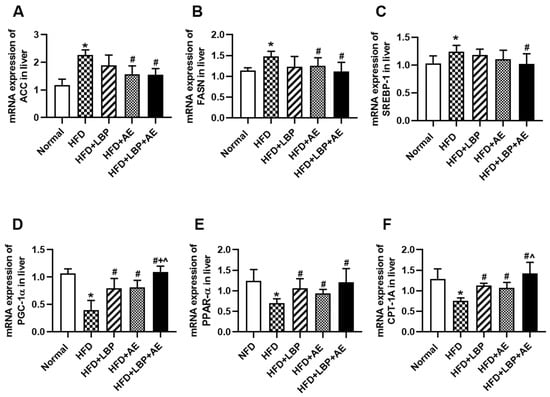
Figure 4.
LBP plus AE affected hepatic fatty acid synthesis and oxidation. (A) mRNA expression of ACC in liver. (B) mRNA expression of FASN in liver. (C) mRNA expression of SREBP1c in liver. (D) mRNA expression of PGC-1α in liver. (E) mRNA expression of PPARα in liver. (F) mRNA expression of CPT-1A in liver. * p < 0.05 vs. the NFD group. # p < 0.05 vs. the HFD group. + p < 0.05 vs. the HFD plus LBP group. ^ p < 0.05 vs. the HFD plus AE group.
3.4. Supplementation of LBP with AE Alleviated NASH via AMPK/PPARα/PGC-1α Pathway
To further explore the effect of supplementation of LBP with AE on the AMPK/PPARα/PGC-1α pathway in NASH, proteins were extracted from the liver tissue and the expressions of AMPK, PPARα, and PGC-1α were determined (Figure 5A). HFD decreased the expression of PGC-1α (Figure 5B). Compared with the HFD group, either LBP or AE e treatment did not cause significant changes, while LBP plus AE upregulated PGC-1α at a significant level of p < 0.05. Rats in the AE and LBP plus AE group exerted a higher expression of p-AMPK (Figure 5C). In addition, all treatments upregulated the levels of PPARα, and LBP plus AE was more effective than AE alone (Figure 5D).
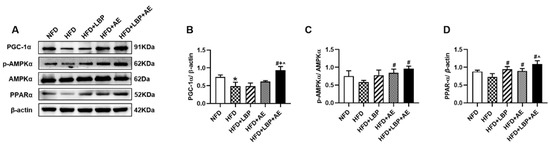
Figure 5.
LBP plus AE activated AMPK/PPARα/PGC-1α pathway to ameliorate NASH. (A–D) Western blot images and quantitative band density analyses in different groups. * p < 0.05 vs. the NFD group. # p < 0.05 vs. the HFD group. + p < 0.05 vs. the HFD plus LBP group. ^ p < 0.05 vs. the HFD plus AE group. Three samples of mice in each group were used and western blot was repeated three times.
4. Discussion
This research determined the protective effect of supplementation of LBP, AE alone, and the combination against NASH, and further revealed that the combination therapy was more effective in ameliorating NASH by inhibiting DNL and activating AMPK/PPARα/PGC-1α pathway (Figure 6).
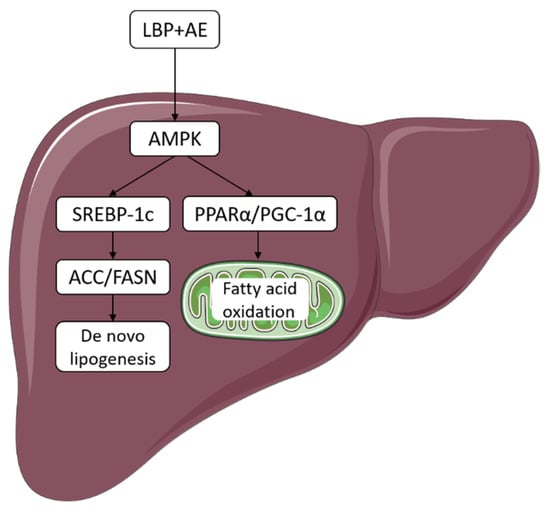
Figure 6.
