Special Diets in Infants and Children and Impact on Gut Microbioma
Abstract
:1. Gut Microbiota in Infants and Children: What Influences It?
2. Methods
3. Gut Microbiota in Infants and Toddlers under Standard Diets
3.1. Breast Milk vs. Formula Milk and Gut Microbiota
3.2. Gut Microbiota and Complementary Feeding
4. Gut Microbiota in Infants under Special Diets
4.1. Special Formulas for the Treatment of Cow’s Milk Allergy
4.2. Special Formula for Functional Gastrointestinal Symptoms
4.3. Phenylketonuria and Inborn Errors of Metabolism Formulas
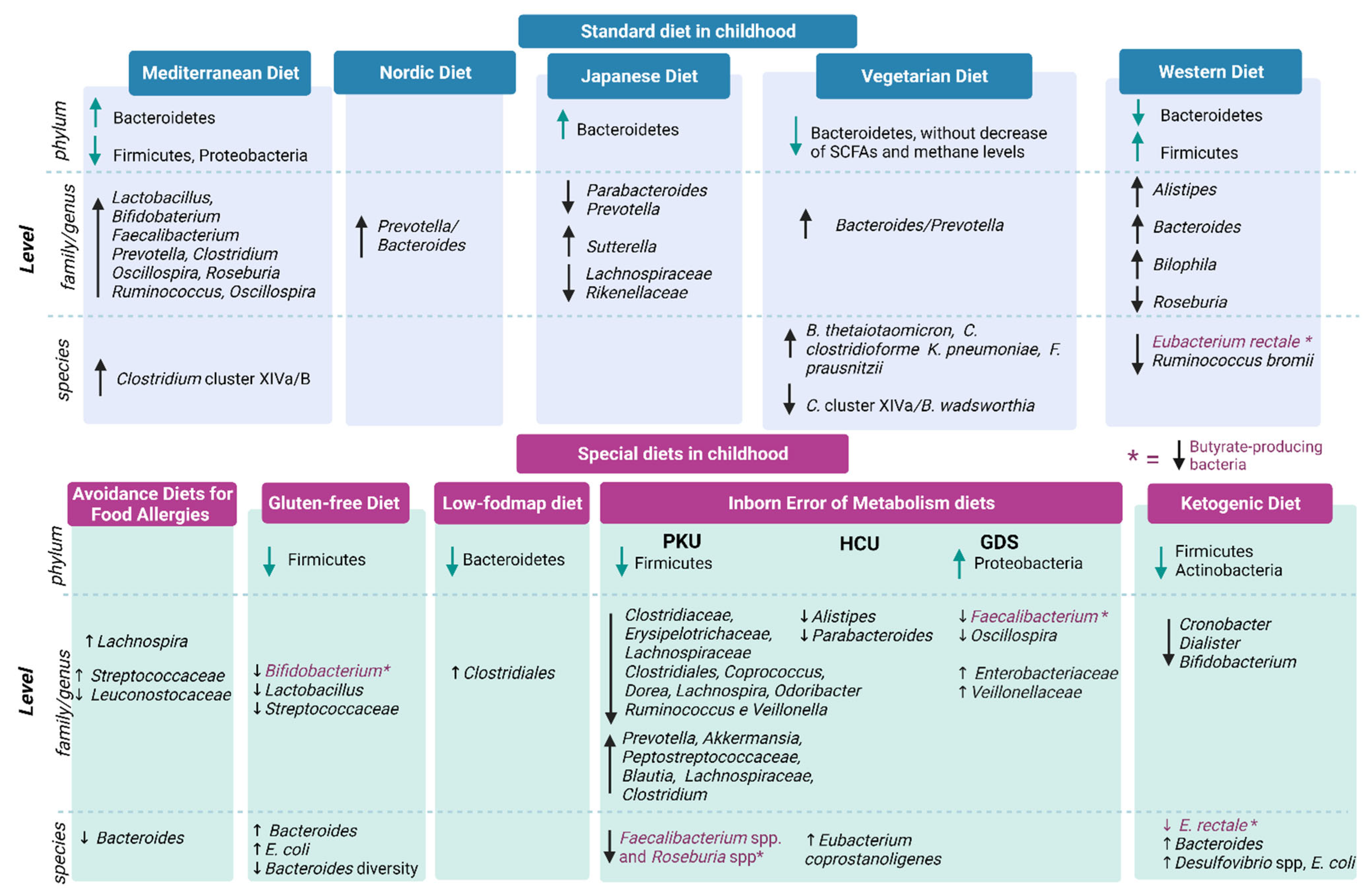
5. Gut Microbiota in Childhood
Standard Diets during Childhood
Mediterranean Diet, Japanese Diet, Nordic Diet and Atlantic Diet
6. Main Special Diets during Childhood
6.1. Avoidance Diets for Food Allergies
6.2. Gluten-Free Diet
6.3. Low-FODMAP Diet
6.4. Inborn Errors of Metabolism (IEMs) Diets
6.5. Ketogenic Diets
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Passos, M.D.C.F.; Moraes-Filho, J.P. Intestinal Microbiota in Digestive Diseases. Arq. Gastroenterol. 2017, 54, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.R.; Guarner, F. Linking the Gut Microbiota to Human Health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Jethwani, P.; Grover, K. Gut Microbiota in Health and Diseases—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1586–1599. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahl, M.I.; Licht, T.R. Settlers of Our Inner Surface—Factors Shaping the Gut Microbiota from Birth to Toddlerhood. FEMS Microbiol. Rev. 2021, 45, fuab001. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [Green Version]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of Intestinal Microbiota during Early Life: A Longitudinal, Explorative Study of a Large Cohort of Danish Infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 1–14. [Google Scholar] [CrossRef]
- Chong, C.; Bloomfield, F.; O’Sullivan, J. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [Green Version]
- Bittinger, K.; Zhao, C.; Li, Y.; Ford, E.; Friedman, E.S.; Ni, J.; Kulkarni, C.V.; Cai, J.; Tian, Y.; Liu, Q.; et al. Bacterial Colonization Reprograms the Neonatal Gut Metabolome. Nat. Microbiol. 2020, 5, 838–847. [Google Scholar] [CrossRef]
- Obermajer, T.; Grabnar, I.; Benedik, E.; Tušar, T.; Pikel, T.R.; Mis, N.F.; Matijašić, B.B.; Rogelj, I. Microbes in Infant Gut Development: Placing Abundance Within Environmental, Clinical and Growth Parameters. Sci. Rep. 2017, 7, 11230. [Google Scholar] [CrossRef] [Green Version]
- Jian, C.; Luukkonen, P.; Yki-Järvinen, H.; Salonen, A.; Korpela, K. Quantitative PCR Provides a Simple and Accessible Method for Quantitative Microbiota Profiling. PLoS ONE 2020, 15, e0227285. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First Foods and Gut Microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization, WHO. Available online: https://www.who.int/health-topics/com-plementary-feeding#tab=tab_2 (accessed on 1 May 2022).
- Simpson, H.L.; Campbell, B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.C.C.; Totten, S.M.; Huang, J.O.; Nagshbandi, S.; Kirmiz, N.; Garrido, D.A.; Lewis, Z.T.; Wu, L.D.; Smilowitz, J.T.; German, J.B.; et al. Identification of Oligosaccharides in Feces of Breast-Fed Infants and Their Correlation with the Gut Microbial Community. Mol. Cell. Proteom. 2016, 15, 2987–3002. [Google Scholar] [CrossRef] [Green Version]
