Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency
Abstract
:1. Introduction
2. Iron Deficiency, the Most Common Comorbidity of Heart Failure
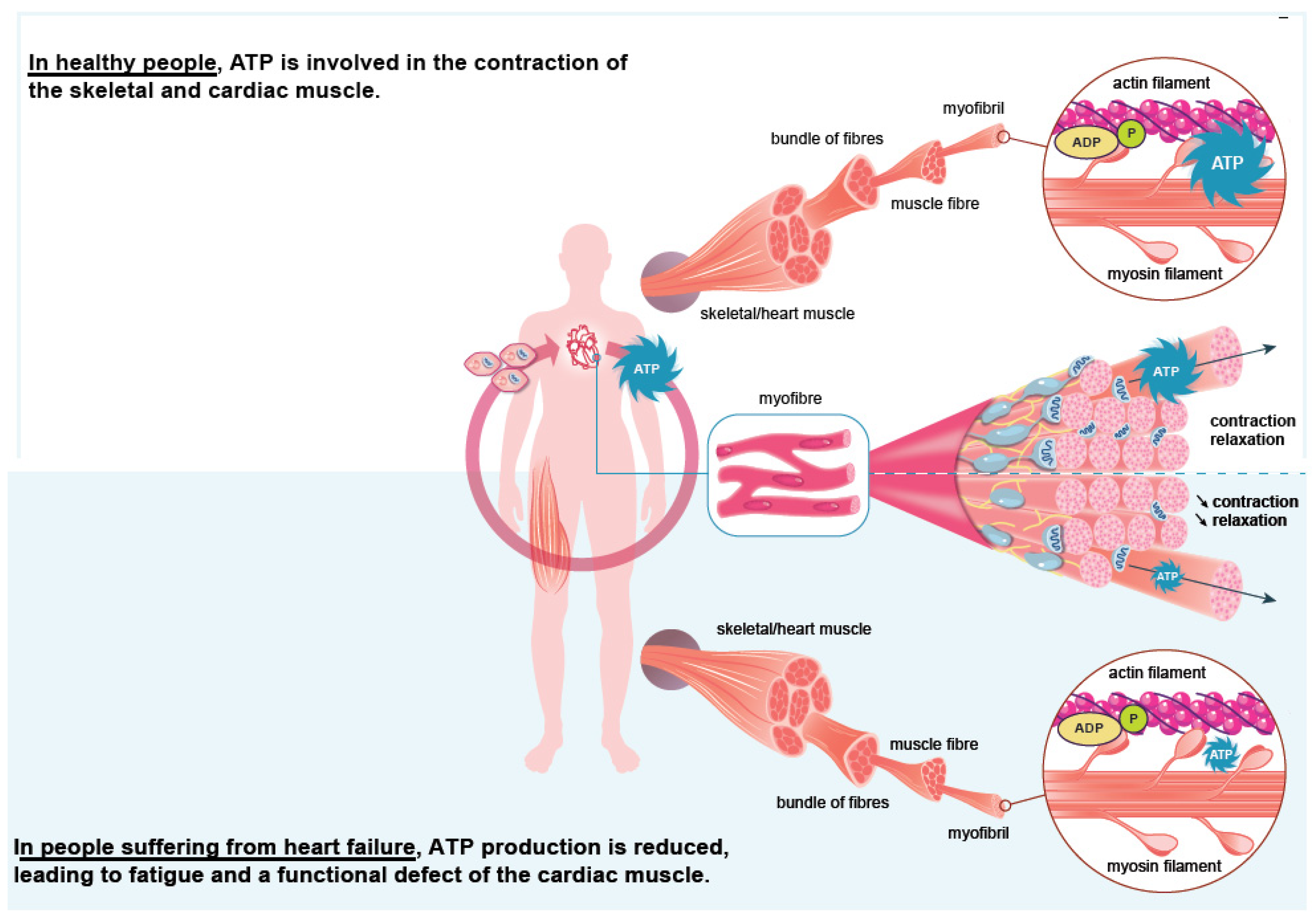
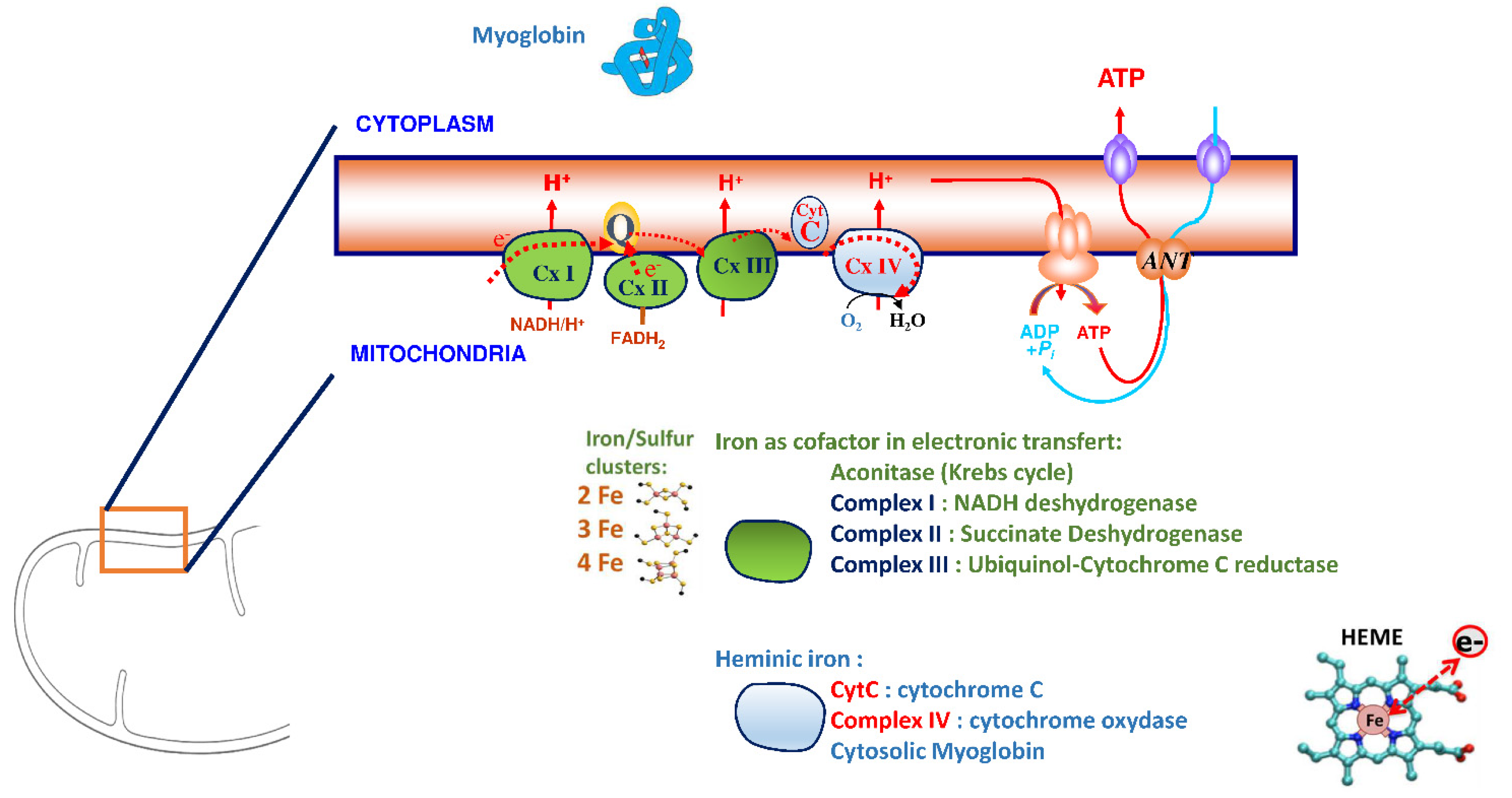
3. Iron Is an Essential Element for the Correct Functioning of the Heart Muscle
4. Iron, a Finely Regulated Element during Inflammation: Role of Hepcidin
5. Biological Diagnosis of Iron Deficiency
6. Iron Supplementation in Heart Failure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gibelin, P. Insuffisance Cardiaque: Aspects Épidémiologiques, Cliniques et Pronostiques. EMC Cardiol. 2018, 13, 11-024-A-10. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology Practical Guidance on the Use of Natriuretic Peptide Concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Assurance Maladie. Améliorer La Qualité Du Système de Santé et Maîtriser Les Dépenses. Propositions de l’Assurance Maladie Pour 2022. Juillet 2021. Rapport Au Ministre Chargé de La Sécurité Sociale et Au Parlement Sur l’évolution Des Charges et Des Produits de l’Assurance Maladie Au Titre de 2022. Available online: https://assurance-maladie.ameli.fr/sites/default/files/2021-07_rapport-propositions-pour-2022_assurance-maladie_3.pdf (accessed on 1 August 2022).