LBP plus AE improved NASH through AMPK activation and AMPK/PPARα/PGC-1α pathway. LBP plus AE activated AMPK to ameliorate NASH in two ways. On the one hand, the level of ACC and FASN decreased after SREBP-1c downregulation, thus inhibiting DNL. On the other hand, PGC-1α and PPARα can cooperate to promote FAO.
NASH, the inflammatory subtype of NAFLD, is considered a major health issue with significant economic impact. Unfortunately, there are no pharmaceuticals for NASH that the FDA has approved in current times [28]. Vitamin E as well as pioglitazone have proved effective against NASH in randomized controlled trials [29], yet the two drugs are limited because of side effects [30,31]. Although some other drugs are in phase Ⅱ and Ⅲ trials, efficacy, safety, and other aspects still need further research [32]. Therefore, developing other new promising therapeutic strategies is still needed at present. LBP, one of the most functional components of Lycium barbarum, has been reported to exert benefits on NASH. Our observations that LBP produced obvious improvement in hepatic steatosis and inflammation are consistent with the results of other studies [13,14,15]. Previous studies have highlighted the positive effect of AE on people with dyslipidemia [33], which is one of the features of NASH. Our results showed that AE not only lowered TG and TC, but also improved liver function and inflammation. Then, we began to explore the synergetic effect of LBP and AE on NASH. Notably, the combined effect was better than the single effect. In addition, we also found the weight of mice in the HFD group drops in the late stage, which is consistent with the animal experiment by Eun Soo Lee et al. [34]. As for the reason, we analyze that on the one hand, it may be the abnormal liver fat metabolism in the late stage of NASH, resulting in the disorder of animal lipid metabolism, which then causes the occurrence of this phenomenon. On the other hand, changes in food intake may lead to changes in body weight, which has also appeared in our other work [35].
DNL is one method of lipid acquisition for the liver. At first, ACC catalyzes the formation of malonyl-CoA from acetyl-CoA. Then, malonyl-CoA is converted to palmitic acid by FASN. After desaturation, elongation, esterification, and other steps, triglyceride is finally formed. By isotope analysis, subjects with NAFLD are found to have high levels of DNL [36]. In an open-label study, individuals with NASH were given ACC inhibitor GS-0976 and their hepatic DNL as well as markers of liver damage were reduced after 12 weeks [37]. In another 12-week trial, 3 mg FASN inhibitor FT-4101 has been shown to reduce hepatic DNL and steatosis [38]. In our study, we found that expression of ACC and FASN declined after AE and LBP plus AE treatments. In addition, LBP plus AE was more effective than AE in trend. SREBP1c, a key transcription factor in the regulation of DNL, was reported to be an effective target to treat NAFLD after its downregulation [39]. As an upstream of SREBP1c, AMPK is able to phosphorylate SREBP-1c, which inhibits the transcription of the factor and improves hepatic steatosis [40]. Berberine and Antrodan have been demonstrated to be effective on NAFLD through AMPK-SREBP-1c pathway [41,42]. LBP has also been shown to regulate DNL by AMPK/SREBP-1c pathway [43], then we began to investigate if LBP combined with AE would also work through the pathway. Our study showed that LBP and AE alone tended to reduce the expression of SREBP-1c level in the liver with no statistical significance, while LBP plus AE significantly lowered the hepatic expression of SREBP-1c (p < 0.05). Therefore, LBP plus AE can activate AMPK, thereby reducing the expression of hepatic SREBP-1c levels and inhibiting DNL, thereby ameliorating NASH.
