Trajectories of Lipid Profile and Risk of Carotid Atherosclerosis Progression: A Longitudinal Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
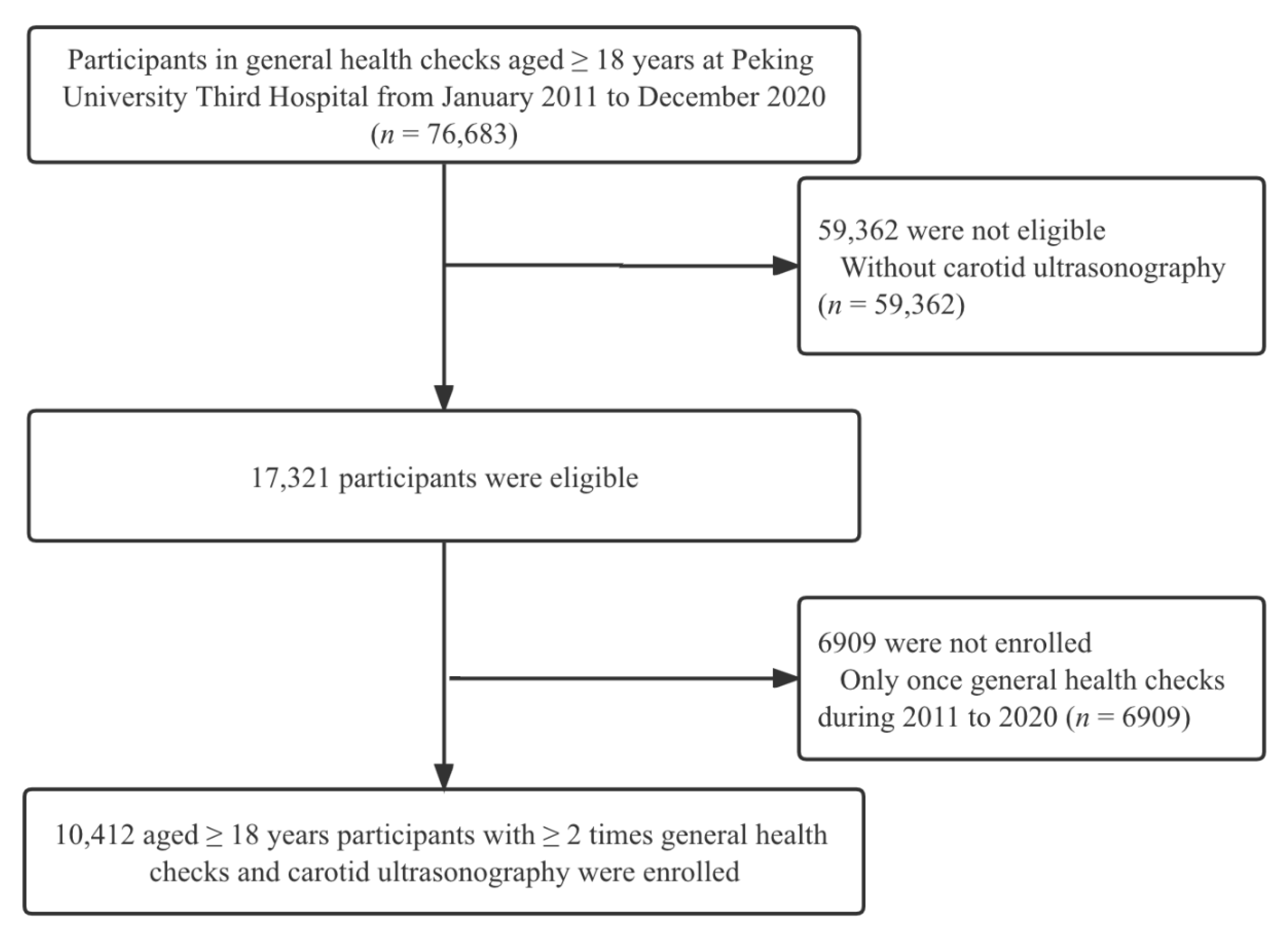
2.1. Study Design and Setting
2.2. Characteristics and Measurement
2.3. Carotid Ultrasonography Examination
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
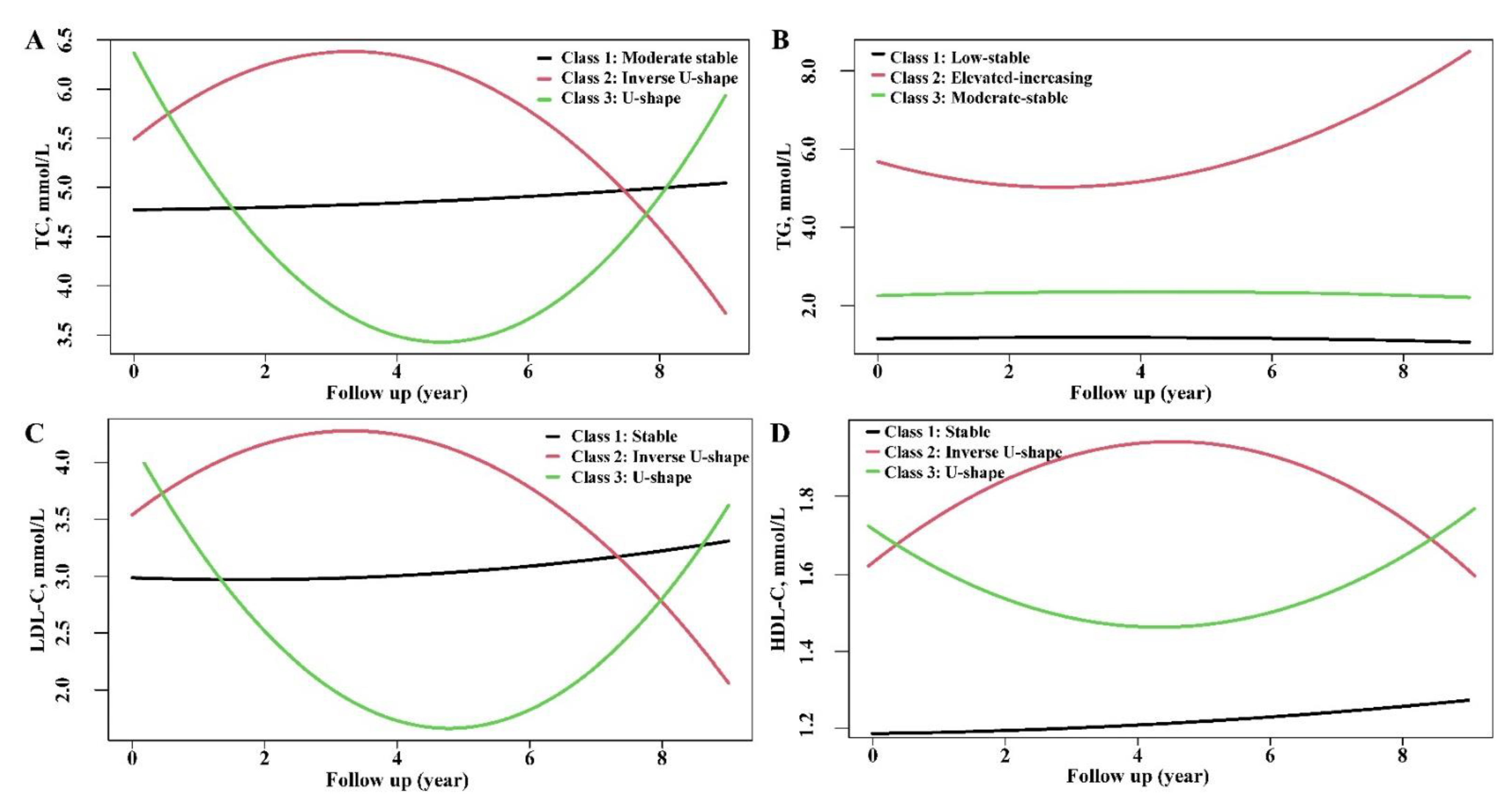
3.2. Characterization of Trajectories of Lipid Profiles
3.3. Association of Lipid Trajectories with CAS Progression
3.4. Sensitivity Analysis
4. Discussion
4.1. Independent and Joint Effects of Baseline and Trajectories of Lipid
4.2. Comparisons with Existing Studies
4.3. Diet and Nutrition as Drivers of Lipid Levels
4.4. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 July 2022).