- WHO. Protecting, Promoting, and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: The Revised Baby-Friendly Hospital Initiative 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-Feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Sakanaka, M.; Gotoh, A.; Yoshida, K.; Odamaki, T.; Koguchi, H.; Xiao, J.; Kitaoka, M.; Katayama, T. Varied Pathways of Infant Gut-Associated Bifidobacterium to Assimilate Human Milk Oligosaccharides: Prevalence of the Gene Set and Its Correlation with Bifidobacteria-Rich Microbiota Formation. Nutrients 2019, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-Mediated Immune System Imprinting Early in Life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.-M.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the Gut Microbiome and Risk of Asthma in Childhood. Nat. Commun. 2018, 9, 141. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, Prebiotic, and Brain Development. Nutrients 2017, 9, 1247. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, Z.; Zhang, W. Comparison of the Gut Microbiota in Healthy Infants With Different Delivery Modes and Feeding Types: A Cohort Study. Front. Microbiol. 2022, 13, 868227. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk to Require Antibiotics. mBio 2020, 11, e03196-19. [Google Scholar] [CrossRef] [Green Version]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, K.; Hultman, J.; Satokari, R.; Rautava, S.; Lamendella, R.; Wright, J.; McLimans, C.J.; Kelleher, S.L.; Virta, M. Formula alters preterm infant gut microbiota and increases its antibiotic resistance load. bioRxiv 2019, 782441. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal Microbiome and Metabolome of Infants Fed Bovine MFGM Supplemented Formula or Standard Formula with Breast-Fed Infants as Reference: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 11589. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.; Panasevich, M.R.; Alexander, D.; Vester Boler, B.M.; Rossoni Serao, M.C.; Faber, T.A.; Bauer, L.L.; Fahey, G.C. Fecal Metabolomics of Healthy Breast-Fed versus Formula-Fed Infants before and during In Vitro Batch Culture Fermentation. J. Proteome Res. 2014, 13, 2534–2542. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-Lactic Acid Associated with Bifidobacterium-Dominated Microbiota Significantly Decreases Inflammation in Intestinal Epithelial Cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-Lactic Acid, a Metabolite of Tryptophan, Secreted by Bifidobacterium longum Subspecies Infantis Is Anti-Inflammatory in the Immature Intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 Modulates Enteric Inflammation in Exclusively Breastfed Infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef]
- Shaw, A.G.; Cornwell, E.; Sim, K.; Thrower, H.; Scott, H.; Brown, J.C.S.; Dixon, R.A.; Kroll, J.S. Dynamics of Toxigenic Clostridium perfringens Colonisation in a Cohort of Prematurely Born Neonatal Infants. BMC Pediatr. 2020, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.-A.; van Prehn, J.; Ang, W.; Kneepkens, F.; van der Schoor, S.; de Meij, T. Clostridium difficile Infections in Young Infants: Case Presentations and Literature Review. IDCases 2017, 10, 7–11. [Google Scholar] [CrossRef]
