Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Data Analyses
3. Results
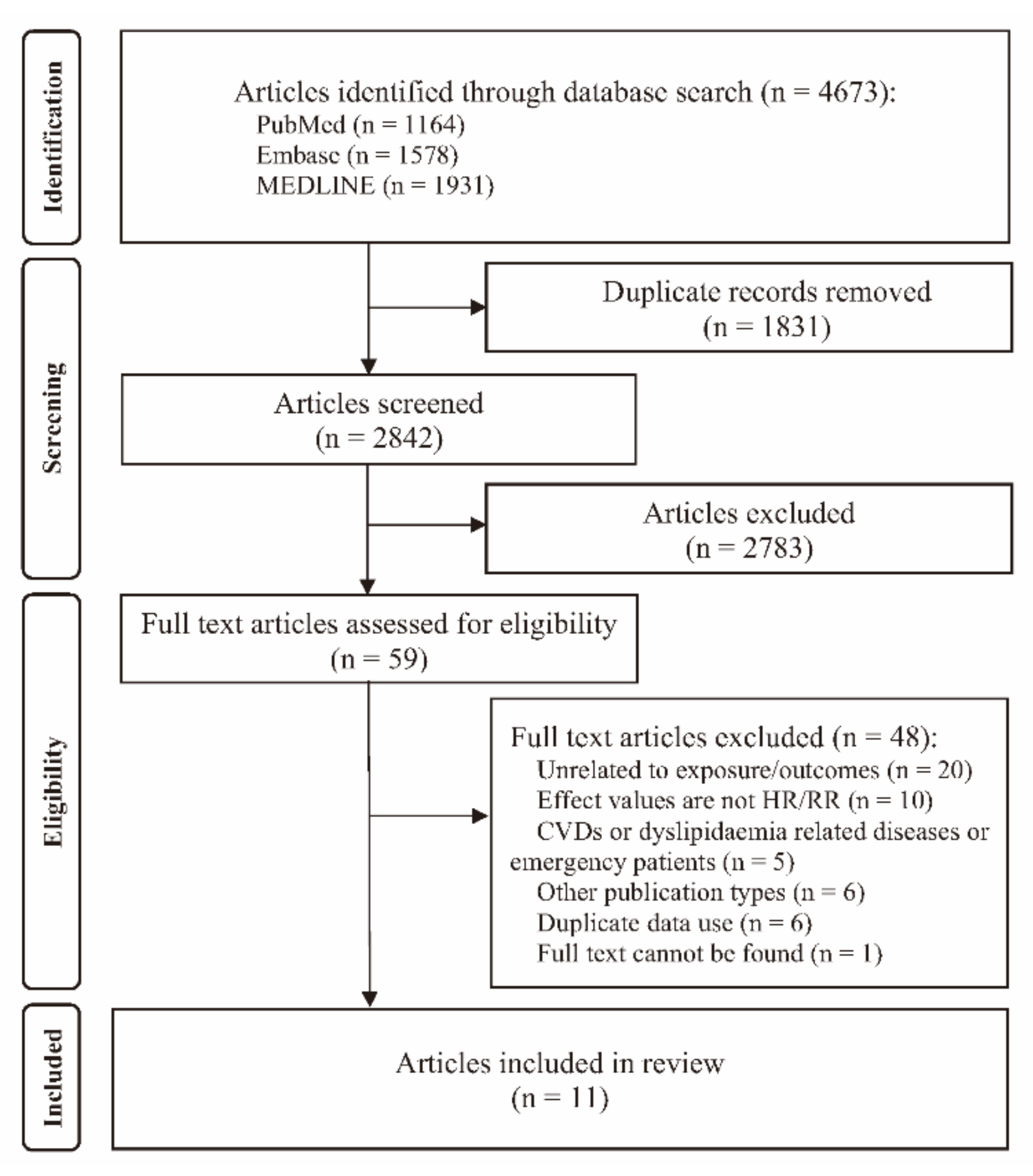
3.1. Identification and Selection of Studies
3.2. Study Characteristics
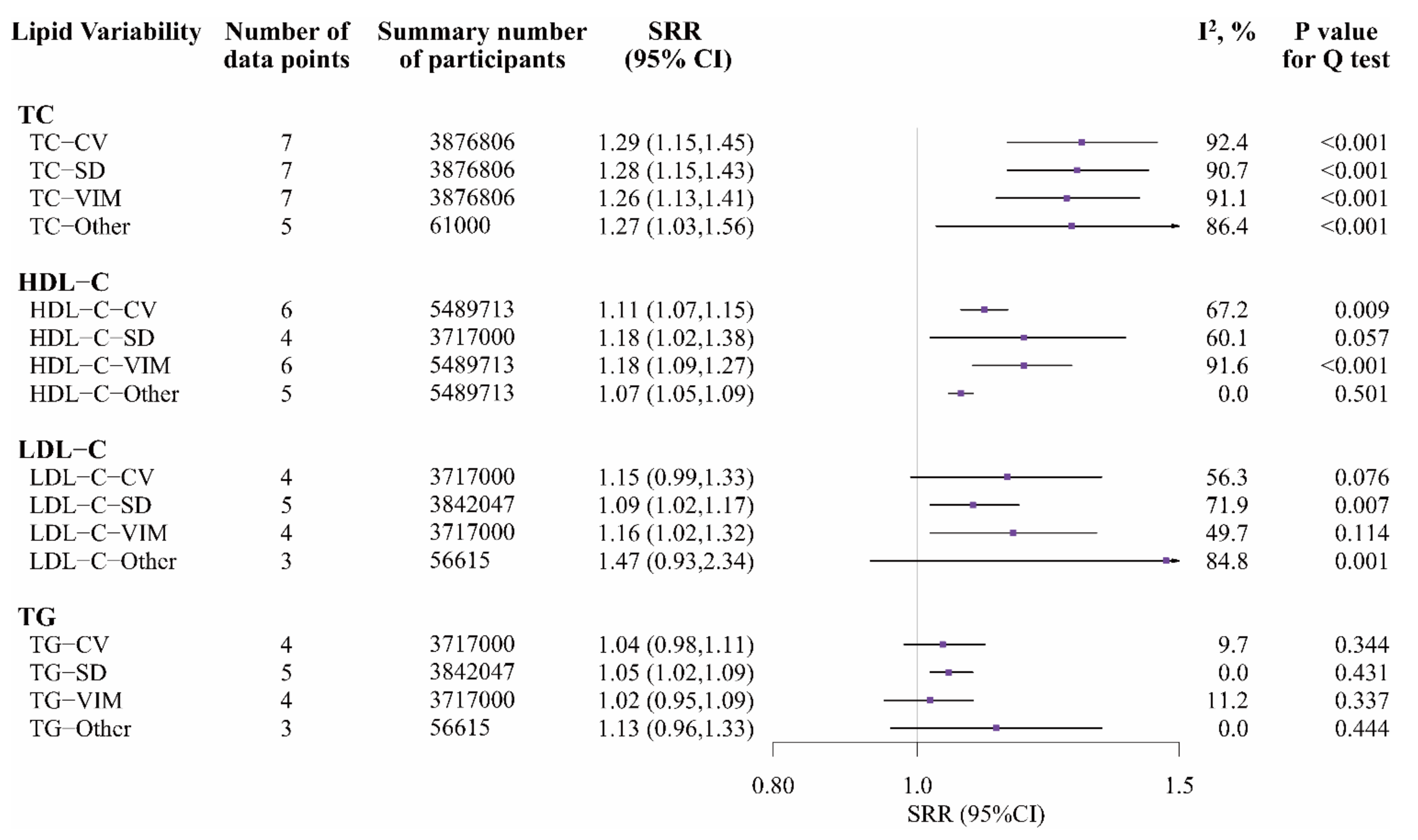
3.3. Lipid Variability and Cardiovascular Diseases
3.4. Lipid Variability and All-Cause Mortality
3.5. Subgroup Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LV | Lipid variability |
| CVDs | Cardiovascular diseases |
| MI | Myocardial infarction |
| CHD | Coronary heart disease |
| HF | Heart failure |
| AF | Atrial fibrillation |
| STEMI | ST-segment elevation myocardial infarction |
| CAD | Coronary artery disease |
| PCI | Percutaneous coronary intervention |
| FH | Familial hypercholesterolemia |
| TC | Total cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| TG | Triglycerides |
| SD | Standard deviation |
| CV | Coefficient of variation |
| VIM | Variation independent of the mean |
| ARV | Average real variability |
| ASV | Average successive variability |
| RMSE | Root mean square error |
| SDR | Standard deviation of the residuals |
| HR | Hazard ratio |
| RR | Relative risk |
| SRR | Summary relative risk |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| NOS | Newcastle–Ottawa quality assessment scale |
| WB income region | The World Bank income region |
| HICs | High-income countries |
| UMICs | Upper-middle-income economies |
| BMI | Body mass index |
| FHS | Framingham Heart Study |
| KNHIS | Korean National Health Insurance System cohort |
| YHIS | Yinzhou Health Information System |
| CDARS | The Clinical Data Analysis and Reporting System |
| NR | Not report |
References
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 November 2021).
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 November 2021).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kim, D.H.; Lee, H.; Hwang, I.C.; Yoon, Y.E.; Park, H.E.; Choi, S.Y.; Kim, Y.J.; Cho, G.Y.; Han, K.; et al. Mildly Abnormal Lipid Levels, but Not High Lipid Variability, Are Associated with Increased Risk of Myocardial Infarction and Stroke in “Statin-Naive” Young Population A Nationwide Cohort Study. Circ. Res. 2020, 126, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Elkind, M.S. Lipids and Cerebrovascular Disease: Research and Practice. Stroke 2015, 46, 3322–3328. [Google Scholar] [CrossRef] [Green Version]