FAO is one of the ways to dispose of lipid in the liver. Hepatic FAO mainly occurs in the mitochondria and acyl-CoA relies on CPT-1A, a rate-limiting enzyme in the outer mitochondrial membrane, to enter mitochondria [44]. The previous study has shown that FAO is reduced in patients with NAFLD [9]. Therefore, augmented FAO appears to be a treatment for NAFLD. Statins prevented the progression of MCDD-induced NASH in a C57BL/6J mice model by increasing mitochondrial and peroxisomal FAO [45], meanwhile TXNIP/VDUP1 was also effective against steatohepatitis via FAO [46]. PPARα is considered as a major regulator of hepatic fatty acid metabolism [8,47]. Activating PPARα has been proved to have a therapeutic effect on the NASH not only in the animal model [48], but also in clinical trials [49]. PGC-1 family members are recognized as multifunctional transcriptional coregulators in metabolic pathways [50]. As PPARα and PGC-1α can interact to regulate FAO in mitochondrion, which plays an important role in hepatic lipid disposal [25], a recent study has found that Nuciferine was beneficial to hepatic steatosis by activating the PPARα/PGC1α pathway [51]. Being an upstream of PGC-1α [52], AMPK acts as a key regulator in homeostasis by regulating related enzymes. Cordycepin is able to improve NASH by activating AMPK [53]. The AMP-activated protein kinase (AMPK) and Sirtuin 1 (SIRT1) are partners which have similar functions in metabolism and energy balance, and both can regulate PGC-1α [54]. As LBP [13] and exercise [55] have been reported to influence SIRT1, related work about the combination therapy and SIRT1 is underway. Since AE has been shown to regulate NAFLD by AMPK-PPAR-α pathway [56] and LBP can prominently increase the expression of PGC-1α [43], we wondered if LBP combined with AE would also work through the pathway. Then we found that LBP plus AE promoted FAO via AMPK/PPARα/PGC-1α pathway.
Rather than an independent disease, NAFLD is closely related to metabolic disorders such as obesity and insulin resistance [57]. Due to the inaccurate nomenclature of NAFLD, a group of experts has suggested replacing the name with metabolic (disfunction) associated fatty liver disease (MAFLD), more accurately reflecting pathogenesis and better helping the development of treatment as well as clinical trial design [58]. Thus, metabolic health has become a key point in the therapy of NAFLD/NASH. LBP has been reported to be effective against insulin resistance by C57BL/6J mice consuming a high-fat diet as well as HepG2 cells [59], and also against obesity symptoms by altering the composition of intestinal flora as well as promoting the metabolism of SCFAs [60]. Similarly, aerobic exercise has also been demonstrated to improve metabolic abnormalities [61,62]. We previously showed that LBP plus AE reduced body weight and improved insulin resistance [27], thereby LBP plus AE is a good choice for the therapy of NAFLD/NASH.
5. Conclusions
In summary, our study identified effective effects of supplementation of LBP combined with AE on NASH induced by a high-fat diet in a Sprague–Dawley rat model. LBP plus AE not only promoted DNL by activating AMPK to affect the expression of related enzymes, but also enhanced FAO by AMPK/PPARα/PGC-1α pathway.
Author Contributions
J.-J.Y. conceived and designed this research. D.-D.L. and J.-M.M. conducted the experiments and prepared the manuscript. M.-J.L., L.-L.G. and Y.-N.F. conducted the animal experiments. Y.-N.Z. and X.-J.T. were responsible for data analyses. J.-J.Y. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number: 82060597) and key research project of Ningxia (grant number: 2021BEG03031).