- Instruction Du 27 Juillet 2021 Relative Au CAQES: Modalités Du Suivi Des Contrats Actuels, de La Phase Transitoire En 2021 et de La Mise En Œuvre Des Nouveaux CAQES En 2022. Available online: http://www.omedit-idf.fr/wp-content/uploads/instruction-CAQES-2021_27072021.pdf (accessed on 27 July 2021).
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Shiba, N.; Shimokawa, H. Chronic Kidney Disease and Heart Failure--Bidirectional Close Link and Common Therapeutic Goal. J. Cardiol. 2011, 57, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Komajda, M. Diabète et Insuffisance Cardiaque: Données Épidémiologiques et Implications Thérapeutiques. Bull Acad. Natl. Med. 2018, 202, 909–916. [Google Scholar] [CrossRef]
- Rutten, F.H.; Cramer, M.J.; Grobbee, D.E.; Sachs, A.P.; Kirkels, J.H.; Lammers, J.W.; Hoes, A.W. Unrecognized Heart Failure in Elderly Patients with Stable Chronic Obstructive Pulmonary Disease. Eur. Heart J. 2005, 26, 1887–1894. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Chopra, V.K.; Anker, S.D. Anaemia, Iron Deficiency and Heart Failure in 2020: Facts and Numbers. ESC Heart Fail. 2020, 7, 2007–2011. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Guide Du Parcours de Soins. Insuffisance Cardiaque. Available online: https://www.has-sante.fr/jcms/c_1242988/fr/guide-parcours-de-soins-insuffisance-cardiaque (accessed on 24 July 2014).
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron Deficiency in Chronic Heart Failure: An International Pooled Analysis. Am. Heart J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum Ferritin Is Derived Primarily from Macrophages through a Nonclassical Secretory Pathway. Blood 2010, 116, 1574–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, M.; Chrysohoou, C.; Georgilas, T.A.; Giamouzis, G.; Giannakoulas, G.; Karavidas, A.; Papadopoulos, C.; Patsilinakos, S.; Tziakas, D.; Parissis, J. Management of Iron Deficiency in Chronic Heart Failure: Practical Considerations for Clinical Use and Future Directions. Eur. J. Intern. Med. 2019, 65, 17–25. [Google Scholar] [CrossRef]
- Martens, P.; Dupont, M.; Dauw, J.; Nijst, P.; Herbots, L.; Dendale, P.; Vandervoort, P.; Bruckers, L.; Tang, W.H.W.; Mullens, W. The Effect of Intravenous Ferric Carboxymaltose on Cardiac Reverse Remodelling Following Cardiac Resynchronization Therapy-the IRON-CRT Trial. Eur. Heart J. 2021, 42, 4905–4914. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron Deficiency: An Ominous Sign in Patients with Systolic Chronic Heart Failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef]
- Okonko, D.O.; Mandal, A.K.; Missouris, C.G.; Poole-Wilson, P.A. Disordered Iron Homeostasis in Chronic Heart Failure: Prevalence, Predictors, and Relation to Anemia, Exercise Capacity, and Survival. J. Am. Coll Cardiol. 2011, 58, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A Precious Metal: Iron, an Essential Nutrient for All Cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Loncar, G.; Obradovic, D.; Thiele, H.; Haehling, S.; Lainscak, M. Iron Deficiency in Heart Failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert Consensus Document: Mitochondrial Function as a Therapeutic Target in Heart Failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron Deficiency Impairs Contractility of Human Cardiomyocytes through Decreased Mitochondrial Function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rineau, E.; Gaillard, T.; Gueguen, N.; Procaccio, V.; Henrion, D.; Prunier, F.; Lasocki, S. Iron Deficiency without Anemia Is Responsible for Decreased Left Ventricular Function and Reduced Mitochondrial Complex I Activity in a Mouse Model. Int. J. Cardiol. 2018, 266, 206–212. [Google Scholar] [CrossRef]