- Han, B.H.; Han, K.; Yoon, K.H.; Kim, M.K.; Lee, S.H. Impact of Mean and Variability of High-Density Lipoprotein-Cholesterol on the Risk of Myocardial Infarction, Stroke, and Mortality in the General Population. J. Am. Heart Assoc. 2020, 9, e015493. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.D.L.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, J.; Lloyd-Jones, D.J.J. Novel Lipid-Lowering Therapies to Reduce Cardiovascular Risk. JAMA 2021, 326, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.; Pencina, K.; Lloyd-Jones, D.; Catapano, A.; Thanassoulis, G.; Sniderman, A.J.C. The Expected 30-Year Benefits of Early Versus Delayed Primary Prevention of Cardiovascular Disease by Lipid Lowering. Circulation 2020, 142, 827–837. [Google Scholar] [CrossRef]
- Simpson, W.G. Biomarker variability and cardiovascular disease residual risk. Curr. Opin. Cardiol. 2019, 34, 413–417. [Google Scholar] [CrossRef]
- Messerli, F.H.; Hofstetter, L.; Rimoldi, S.F.; Rexhaj, E.; Bangalore, S. Risk Factor Variability and Cardiovascular Outcome: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2596–2603. [Google Scholar] [CrossRef]
- Bangalore, S.; Breazna, A.; DeMicco, D.A.; Wun, C.C.; Messerli, F.H. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: Insights from the TNT trial. J. Am. Coll. Cardiol. 2015, 65, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.K.; Han, K.; Kim, H.S.; Park, Y.M.; Kwon, H.S.; Yoon, K.H.; Lee, S.H. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: A nationwide population-based study. Eur. Heart J. 2017, 38, 3560–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, E.Y.F.; Yu, E.Y.T.; Chin, W.Y.; Barrett, J.K.; Mok, A.H.Y.; Lau, C.S.T.; Wang, Y.; Wong, I.C.K.; Chan, E.W.Y.; Lam, C.L.K. Greater variability in lipid measurements associated with cardiovascular disease and mortality: A 10-year diabetes cohort study. Diabetes Obes. Metab. 2020, 22, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Clark, D., 3rd; Nicholls, S.J.; St John, J.; Elshazly, M.B.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; Puri, R. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur. Heart J. 2018, 39, 2551–2558. [Google Scholar] [CrossRef]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.N.