Institutional Review Board Statement
The study was conducted according to the guidelines for animal research of Ningxia Medical University (China) and was approved by the Ethics Committee on Animal Research of Ningxia Medical University (Permit Number: 2015-127).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povsic, M.; Wong, O.Y.; Perry, R.; Bottomley, J. A Structured Literature Review of the Epidemiology and Disease Burden of Non-Alcoholic Steatohepatitis (NASH). Adv. Ther. 2019, 36, 1574–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Tampi, R.; Priyadarshini, M.; Nader, F.; Younossi, I.M.; Racila, A. Burden of Illness and Economic Model for Patients With Nonalcoholic Steatohepatitis in the United States. Hepatology 2019, 69, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9; quiz e639–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, I.; Byrne, N.M.; Choquette, S.; Hills, A.P.; Chachay, V.S.; Clouston, A.D.; O’Moore-Sullivan, T.M.; Macdonald, G.A.; Prins, J.B.; Hickman, I.J. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 2013, 62, 1625–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, S.F.; Zhao, J.; Li, S.P. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhao, Q.; Zhu, Q.; He, X.; Gao, M.; Wang, Y. Lycium barbarum polysaccharide protects ARPE19 cells against H2O2 induced oxidative stress via the Nrf2/HO1 pathway. Mol. Med. Rep. 2021, 24, 769. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, W.; Li, J.; Li, Y.; Song, H.; Luan, Y.; Qi, H.; Ma, L.; Lu, X.; Yang, Y. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci. Rep. 2016, 6, 36209. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Liong, E.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides improve hepatic injury through NFkappa-B and NLRP3/6 pathways in a methionine choline deficient diet steatohepatitis mouse model. Int. J. Biol. Macromol. 2018, 120, 1480–1489. [Google Scholar] [CrossRef]
- Xiao, J.; Xing, F.; Huo, J.; Fung, M.L.; Liong, E.C.; Ching, Y.P.; Xu, A.; Chang, R.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci. Rep. 2014, 4, 5587. [Google Scholar] [CrossRef] [Green Version]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Elnegamy, T.E.; Soliman, G.S.; Ibrahim, A.A. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine 2020, 99, e19471. [Google Scholar] [CrossRef] [PubMed]
- Slomko, J.; Zalewska, M.; Niemiro, W.; Kujawski, S.; Slupski, M.; Januszko-Giergielewicz, B.; Zawadka-Kunikowska, M.; Newton, J.; Hodges, L.; Kubica, J.; et al. Evidence-Based Aerobic Exercise Training in Metabolic-Associated Fatty Liver Disease: Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Jian, C.; Fu, J.; Cheng, X.; Shen, L.J.; Ji, Y.X.; Wang, X.; Pan, S.; Tian, H.; Tian, S.; Liao, R.; et al. Low-Dose Sorafenib Acts as a Mitochondrial Uncoupler and Ameliorates Nonalcoholic Steatohepatitis. Cell Metab. 2020, 31, 892–908.e811. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puengel, T.; Liu, H.; Guillot, A.; Heymann, F.; Tacke, F.; Peiseler, M. Nuclear Receptors Linking Metabolism, Inflammation, and Fibrosis in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 2668. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Regnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1alpha as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VEGA, R.B.; HUSS, J.M.; KELLY, D.P. The Coactivator PGC-1 Cooperates with Peroxisome Proliferator-Activated Receptor alpha in Transcriptional Control of Nuclear Genes Encoding Mitochondrial Fatty Acid Oxidation Enzymes. Mol. Cell Biol. 2000, 20, 1868–1876. [Google Scholar] [CrossRef] [Green Version]
- Jian-gao, F. Guidelines for management of nonalcoholic fatty liver disease: An updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 2010, 18, 163–166. [Google Scholar] [PubMed]
- Gao, L.L.; Ma, J.M.; Fan, Y.N.; Zhang, Y.N.; Ge, R.; Tao, X.J.; Zhang, M.W.; Gao, Q.H.; Yang, J.J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef]