- Lacour, P.; Dang, P.L.; Morris, D.A.; Parwani, A.S.; Doehner, W.; Schuessler, F.; Hohendanner, F.; Heinzel, F.R.; Stroux, A.; Tschoepe, C.; et al. The Effect of Iron Deficiency on Cardiac Resynchronization Therapy: Results from the RIDE-CRT Study. ESC Heart Fail. 2020, 7, 1072–1084. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of Iron Deficiency on Exercise Capacity and Outcome in Heart Failure with Reduced, Mid-Range and Preserved Ejection Fraction. Acta Cardiol. 2018, 73, 115–123. [Google Scholar] [CrossRef]
- van Veldhuisen, D.J.; Anker, S.D.; Ponikowski, P.; Macdougall, I.C. Anemia and Iron Deficiency in Heart Failure: Mechanisms and Therapeutic Approaches. Nat. Rev. Cardiol. 2011, 8, 485–493. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Leclercq, C.; Deray, G.; Lasocki, S.; Zambrowski, J.J.; Mebazaa, A.; de Groote, P.; Damy, T.; Galinier, M. Iron Deficiency: An Emerging Therapeutic Target in Heart Failure. Heart 2014, 100, 1414–1420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.S.; Enns, C.A. Molecular Mechanisms of Normal Iron Homeostasis. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing Acts: Molecular Control of Mammalian Iron Metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Koulaouzidis, A.; Said, E.; Cottier, R.; Saeed, A.A. Soluble Transferrin Receptors and Iron Deficiency, a Step beyond Ferritin. A Systematic Review. J. Gastrointestin. Liver Dis. 2009, 18, 345–352. [Google Scholar] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral Iron Supplements Increase Hepcidin and Decrease Iron Absorption from Daily or Twice-Daily Doses in Iron-Depleted Young Women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA 2017, 317, 1958–1966. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinchi, F.; Castagna, A.; Costa da Silva, M.; Busti, F.; Marchi, G.; Sparla, R.; Muckenthaler, M.U.; Girelli, D. Intravenous Iron Promotes Low-Grade Inflammation in Anemic Patients By Triggering Macrophage Activation. Blood 2019, 134, 957. [Google Scholar] [CrossRef]
- Palau, P.; Llacer, P.; Dominguez, E.; Tormo, J.P.; Zakarne, R.; Mollar, A.; Martinez, A.; Minana, G.; Santas, E.; Almenar, L.; et al. Iron Deficiency and Short-Term Adverse Events in Patients with Decompensated Heart Failure. Clin. Res. Cardiol. 2021, 110, 1292–1298. [Google Scholar] [CrossRef]
- Martens, P.; Grote Beverborg, N.; van der Meer, P. Iron Deficiency in Heart Failure—Time to Redefine. Eur. J. Prev. Cardiol. 2021, 28, 1647–1649. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Choix des Examens du Métabolisme du Fer en Cas de Suspicion de Carence en Fer. Mars 2011. Available online: http://www.Has-Sante.Fr/Portail/Upload/Docs/Application/Pdf/2011-11/Texte_court__bilan_martial_carence_2011-11-09_17-22-2_135.Pdf (accessed on 21 March 2013).