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women with a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Chêne, G.; Thompson, S.G. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am. J. Epidemiol. 1996, 144, 610–621. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 November 2021).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Kreger, B.E.; Odell, P.M.; D'Agostino, R.B.; Wilson, P.F. Long-term intraindividual cholesterol variability: Natural course and adverse impact on morbidity and mortality—The Framingham Study. Am. Heart J. 1994, 127, 1607–1614. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.R.; Choi, E.K.; Lee, S.H.; Han, K.D.; Lee, S.Y.; Yang, S.; Park, J.; Choi, Y.J.; Lee, H.J.; et al. Visit-to-visit variability of metabolic parameters and risk of heart failure: A nationwide population-based study. Int. J. Cardiol. 2019, 293, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, J.M.; Yu, Z.B.; Li, D.; Wu, M.Y.; Shen, P.; Lin, H.B.; Wang, J.B.; Chen, K. Intra-individual variability of total cholesterol is associated with cardiovascular disease mortality: A cohort study. Nutr. Metab. Cardiovasc. Dis. NMCD 2019, 29, 1205–1213. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.R.; Choi, E.K.; Han, K.D.; Oh, S. Low Lipid Levels and High Variability are Associated With the Risk of New-Onset Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e012771. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Song, Q.; Wang, X. Visit-to-visit variability of lipid measurements and the risk of myocardial infarction and all-cause mortality: A prospective cohort study. Atherosclerosis 2020, 312, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kalani, R.; Bartz, T.M.; Suchy-Dicey, A.; Elkind, M.S.V.; Psaty, B.M.; Leung, L.Y.; Rice, K.; Tirschwell, D.; Longstreth, W.T., Jr. Cholesterol Variability and Cranial Magnetic Resonance Imaging Findings in Older Adults: The Cardiovascular Health Study. Stroke 2020, 51, 69–74. [Google Scholar] [CrossRef]