- Schurks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ 2010, 341, c5702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Shi, W.; Fu, S.; Wang, T.; Zhai, S.; Song, Y.; Han, J. Pioglitazone and bladder cancer risk: A systematic review and meta-analysis. Cancer Med. 2018, 7, 1070–1080. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.S.; Kwon, M.H.; Kim, H.M.; Woo, H.B.; Ahn, C.M.; Chung, C.H. Curcumin analog CUR5-8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metabolism 2020, 103, 154015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Shuang, L.; Jia, M.; Lu, Y.; Lian, D.; Yan, Z.; Jian, Y. Effecf of Lycium barbarum polysacchirdes combined aerobic exercise on plasma free fatty acid profile in rats with nonalcoholic fatty liver disease and prediabetes. Acta Nutr. Sin. 2022, 44, 64–71. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Lawitz, E.J.; Coste, A.; Poordad, F.; Alkhouri, N.; Loo, N.; McColgan, B.J.; Tarrant, J.M.; Nguyen, T.; Han, L.; Chung, C.; et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1983–1991.e1983. [Google Scholar] [CrossRef] [PubMed]
- Beysen, C.; Schroeder, P.; Wu, E.; Brevard, J.; Ribadeneira, M.; Lu, W.; Dole, K.; O’Reilly, T.; Morrow, L.; Hompesch, M.; et al. Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: Results from two early-phase randomized trials. Diabetes Obes. Metab. 2020, 23, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, R.J.; Peng, S.Y.; Yu, W.C.Y.; Chang, V.H. Therapeutic Targeting of Nonalcoholic Fatty Liver Disease by Downregulating SREBP-1C Expression via AMPK-KLF10 Axis. Front. Mol. Biosci. 2021, 8, 751938. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y.; et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARgamma Pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, Y.; Wang, Q.; Yang, Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. Biomed. Res. Int. 2014, 2014, 196198. [Google Scholar] [CrossRef]
- Donde, H.; Ghare, S.; Joshi-Barve, S.; Zhang, J.; Vadhanam, M.V.; Gobejishvili, L.; Lorkiewicz, P.; Srivastava, S.; McClain, C.J.; Barve, S. Tributyrin Inhibits Ethanol-Induced Epigenetic Repression of CPT-1A and Attenuates Hepatic Steatosis and Injury. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 569–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Jang, J.E.; Ko, M.S.; Woo, S.H.; Kim, B.J.; Kim, H.S.; Park, H.S.; Park, I.S.; Koh, E.H.; Lee, K.U. Statins Increase Mitochondrial and Peroxisomal Fatty Acid Oxidation in the Liver and Prevent Non-Alcoholic Steatohepatitis in Mice. Diabetes Metab. J. 2016, 40, 376–385. [Google Scholar] [CrossRef]
- Park, H.S.; Song, J.W.; Park, J.H.; Lim, B.K.; Moon, O.S.; Son, H.Y.; Lee, J.H.; Gao, B.; Won, Y.S.; Kwon, H.J. TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy 2021, 17, 2549–2564. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Hooiveld, G.; Muller, M.; Kersten, S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS ONE 2009, 4, e6796. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.P.; Caffrey, R.; Marioneaux, J.; Santhekadur, P.K.; Bhat, M.; Alonso, C.; Koduru, S.V.; Philip, B.; Jain, M.R.; Giri, S.R.; et al. The PPAR alpha/gamma Agonist Saroglitazar Improves Insulin Resistance and Steatohepatitis in a Diet Induced Animal Model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 9330. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-alpha/gamma Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.D. PGC-1 coactivators in the control of energy metabolism. Acta Biochim. Biophys. Sin. 2011, 43, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARalpha/PPARgamma coactivator-1alpha pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.; Hertz, R.; Bar-Tana, J. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase. Biochem. J. 2004, 384, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Diniz, T.A.; de Lima Junior, E.A.; Teixeira, A.A.; Biondo, L.A.; da Rocha, L.A.F.; Valadão, I.C.; Silveira, L.S.; Cabral-Santos, C.; de Souza, C.O.; Rosa Neto, J.C. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021, 266, 118868. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus, P. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014, 2014, 145641. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef]
- Da Silva Ferreira, G.; Bochi, A.P.G.; Pinto, P.R.; Del Bianco, V.; Rodrigues, L.G.; Morais, M.; Nakandakare, E.R.; Machado, U.F.; Catanozi, S.; Passarelli, M. Aerobic Exercise Training Prevents Insulin Resistance and Hepatic Lipid Accumulation in LDL Receptor Knockout Mice Chronically Fed a Low-Sodium Diet. Nutrients 2021, 13, 2174. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.; Hjelmesaeth, J.; Hertel, J.K.; Gjevestad, E.; Smastuen, M.C.; Johnson, L.K.; Martins, C.; Andersen, E.; Helgerud, J.; Storen, O. Effect of Aerobic Exercise Intensity on Energy Expenditure and Weight Loss in Severe Obesity-A Randomized Controlled Trial. Obesity 2021, 29, 359–369. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).