- Saraf, M.K.; Piccolo, B.D.; Bowlin, A.K.; Mercer, K.E.; LeRoith, T.; Chintapalli, S.V.; Shankar, K.; Badger, T.M.; Yeruva, L. Formula Diet Driven Microbiota Shifts Tryptophan Metabolism from Serotonin to Tryptamine in Neonatal Porcine Colon. Microbiome 2017, 5, 77. [Google Scholar] [CrossRef]
- Fernández-Reina, A.; Urdiales, J.; Sánchez-Jiménez, F. What We Know and What We Need to Know about Aromatic and Cationic Biogenic Amines in the Gastrointestinal Tract. Foods 2018, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Isolauri, E.; Rautava, S.; Salminen, S.; Collado, M.C. Early-Life Nutrition and Microbiome Development. In Nestlé Nutrition Institute Workshop Series; Donovan, S.M., German, J.B., Lönnerdal, B., Lucas, A., Eds.; S. Karger AG: Berlin, Germany, 2019; Volume 90, pp. 151–162. ISBN 978-3-318-06340-0. [Google Scholar]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of Complementary Feeding Is Associated with Gut Microbiota Diversity and Composition and Short Chain Fatty Acid Concentrations over the First Year of Life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, C.; Haszard, J.J.; Lawley, B.; Otal, A.; Taylor, R.W.; Szymlek-Gay, E.A.; Fleming, E.A.; Daniels, L.; Fangupo, L.J.; Tannock, G.W.; et al. Mediation Analysis as a Means of Identifying Dietary Components That Differentially Affect the Fecal Microbiota of Infants Weaned by Modified Baby-Led and Traditional Approaches. Appl. Environ. Microbiol. 2018, 84, e00914-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the Human Infant Intestinal Microbiota after the Introduction of First Complementary Foods in Infant Samples from Five European Centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, M.F.; Andersen, L.B.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity. mSphere 2016, 1, e00069-15. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Frank, D.; Hendricks, A.; Ir, D.; Krebs, N. Protein Intake During Early Complementary Feeding Affects the Gut Microbiota in U.S. Formula-Fed Infants (FS04-03-19). Curr. Dev. Nutr. 2019, 3, nzz048.FS04-03-19. [Google Scholar] [CrossRef]
- Sicherer, S.H. Epidemiology of Food Allergy. J. Allergy Clin. Immunol. 2011, 127, 594–602. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and Management of Food Allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- von Berg, A. The Role of Hydrolysates for Allergy Prevention—Pro. Pediatr. Allergy Immunol. 2013, 24, 720–723. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef] [Green Version]
- Canani, R.B.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef]
- Katz, Y.; Rajuan, N.; Goldberg, M.R.; Eisenberg, E.; Heyman, E.; Cohen, A.; Leshno, M. Early Exposure to Cow’s Milk Protein Is Protective against IgE-Mediated Cow’s Milk Protein Allergy. J. Allergy Clin. Immunol. 2010, 126, 77–82.e1. [Google Scholar] [CrossRef]
- Paparo, L.; Picariello, G.; Bruno, C.; Pisapia, L.; Canale, V.; Sarracino, A.; Nocerino, R.; Carucci, L.; Cosenza, L.; Cozzolino, T.; et al. Tolerogenic Effect Elicited by Protein Fraction Derived From Different Formulas for Dietary Treatment of Cow’s Milk Allergy in Human Cells. Front. Immunol. 2020, 11, 604075. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal Bacteria Protect against Food Allergen Sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [Green Version]