- Wang, A.; Li, H.; Yuan, J.; Zuo, Y.; Zhang, Y.; Chen, S.; Wu, S.; Wang, Y. Visit-to-Visit Variability of Lipids Measurements and the Risk of Stroke and Stroke Types: A Prospective Cohort Study. J. Stroke 2020, 22, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.Q.; Liu, L.; Liu, X.C.; Lo, K.; Tang, S.T.; Feng, Y.Q.; Zhang, B. The association of blood lipid parameters variability with ischemic stroke in hypertensive patients. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 1521–1532. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.K.; Rhee, E.J. Effects of Cardiovascular Risk Factor Variability on Health Outcomes. Endocrinol. Metab. 2020, 35, 217–226. [Google Scholar] [CrossRef]
- Roh, E.; Chung, H.S.; Lee, J.S.; Kim, J.A.; Lee, Y.B.; Hong, S.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; et al. Total cholesterol variability and risk of atrial fibrillation: A nationwide population-based cohort study. PLoS ONE 2019, 14, e0215687. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Lee, S.H.; Oh, S. Effect of the variability of blood pressure, glucose level, total cholesterol level, and body mass index on the risk of atrial fibrillation in a healthy population. Heart Rhythm 2020, 17, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Vedre, A.; Pathak, D.R.; Crimp, M.; Lum, C.; Koochesfahani, M.; Abela, G.S. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis 2009, 203, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Besterman, E.M. Experimental coronary atherosclerosis in rabbits. Atherosclerosis 1970, 12, 75–83. [Google Scholar] [CrossRef]
- Chen, H.; Ren, J.Y.; Xing, Y.; Zhang, W.L.; Liu, X.; Wu, P.; Wang, R.J.; Luo, Y. Short-term withdrawal of simvastatin induces endothelial dysfunction in patients with coronary artery disease: A dose-response effect dependent on endothelial nitric oxide synthase. Int. J. Cardiol. 2009, 131, 313–320. [Google Scholar] [CrossRef]
- Boey, E.; Gay, G.M.; Poh, K.K.; Yeo, T.C.; Tan, H.C.; Lee, C.H. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis 2016, 244, 86–92. [Google Scholar] [CrossRef]
- Bangalore, S.; Fayyad, R.; Messerli, F.H.; Laskey, R.; DeMicco, D.A.; Kastelein, J.J.; Waters, D.D. Relation of Variability of Low-Density Lipoprotein Cholesterol and Blood Pressure to Events in Patients with Previous Myocardial Infarction from the IDEAL Trial. Am. J. Cardiol. 2017, 119, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E.; Rueda-Ochoa, O.L. Methodological challenges in studies of the role of blood lipids variability in the incidence of cardiovascular disease. Lipids Health Dis. 2021, 20, 51. [Google Scholar] [CrossRef] [PubMed]