- National Center for Cardiovascular Diseases. Annual Report on Cardiovascular Health and Diseases in China 2019; Science Press: Beijing, China, 2020. [Google Scholar]
- Daghem, M.; Bing, R.; Fayad, Z.A.; Dweck, M.R. Noninvasive Imaging to Assess Atherosclerotic Plaque Composition and Disease Activity: Coronary and Carotid Applications: Coronary and Carotid Applications. JACC Cardiovasc. Imaging 2020, 13, 1055–1068. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Kissela, B.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Ferioli, S.; Adeoye, O.; Broderick, J.P.; et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013, 40, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, Y.C.; Gonzalez, N.R. Management of Extracranial Carotid Artery Disease. Cardiol. Clin. 2014, 33, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Peters, S.A.; den Ruijter, H.M.; Bots, M.L.; Moons, K.G. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: A systematic review. Heart 2012, 98, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.M.; Nambi, V.; Naqvi, T.Z.; Feinstein, S.B.; Kim, E.S.H.; Park, M.M.; Becher, H.; Sillesen, H. Recommendations for the Assessment of Carotid Arterial Plaque by Ultrasound for the Characterization of Atherosclerosis and Evaluation of Cardiovascular Risk: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, Y.; Jellinger, P.S.; Guerin, C.K.; Bloomgarden, Z.T.; Brinton, E.A.; Budoff, M.J.; Davidson, M.H.; Einhorn, D.; Fazio, S.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Management of Dyslipidemia and Prevention of Cardiovascular Disease Algorithm—2020 Executive Summary. Endocr. Pract. 2020, 26, 1196–1224. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Chamnan, P.; Simmons, R.K.; Sharp, S.J.; Khaw, K.-T.; Wareham, N.J.; Griffin, S.J. Repeat cardiovascular risk assessment after four years: Is there improvement in risk prediction? PLoS ONE 2016, 11, e0147417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayimu, A.; Wang, C.; Li, J.; Fan, B.; Ji, X.; Zhang, T.; Xue, F. Trajectories of Lipids Profile and Incident Cardiovascular Disease Risk: A Longitudinal Cohort Study. J. Am. Heart Assoc. 2019, 8, e013479. [Google Scholar] [CrossRef]
- Koohi, F.; Ahmadi, N.; Hadaegh, F.; Safiee, S.; Azizi, F.; Khalili, D. Trajectories of cardiovascular disease risk and their association with the incidence of cardiovascular events over 18 years of follow-up: The Tehran Lipid and Glucose study. J. Transl. Med. 2021, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; for the STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev. Med. 2007, 45, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Joint Committee for Guideline Revision. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J. Geriatr. Cardiol. 2019, 16, 182–241. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Brunelli, N.; Altamura, C.; Costa, C.M.; Altavilla, R.; Palazzo, P.; Maggio, P.; Marcosano, M.; Vernieri, F. Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness. Int. J. Environ. Res. Public Health 2022, 19, 758. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Fan, J.; Liu, J.; Wang, W.; Wang, M.; Sun, J.; Liu, J.; Xie, W.; Zhao, F.; Li, Y.; et al. Cholesterol-overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: A community-based cohort study. J. Am. Coll. Cardiol. 2015, 65, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, S.S.; Wolters, F.J.; Swanson, S.A.; Koudstaal, P.J.; Hofman, A.; Tiemeier, H.; Ikram, M.A. 10-year trajectories of depressive symptoms and risk of dementia: A population-based study. Lancet Psychiatry 2016, 3, 628–635. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Jacobson, T.A.; Martin, S.S.; Jackson, E.J.; Le, N.A.; Davidson, M.H.; Vesper, H.W.; Frikke-Schmidt, R.; Ballantyne, C.M.; Remaley, A.T. Lipid measurements in the management of cardiovascular diseases: Practical recommendations a scientific statement from the national lipid association writing group. J. Clin. Lipidol. 2021, 15, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Strategies to alter the trajectory of atherosclerotic cardiovascular disease. Curr. Opin. Lipidol. 2019, 30, 438–445. [Google Scholar] [CrossRef]
- Messerli, F.H.; Hofstetter, L.; Rimoldi, S.F.; Rexhaj, E.; Bangalore, S. Risk Factor Variability and Cardiovascular Outcome: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2596–2603. [Google Scholar] [CrossRef]
- Brandts, J.; Ray, K.K. Low Density Lipoprotein Cholesterol-Lowering Strategies and Population Health: Time to Move to a Cumulative Exposure Model. Circulation 2020, 141, 873–876. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Joint Committee for Guideline Revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J. Geriatr. Cardiol. 2018, 15, 1–29. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Koohi, F.; Khalili, D.; Mansournia, M.A.; Hadaegh, F.; Soori, H. Multi-trajectories of lipid indices with incident cardiovascular disease, heart failure, and all-cause mortality: 23 years follow-up of two US cohort studies. J. Transl. Med. 2021, 19, 286. [Google Scholar] [CrossRef]
- Tsai, C.W.; Huang, H.C.; Chiang, H.Y.; Chung, C.W.; Chang, S.N.; Chu, P.L.; Kuo, C.C. Longitudinal lipid trends and adverse outcomes in patients with CKD: A 13-year observational cohort study. J. Lipid Res. 2019, 60, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Na, M.; Lichtenstein, A.H.; Xing, A.; Chen, S.; Wu, S.; Gao, X. Habitual Night Eating Was Positively Associated with Progress of Arterial Stiffness in Chinese Adults. J. Am. Heart Assoc. 2020, 9, e016455. [Google Scholar] [CrossRef]