- Canani, R.B.; Gilbert, J.A.; Nagler, C.R. The Role of the Commensal Microbiota in the Regulation of Tolerance to Dietary Allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Di Costanzo, M.; Buono, A.; Bruno, C.; Canani, R.B. Targeting Food Allergy with Probiotics. Probiotics Child Gastrointest. Health 2019, 1125, 57–68. [Google Scholar] [CrossRef]
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Nocerino, R.; Bedogni, G.; Carucci, L.; Cosenza, L.; Cozzolino, T.; Paparo, L.; Palazzo, S.; Riva, L.; Verduci, E.; Canani, R.B. The Impact of Formula Choice for the Management of Pediatric Cow’s Milk Allergy on the Occurrence of Other Allergic Manifestations: The Atopic March Cohort Study. J. Pediatr. 2021, 232, 183–191.e3. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Lynch, S.V. Microbiota in Allergy and Asthma and the Emerging Relationship with the Gut Microbiome. Cell Host Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Perezabad, L.; López-Abente, J.; Alonso-Lebrero, E.; Seoane, E.; Pion, M.; Correa-Rocha, R. The Establishment of Cow’s Milk Protein Allergy in Infants Is Related with a Deficit of Regulatory T Cells (Treg) and Vitamin D. Pediatr. Res. 2017, 81, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreffler, W.G.; Wanich, N.; Moloney, M.; Nowak-Wegrzyn, A.; Sampson, H.A. Association of Allergen-Specific Regulatory T Cells with the Onset of Clinical Tolerance to Milk Protein. J. Allergy Clin. Immunol. 2009, 123, 43–52.e7. [Google Scholar] [CrossRef] [PubMed]
- Ruohtula, T.; de Goffau, M.C.; Nieminen, J.K.; Honkanen, J.; Siljander, H.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Ilonen, J.; Niemelä, O.; et al. Maturation of Gut Microbiota and Circulating Regulatory T Cells and Development of IgE Sensitization in Early Life. Front. Immunol. 2019, 10, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Brabec, B.; Chichlowski, M.; Harris, C.L.; Moore, N.; Wampler, J.L.; Vanderhoof, J.; Rose, D.; Hutkins, R. Stool Microbiome, PH and Short/Branched Chain Fatty Acids in Infants Receiving Extensively Hydrolyzed Formula, Amino Acid Formula, or Human Milk through Two Months of Age. BMC Microbiol. 2020, 20, 337. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by Non-IgE-Mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Wegrzyn, A.; Czerkies, L.; Reyes, K.; Collins, B.; Heine, R.G. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients 2019, 11, 1447. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Żołnowska, M.; Canani, R.B.; Ludman, S.; Tengelyi, Z.; Moreno-Álvarez, A.; Goh, A.E.N.; Gosoniu, M.L.; Kirwan, B.-A.; Tadi, M.; et al. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow’s Milk Protein Allergy: A Randomized, Multi-Center Trial. Nutrients 2022, 14, 530. [Google Scholar] [CrossRef]
- Burks, A.W.; Harthoorn, L.F.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B.; et al. Synbiotics-Supplemented Amino Acid-Based Formula Supports Adequate Growth in Cow’s Milk Allergic Infants. Pediatr. Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Candy, D.C.A.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A Synbiotic-Containing Amino-Acid-Based Formula Improves Gut Microbiota in Non-IgE-Mediated Allergic Infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Hilvo, M. Maternal Elimination Diet and Symptoms of Cow’s Milk Allergy in Breastfed Infants. JAMA Pediatr. 2021, 175, 425–426. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A Workshop Report on the Development of the Cow’s Milk-Related Symptom Score Awareness Tool for Young Children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D. Althera Study Group Treating Cow’s Milk Protein Allergy: A Double-Blind Randomized Trial Comparing Two Extensively Hydrolysed Formulas with Probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef]
- Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease-Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Salvatore, S. Infant Formula with Partially Hydrolyzed Proteins in Functional Gastrointestinal Disorders. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, C.; Bradatan, E.; Soulaines, P.; Nocerino, R.; Berni-Canani, R. Tolerance and Growth in Children with Cow’s Milk Allergy Fed a Thickened Extensively Hydrolyzed Casein-Based Formula. BMC Pediatr. 2016, 16, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; De Greef, E.; Hauser, B. Paradice Study Group Safety and Tolerance of a New Extensively Hydrolyzed Rice Protein-Based Formula in the Management of Infants with Cow’s Milk Protein Allergy. Eur. J. Pediatr. 2014, 173, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.; Biagioli, E.; Sorrenti, M.; Lingua, C.; Moja, L.; Banks, S.S.; Ceratto, S.; Savino, F. Dietary Modifications for Infantile Colic. Cochrane Database Syst. Rev. 2018, 10, CD011029. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Benninga, M.; Broekaert, I.; Falconer, J.; Gottrand, F.; Guarino, A.; Lifschitz, C.; Lionetti, P.; Orel, R.; Papadopoulou, A.; et al. Functional Gastro-Intestinal Disorder Algorithms Focus on Early Recognition, Parental Reassurance and Nutritional Strategies. Acta Paediatr. 2016, 105, 244–252. [Google Scholar] [CrossRef]
- Daelemans, S.; Peeters, L.; Hauser, B.; Vandenplas, Y. Recent Advances in Understanding and Managing Infantile Colic. F1000Research 2018, 7, F1000 Faculty Rev-1426. [Google Scholar] [CrossRef] [Green Version]
- Xinias, I.; Analitis, A.; Mavroudi, A.; Roilides, I.; Lykogeorgou, M.; Delivoria, V.; Milingos, V.; Mylonopoulou, M.; Vandenplas, Y. Innovative Dietary Intervention Answers to Baby Colic. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 100–106. [Google Scholar] [CrossRef] [Green Version]
- de Weerth, C.; Fuentes, S.; de Vos, W.M. Crying in Infants: On the Possible Role of Intestinal Microbiota in the Development of Colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Pärtty, A.; Kalliomäki, M.; Endo, A.; Salminen, S.; Isolauri, E. Compositional Development of Bifidobacterium and Lactobacillus Microbiota Is Linked with Crying and Fussing in Early Infancy. PLoS ONE 2012, 7, e32495. [Google Scholar] [CrossRef] [Green Version]
- Savino, F.; Cordisco, L.; Tarasco, V.; Calabrese, R.; Palumeri, E.; Matteuzzi, D. Molecular Identification of Coliform Bacteria from Colicky Breastfed Infants. Acta Paediatr. 2009, 98, 1582–1588. [Google Scholar] [CrossRef]
- Hall, B.; Chesters, J.; Robinson, A. Infantile Colic: A Systematic Review of Medical and Conventional Therapies. J. Paediatr. Child Health 2012, 48, 128–137. [Google Scholar] [CrossRef]