| Authors (Year) | Country | Cohort | WB Income Region | Study Period | Mean/Median Follow-Up Years | Number of Participants | Age (Years) | Female (%) | Lipids | Metrics of Variability | Numbers of Causes of Outcome(s) | Comparison |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kreger, et al. (1994) [24] | America | FHS | HICs | 1948–1985 | NR | 2912 | 30–62 | 51.7 | TC | RMSE | CVDs (CHD); all-cause mortality | Extreme quartiles |
| Kim, et al. (2017) [14] | South Korea | KNHIS | HICs | 2002–2015 | 8.3 | 3,656,648 | ≥20 | 32.4 | TC | CV; SD; VIM | CVDs (stroke/MI); all-cause mortality | Extreme quartiles |
| Kwon, et al. (2019) [25] | South Korea | KNHIS | HICs | 2009–2015 | 5.3 | 3,820,191 | ≥40 | 47.1 | TC | CV; SD; VIM | CVDs (HF) | Extreme quartiles |
| Zhu, et al. (2019) [26] | China | YHIS | UMICs | 2010–2017 | 4.3 | 32,237 | ≥40 | NR | TC | CV; SD; VIM; ASV | All-cause mortality | Extreme quartiles |
| Lee, et al. (2019) [27] | South Korea | KNHIS | HICs | 2009–2015 | 5.4 | 3,660,385 | 43.4 | 31.8 | TC; HDL-C; LDL-C; TG | CV; SD; VIM | CVDs (AF) | Extreme quartiles |
| Liu, et al. (2020) [28] | China | Kailuan cohort | UMICs | 2006–2017 | 7.0 | 51,620 | 52.8 ± 11.8 | 24.0 | TC; HDL-C; LDL-C; TG | CV; SD; VIM; ARV | CVDs (MI); all-cause mortality | Extreme quartiles, Per SD |
| Han, et al. (2020) [6] | South Korea | KNHIS | HICs | 2009–2017 | 5.1 | 5,433,098 | ≥20 | 34.2 | HDL-C | CV; VIM; ARV | CVDs (stroke/M); all-cause mortality | Extreme quartiles |
| Kalani, et al. (2020) [29] | America | The Cardiovascular Health Study | HICs | 1989–1998 | 5.2 | 1473 | 73.8 ± 4.4 | 60.1 | TC | SDR | CVDs (stroke) | Per unit |
| Wang, et al. (2020) [30] | China | Kailuan Cohort | UMICs | 2006–2016 | 6.0 | 51,620 | 52.8 ± 11.8 | 24.0 | TC; HDL-C; LDL-C; TG | CV; SD; VIM; ARV | CVDs (stroke) | Extreme quartiles, Per SD |
| Wan, et al. (2020) [15] | China (Hong Kong) | CDARS | HICs | 2008–2017 | 6.5 | 125,047 | 64.3 ± 9.7 | 54.5 | TG; LDL-C | SD | CVDs; all-cause mortality | Extreme quintiles |