- Pot, G.K.; Hardy, R.; Stephen, A.M. Irregularity of energy intake at meals: Prospective associations with the metabolic syndrome in adults of the 1946 British birth cohort. Br. J. Nutr. 2016, 115, 315–323. [Google Scholar] [CrossRef] [Green Version]

| Variables | Total (n = 10,412) | Normal (n = 5583) | Carotid Intima-Media Thickness (n = 1373) | Carotid Artery Plaque (n = 3456) | p for Trend |
|---|---|---|---|---|---|
| Age, years | 50 (40–61) | 42 (34–50) | 54 (48–61) | 63 (55–73) | 0.000 |
| Male, n (%) | 5421 (52.1) | 2712 (48.6) | 733 (53.4) | 1976 (57.2) | 0.000 |
| BMI, kg/m2 | 24.42 (22.21–26.71) | 23.87 (23.60–26.21) | 24.85 (22.76–26.95) | 25.07 (23.11–27.23) | 0.000 |
| Hypertension, n (%) | 1584 (15.2) | 343 (6.1) | 214 (15.6) | 1027 (29.7) | 0.000 |
| Diabetes, n (%) | 505 (4.9) | 95 (1.7) | 51 (3.7) | 359 (10.4) | 0.000 |
| SBP, mmHg | 127 (115–140) | 121 (111–132) | 129 (117–140) | 137 (124−149) | 0.000 |
| DBP, mmHg | 77 (69−84) | 74 (67−82) | 79 (71−86) | 80 (72−87) | 0.000 |
| FBG, mmol/L | 5.20 (4.80−5.70) | 5.00 (4.70−5.40) | 5.20 (4.90−5.70) | 5.50 (5.10−6.30) | 0.000 |
| TC, mmol/L | 4.82 (4.21−5.43) | 4.70 (4.16−5.31) | 5.01 (4.41−5.58) | 4.94 (4.24−5.58) | 0.000 |
| TG, mmol/L | 1.35 (0.96−1.96) | 1.25 (0.89−1.85) | 1.45 (1.04−2.02) | 1.49 (1.08−2.11) | 0.000 |
| HDL-C, mmol/L | 1.26 (1.08–1.49) | 1.27 (1.08–1.49) | 1.28 (1.10–1.50) | 1.25 (1.07–1.47) | 0.009 |
| LDL-C, mmol/L | 3.03 (2.49–3.58) | 2.95 (2.46–3.47) | 3.20 (2.68–3.74) | 3.10 (2.46–3.72) | 0.000 |
| Variables | Total Cholesterol | Triglyceride | LDL-C | HDL-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class 1: Moderate-Stable (n = 9917) | Class 2: Inverse U-Shape (n = 222) | Class 3: U-Shape (n = 257) | Class 1: Low-Stable (n = 7216) | Class 2: Elevated-Increasing (n = 334) | Class 3: Moderate-Stable (n = 2846) | Class 1: Stable (n = 9909) | Class 2: Inverse U-Shape (n = 148) | Class 3: U-Shape (n = 339) | Class 1: Stable (n = 8731) | Class 2: Inverse U-Shape (n = 359) | Class 3: U-Shape (n = 1306) | |
| Age, years | 50 (39–61) | 54 (48–64) | 58 (53–65) | 49 (38–61) | 51 (42–58) | 53 (43–62) | 50 (39–61) | 54 (47–64) | 58 (52–65) | 50 (39–61) | 52 (42–62) | 53 (44–63) |
| Male | 5227 (52.7) | 75 (33.8) | 108 (42.0) | 3380 (46.8) | 249 (74.6) | 1781 (62.6) | 5209 (52.6) | 58 (39.2) | 143 (42.2) | 3685 (42.2) | 80 (22.3) | 284 (21.7) |