- Iacovou, M.; Ralston, R.A.; Muir, J.; Walker, K.Z.; Truby, H. Dietary Management of Infantile Colic: A Systematic Review. Matern. Child Health J. 2012, 16, 1319–1331. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal Diet Alters Fecal Microbiota and Metabolome Profiles at Different Ages in Infants Fed Breast Milk or Formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Baumann-Dudenhoeffer, A.M.; D’Souza, A.W.; Tarr, P.I.; Warner, B.B.; Dantas, G. Infant Diet and Maternal Gestational Weight Gain Predict Early Metabolic Maturation of Gut Microbiomes. Nat. Med. 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- MacDonald, A.; Cochrane, B.; Wopereis, H.; Loveridge, N. Specific Prebiotics in a Formula for Infants with Phenylketonuria. Mol. Genet. Metab. 2011, 104, S55–S59. [Google Scholar] [CrossRef]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut Microbiome-Mediterranean Diet Interactions in Improving Host Health. F1000Research 2019, 8, 699. [Google Scholar] [CrossRef] [Green Version]
- La Fauci, V.; Alessi, V.; Assefa, D. Mediterranean Diet: Knowledge and Adherence in Italian Young People. Clin. Ter. 2020, 171, e437–e443. [Google Scholar] [CrossRef]
- Pereira-da-Silva, L.; Rêgo, C.; Pietrobelli, A. The Diet of Preschool Children in the Mediterranean Countries of the European Union: A Systematic Review. Int. J. Environ. Res. Public. Health 2016, 13, 572. [Google Scholar] [CrossRef] [Green Version]
- Pignanelli, M.; Just, C.; Bogiatzi, C.; Dinculescu, V.; Gloor, G.B.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; et al. Mediterranean Diet Score: Associations with Metabolic Products of the Intestinal Microbiome, Carotid Plaque Burden, and Renal Function. Nutrients 2018, 10, 779. [Google Scholar] [CrossRef] [Green Version]
- Krznarić, Ž.; Karas, I.; Ljubas Kelečić, D.; Vranešić Bender, D. The Mediterranean and Nordic Diet: A Review of Differences and Similarities of Two Sustainable, Health-Promoting Dietary Patterns. Front. Nutr. 2021, 8, 683678. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Hanhineva, K. Biomarkers of a Healthy Nordic Diet—From Dietary Exposure Biomarkers to Microbiota Signatures in the Metabolome. Nutrients 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, Y.; Kusumoto, K.-I.; Oguma, T.; Nagai, T.; Furukawa, S.; Suzuki, C.; Satomi, M.; Magariyama, Y.; Takamine, K.; Tamaki, H. Ethnic Fermented Foods and Alcoholic Beverages of Japan. In Ethnic Fermented Foods and Alcoholic Beverages of Asia; Tamang, J.P., Ed.; Springer: New Delhi, India, 2016; pp. 193–236. ISBN 978-81-322-2800-4. [Google Scholar]
- Kushida, M.; Sugawara, S.; Asano, M.; Yamamoto, K.; Fukuda, S.; Tsuduki, T. Effects of the 1975 Japanese Diet on the Gut Microbiota in Younger Adults. J. Nutr. Biochem. 2019, 64, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Pérez, C.; Sánchez-Bao, A.; Bellido-Guerrero, D.; Casanueva, F.F. The Southern European Atlantic Diet. Minerva Endocrinol. 2021, 46, 145–160. [Google Scholar] [CrossRef]
- Vaz-Velho, M.; Pinheiro, R.; Rodrigues, A. The Atlantic Diet—Origin and Features. Int. J. Food Stud. 2016, 5, 106–119. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Izquierdo, A.G.; Rodriguez-Carnero, G.; Fernández-Pombo, A.; Iglesias, A.; Carreira, M.C.; Tejera, C.; Bellido, D.; Martinez-Olmos, M.A.; Leis, R.; et al. Epigenetic Effects of Healthy Foods and Lifestyle Habits from the Southern European Atlantic Diet Pattern: A Narrative Review. Adv. Nutr. 2022, 00, 1–23. [Google Scholar] [CrossRef]
- Association of Dietary Type with Fecal Microbiota in Vegetarians and Omnivores in Slovenia. Available online: https://pubmed.ncbi.nlm.nih.gov/24173964/ (accessed on 5 July 2022).