- Cacoub, P.; Vandewalle, C.; Peoc’h, K. Using Transferrin Saturation as a Diagnostic Criterion for Iron Deficiency: A Systematic Review. Crit. Rev. Clin. Lab. Sci. 2019, 56, 526–532. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Ghafourian, K.; Shapiro, J.S.; Goodman, L.; Ardehali, H. Iron and Heart Failure: Diagnosis, Therapies, and Future Directions. JACC Basic Transl. Sci. 2020, 5, 300–313. [Google Scholar] [CrossRef]
- Pezel, T.; Audureau, E.; Mansourati, J.; Baudry, G.; Ben Driss, A.; Durup, F.; Fertin, M.; Godreuil, C.; Jeanneteau, J.; Kloeckner, M.; et al. Diagnosis and Treatment of Iron Deficiency in Heart Failure: OFICSel Study by the French Heart Failure Working Group. ESC Heart Fail. 2021, 8, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Wienbergen, H.; Pfister, O.; Hochadel, M.; Michel, S.; Bruder, O.; Remppis, B.A.; Maeder, M.T.; Strasser, R.; von Scheidt, W.; Pauschinger, M.; et al. Usefulness of Iron Deficiency Correction in Management of Patients With Heart Failure [from the Registry Analysis of Iron Deficiency-Heart Failure (RAID-HF) Registry]. Am. J. Cardiol. 2016, 118, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.M.; Schrage, B.; Benson, L.; Fudim, M.; Corovic Cabrera, C.; Dahlström, U.; Rosano, G.M.C.; Jankowska, E.A.; Anker, S.D.; Lund, L.H.; et al. Phenotyping Heart Failure Patients for Iron Deficiency and Use of Intravenous Iron Therapy: Data from the S Wedish H Eart F Ailure R Egistry. Eur. J. Heart Fail. 2021, 23, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Nicolas, G.; Peoc’h, K. Iron Deficiency Markers in Patients Undergoing Iron Replacement Therapy: A 9-Year Retrospective Real-World Evidence Study Using Healthcare Databases. Sci. Rep. 2020, 10, 14983. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [Green Version]
- DeLoughery, T.G. Safety of Oral and Intravenous Iron. Acta Haematol. 2019, 142, 8–12. [Google Scholar] [CrossRef]
- Auerbach, M.; Rodgers, G.M. Intravenous Iron. N. Engl. J. Med. 2007, 357, 93–94. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The Safety of Intravenous Iron Preparations. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial Effects of Long-Term Intravenous Iron Therapy with Ferric Carboxymaltose in Patients with Symptomatic Heart Failure and Iron Deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef]
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients with Symptomatic Chronic Heart Failure and Iron Deficiency FERRIC-HF: A Randomized, Controlled, Observer-Blinded Trial. J. Am. Coll Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Bohm, M.; Doletsky, A.; Voors, A.A.; Macdougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Kirwan, B.A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Luscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of Ferric Carboxymaltose on Hospitalisations and Mortality Rates in Iron-Deficient Heart Failure Patients: An Individual Patient Data Meta-Analysis. Eur. J. Heart Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Colet, J.C.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Mori, C.; von Eisenhart Rothe, B.; Pocock, S.; et al. Rationale and Design of Ferinject Assessment in Patients with IRon Deficiency and Chronic Heart Failure (FAIR-HF) Study: A Randomized, Placebo-Controlled Study of Intravenous Iron Supplementation in Patients with and without Anaemia. Eur. J. Heart Fail. 2009, 11, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Arutyunov, G.; Bylova, N.; Ivleva, A.; Kobalava, Z. The Safety of Intravenous (IV) Ferric Carboxymaltose versus IV Iron Sucrose on Patients with Chronic Heart Failure (CHF) and Chronic Kidney Disease (CKD) with Iron Deficincy (ID). Eur. J. Heart Fail. 2009, 8, ii71. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017, 23, 628–651. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manceau, H.; Ausseil, J.; Masson, D.; Feugeas, J.-P.; Sablonniere, B.; Guieu, R.; Puy, H.; Peoc’h, K. Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients 2022, 14, 3214. https://doi.org/10.3390/nu14153214
Manceau H, Ausseil J, Masson D, Feugeas J-P, Sablonniere B, Guieu R, Puy H, Peoc’h K. Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients. 2022; 14(15):3214. https://doi.org/10.3390/nu14153214
Chicago/Turabian StyleManceau, Hana, Jérome Ausseil, Damien Masson, Jean-Paul Feugeas, Bernard Sablonniere, Régis Guieu, Hervé Puy, and Katell Peoc’h. 2022. "Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency" Nutrients 14, no. 15: 3214. https://doi.org/10.3390/nu14153214
APA StyleManceau, H., Ausseil, J., Masson, D., Feugeas, J.-P., Sablonniere, B., Guieu, R., Puy, H., & Peoc’h, K. (2022). Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients, 14(15), 3214. https://doi.org/10.3390/nu14153214