| BMI, kg/m2 | 24.39 (22.19–26.67) | 24.55 (22.47–27.17) | 25.36 (22.91–27.67) | 23.71 (21.58–25.91) | 26.06 (24.24–27.98) | 25.84 (23.95–28.06) | 24.39 (22.18–26.65) | 25.19 (23.06–27.26) | 25.33 (22.85–27.73) | 24.80 (22.68–27.04) | 22.19 (20.31–24.27) | 22.23 (20.41–24.31) |
| Hypertension | 1482 (14.9) | 34 (15.3) | 67 (26.1) | 938 (13.0) | 80 (24.0) | 565 (19.9) | 1475 (14.9) | 20 (13.5) | 88 (26.0) | 1375 (15.7) | 47 (13.1) | 161 (12.3) |
| Diabetes | 472 (4.8) | 9 (4.1) | 24 (9.3) | 320 (4.4) | 37 (11.1) | 148 (5.2) | 471 (4.8) | 5 (3.4) | 29 (8.6) | 446 (5.1) | 14 (3.9) | 45 (3.4) |
| SBP, mmHg | 126 (115–139) | 130 (117–142) | 137 (124–148) | 124 (113–138) | 135 (123–147) | 131 (121–144) | 126 (115–139) | 131 (119–144) | 135 (122–146) | 127 (116–140) | 122 (110–134) | 123 (111–137) |
| DBP, mmHg | 77 (69–84) | 78 (70–87) | 81 (74–88) | 75 (68–83) | 84 (77–91) | 80 (73–87) | 77 (69–84) | 77 (70–86) | 81 (73–88) | 78 (70–85) | 74 (66–82) | 74 (67–82) |
| Baseline biochemical profiles | ||||||||||||
| TC, mmol/L | 4.77 (4.19–5.36) | 6.32 (5.55–7.04) | 6.56 (5.98–7.23) | 4.69 (4.11–5.31) | 5.34 (4.55–6.15) | 5.10 (4.49–5.72) | 4.77 (4.19–5.36) | 6.10 (5.09–6.90) | 6.32 (5.70–6.84) | 4.75 (4.16–5.38) | 4.94 (4.30–5.48) | 5.18 (4.63–5.77) |
| TG, mmol/L | 1.33 (0.95–1.93) | 1.67 (1.24–2.48) | 2.05 (1.43–2.96) | 1.10 (0.84–1.42) | 5.17 (3.93–6.95) | 2.29 (1.92–2.82) | 1.33 (0.95–1.94) | 1.78 (1.26–2.35) | 1.72 (1.28–2.33) | 1.46 (1.04–2.09) | 1.00 (0.78–1.36) | 0.92 (0.72–1.21) |
| HDL-C, mmol/L | 1.26 (1.08–1.49) | 1.36 (1.16–1.58) | 1.27 (1.11–1.52) | 1.35 (1.16–1.57) | 0.99 (0.87–1.12) | 1.11 (0.98–1.27) | 1.26 (1.08–1.49) | 1.26 (1.05–1.46) | 1.28 (1.15–1.49) | 1.21 (1.05–1.38) | 1.63 (1.49–1.77) | 1.80 (1.71–1.96) |
| LDL-C, mmol/L | 2.99 (2.47–3.52) | 4.15 (3.44–4.87) | 4.37 (3.82–4.84) | 2.95 (2.43–3.50) | 2.64 (2.02–3.30) | 3.27 (2.72–3.80) | 2.99 (2.46–3.51) | 4.14 (2.99–5.01) | 4.37 (3.91–4.82) | 3.05 (2.52–3.60) | 2.86 (2.32–3.41) | 2.94 (2.43–3.51) |
| FBG, mmol/L | 5.2 (4.8–5.7) | 5.4 (5.0–6.0) | 5.6 (5.2–6.4) | 5.1 (4.8–5.5) | 5.6 (5.1–6.8) | 5.4 (5.0–6.0) | 5.2 (4.8–5.7) | 5.3 (5.0–6.1) | 5.5 (5.1–6.2) | 5.2 (4.9–5.7) | 5.1 (4.7–5.5) | 5.1 (4.7–5.5) |
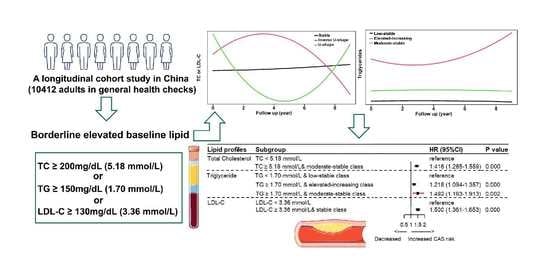
| Baseline Lipid Profiles and Longitudinal Trajectory | n | CAS Progression | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |||
| TC | ||||||||
| Class 1: Moderate-stable | 9917 | 1738 | Reference | Reference | Reference | |||
| Class 2: Inverse U-shape | 222 | 40 | 0.905 (0.661–1.239) | 0.534 | 0.876 (0.640–1.201) | 0.411 | 0.849 (0.617–1.169) | 0.317 |
| Class 3: U-shape | 257 | 36 | 0.910 (0.654–1.267) | 0.577 | 0.835 (0.599–1.164) | 0.287 | 0.824 (0.585–1.159) | 0.266 |
| TG | ||||||||
| Class 1: Low-stable | 7216 | 1185 | Reference | Reference | Reference | |||