| Huang, et al. (2021) [31] | China | Liaobu Community Study | UMICs | 2013–2018 | 4.2 | 4995 | 62.7 ± 12.6 | 55.2 | TC; LDL-C; HDL-C; TG | CV; SD; VIM; ASV | CVDs (stroke) | Extreme quartiles |
| Characteristics of Studies and Populations | Number of Data Points | SRR (95% CI) | Number of Data Points | SRR (95% CI) | Number of Data Points | SRR (95% CI) |
|---|---|---|---|---|---|---|
| TC-CV | TC-SD | TC-VIM | ||||
| Global analysis | 7 | 1.29 (1.15, 1.45) | 7 | 1.28 (1.15, 1.43) | 7 | 1.26 (1.13, 1.41) |
| Subtypes of CVDs | ||||||
| MI | 2 | 1.39 (1.03, 1.87) | 2 | 1.35 (1.03, 1.77) | 2 | 1.39 (1.08, 1.79) |
| Stroke | 3 | 1.56 (1.07, 2.28) | 3 | 1.59 (1.12, 2.27) | 3 | 1.49 (1.06, 2.10) |
| AF | 1 | 1.10 (1.06, 1.13) | 1 | 1.09 (1.06, 1.13) | 1 | 1.08 (1.04, 1.12) |
| HF | 1 | 1.17 (1.13, 1.22) | 1 | 1.18 (1.13, 1.23) | 1 | 1.17 (1.12, 1.22) |
| Gender * | ||||||
| Male | 4 | 1.08 (1.05, 1.11) | 3 | 1.09 (1.07, 1.10) | 3 | 1.08 (1.07, 1.10) |
| Female | 4 | 1.09 (0.99, 1.19) | 3 | 1.06 (1.03, 1.08) | 3 | 1.05 (1.01, 1.09) |
| Adjusted for mean lipid level | ||||||
| Yes | 6 | 1.25 (1.12, 1.40) | 6 | 1.24 (1.13, 1.37) | 6 | 1.23 (1.11, 1.36) |
| No | 1 | 3.83 (2.03, 7.25) | 1 | 4.43 (2.29, 8.56) | 1 | 3.87 (2.04, 7.32) |
| Adjusted for lipid-lowering medication | ||||||
| Yes | 5 | 1.43 (1.17, 1.75) | 4 | 1.52 (1.23, 1.86) | 4 | 1.49 (1.23, 1.81) |
| No | 2 | 1.13 (1.06, 1.21) | 3 | 1.13 (1.07, 1.21) | 3 | 1.12 (1.04, 1.19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Hou, L.; Zhu, S.; Yi, Q.; Liu, W.; Zhao, Y.; Wu, F.; Li, X.; Pan, A.; Song, P. Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2022, 14, 2450. https://doi.org/10.3390/nu14122450
Li S, Hou L, Zhu S, Yi Q, Liu W, Zhao Y, Wu F, Li X, Pan A, Song P. Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients. 2022; 14(12):2450. https://doi.org/10.3390/nu14122450
Chicago/Turabian StyleLi, Shuting, Leying Hou, Siyu Zhu, Qian Yi, Wen Liu, Yang Zhao, Feitong Wu, Xue Li, An Pan, and Peige Song. 2022. "Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies" Nutrients 14, no. 12: 2450. https://doi.org/10.3390/nu14122450
APA StyleLi, S., Hou, L., Zhu, S., Yi, Q., Liu, W., Zhao, Y., Wu, F., Li, X., Pan, A., & Song, P. (2022). Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients, 14(12), 2450. https://doi.org/10.3390/nu14122450