| Class 2: Elevated-increasing | 334 | 69 | 1.497 (1.174–1.908) | 0.000 | 1.442 (1.129–1.841) | 0.003 | 1.417 (1.101–1.824) | 0.007 |
| Class 3: Moderate-stable | 2846 | 560 | 1.192 (1.078–1.318) | 0.000 | 1.144 (1.034–1.266) | 0.009 | 1.084 (0.974–1.207) | 0.139 |
| LDL-C | ||||||||
| Class 1: Stable | 9909 | 1730 | Reference | Reference | Reference | |||
| Class 2: U-shape | 339 | 57 | 1.076 (0.826–1.402) | 0.585 | 0.993 (0.761–1.295) | 0.960 | 0.978 (0.744–1.286) | 0.874 |
| Class 3: Inverse U-shape | 148 | 27 | 0.832 (0.568–1.219) | 0.345 | 0.788 (0.538–1.155) | 0.221 | 0.759 (0.514–1.121) | 0.165 |
| HDL-C | ||||||||
| Class 1: Stable | 8731 | 1544 | Reference | Reference | Reference | |||
| Class 2: Inverse U-shape | 359 | 71 | 1.079 (0.851–1.369) | 0.530 | 1.122 (0.883–1.427) | 0.346 | 1.217 (0.953–1.554) | 0.116 |
| Class 3: U-shape | 1306 | 199 | 0.983 (0.848–1.140) | 0.819 | 1.023 (0.879–1.191) | 0.766 | 1.089 (0.930–1.275) | 0.292 |
| Baseline borderline high levels | ||||||||
| TC ≥ 5.18 mmol/L | 3613 | 741 | 1.371 (1.248–1.506) | 0.000 | 1.331 (1.210–1.464) | 0.000 | 1.316 (1.194–1.451) | 0.000 |
| TG ≥ 1.70 mmol/L | 3508 | 671 | 1.193 (1.084–1.312) | 0.000 | 1.136 (1.031–1.251) | 0.010 | 1.061 (0.957–1.176) | 0.263 |
| LDL-C ≥ 3.36 mmol/L | 3592 | 740 | 1.461 (1.330–1.605) | 0.000 | 1.417 (1.289–1.557) | 0.000 | 1.382 (1.254–1.523) | 0.000 |
| HDL-C < 1.04 mmol/L | 1991 | 399 | 1.107 (0.994–1.242) | 0.063 | 1.067 (0.951–1.197) | 0.271 | 1.021 (0.998–1.217) | 0.739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, Y.; Tao, L.; Yang, L.; Liu, D.; Wang, Y.; Hao, X.; He, H.; Che, Y.; Wang, P.; et al. Trajectories of Lipid Profile and Risk of Carotid Atherosclerosis Progression: A Longitudinal Cohort Study. Nutrients 2022, 14, 3243. https://doi.org/10.3390/nu14153243
Yu H, Li Y, Tao L, Yang L, Liu D, Wang Y, Hao X, He H, Che Y, Wang P, et al. Trajectories of Lipid Profile and Risk of Carotid Atherosclerosis Progression: A Longitudinal Cohort Study. Nutrients. 2022; 14(15):3243. https://doi.org/10.3390/nu14153243
Chicago/Turabian StyleYu, Haixu, Yanguang Li, Liyuan Tao, Lincheng Yang, Dan Liu, Yang Wang, Xiaoyan Hao, Honghai He, Ying Che, Peng Wang, and et al. 2022. "Trajectories of Lipid Profile and Risk of Carotid Atherosclerosis Progression: A Longitudinal Cohort Study" Nutrients 14, no. 15: 3243. https://doi.org/10.3390/nu14153243
APA StyleYu, H., Li, Y., Tao, L., Yang, L., Liu, D., Wang, Y., Hao, X., He, H., Che, Y., Wang, P., Zhao, W., & Gao, W. (2022). Trajectories of Lipid Profile and Risk of Carotid Atherosclerosis Progression: A Longitudinal Cohort Study. Nutrients, 14(15), 3243. https://doi.org/10.3390/nu14153243