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Ruengsomwong, S.; La-Ongkham, O.; Jiang, J.; Wannissorn, B.; Nakayama, J.; Nitisinprasert, S. Microbial Community of Healthy Thai Vegetarians and Non-Vegetarians, Their Core Gut Microbiota, and Pathogen Risk. J. Microbiol. Biotechnol. 2016, 26, 1723–1735. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A Vegan or Vegetarian Diet Substantially Alters the Human Colonic Faecal Microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Hojsak, I.; Benninga, M.A.; Hauser, B.; Kansu, A.; Kelly, V.B.; Stephen, A.M.; Morais Lopez, A.; Slavin, J.; Tuohy, K. Benefits of Dietary Fibre for Children in Health and Disease. Arch. Dis. Child. 2022, 0, 1–7. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [Green Version]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Available online: https://pubmed.ncbi.nlm.nih.gov/33642988/ (accessed on 22 June 2022).
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.-A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Ni, C.; et al. A Phenotypically and Functionally Distinct Human TH2 Cell Subpopulation Is Associated with Allergic Disorders. Sci. Transl. Med. 2017, 9, eaam9171. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on Allergen Immunotherapy: IgE-Mediated Food Allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [Green Version]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and Cellular Mechanisms of Food Allergy and Food Tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Canani, R.B. Specific Gut Microbiome Signatures and the Associated Pro-Inflamatory Functions Are Linked to Pediatric Allergy and Acquisition of Immune Tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial Signature in IgE-Mediated Food Allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Elizur, A.; Rajuan, N.; Goldberg, M.R.; Leshno, M.; Cohen, A.; Katz, Y. Natural Course and Risk Factors for Persistence of IgE-Mediated Cow’s Milk Allergy. J. Pediatr. 2012, 161, 482–487.e1. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Goedert, J.J.; Pu, A.; Yu, G.; Shi, J. Allergy Associations with the Adult Fecal Microbiota: Analysis of the American Gut Project. EBioMedicine 2016, 3, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Odamaki, T.; Xiao, J.-Z.; Sakamoto, M.; Kondo, S.; Yaeshima, T.; Iwatsuki, K.; Togashi, H.; Enomoto, T.; Benno, Y. Distribution of Different Species of the Bacteroides fragilis Group in Individuals with Japanese Cedar Pollinosis. Appl. Environ. Microbiol. 2008, 74, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-Life Gut Microbiome Composition and Milk Allergy Resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for Cow’s Milk Protein Allergy: A Systematic Review of Randomized Controlled Trials. Eur. J. Pediatr. 2019, 178, 1139–1149. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Wang, J. Current Understanding of Egg Allergy. Pediatr. Clin. N. Am. 2011, 58, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-Life Gut Microbiome and Egg Allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef]
- Gallegos, C.; Merkel, R. Current Evidence in the Diagnosis and Treatment of Children with Celiac Disease. Gastroenterol. Nurs. 2019, 42, 41–48. [Google Scholar] [CrossRef]
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Nadal, I.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the Composition of the Duodenal Microbiota of Children with Coeliac Disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal Bacteroides Species Associated with Coeliac Disease. J. Clin. Pathol. 2010, 63, 1105–1111. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and Faecal Microbiota of Celiac Children: Molecular, Phenotype and Metabolome Characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalances in Faecal and Duodenal Bifidobacterium Species Composition in Active and Non-Active Coeliac Disease. BMC Microbiol. 2008, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a Gluten-Free Diet on Gut Microbiota and Immune Function in Healthy Adult Human Subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Golfetto, L.; de Senna, F.D.; Hermes, J.; Beserra, B.T.S.; França, F.D.S.; Martinello, F. Lower Bifidobacteria Counts in Adult Patients with Celiac Disease on a Gluten-Free Diet. Arq. Gastroenterol. 2014, 51, 139–143. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel Players in Coeliac Disease Pathogenesis: Role of the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; Vos, W.M. de Global and Deep Molecular Analysis of Microbiota Signatures in Fecal Samples From Patients With Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An Irritable Bowel Syndrome Subtype Defined by Species-Specific Alterations in Faecal Microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Naseri, K.; Dabiri, H.; Rostami-Nejad, M.; Yadegar, A.; Houri, H.; Olfatifar, M.; Sadeghi, A.; Saadati, S.; Ciacci, C.; Iovino, P.; et al. Influence of Low FODMAP-Gluten Free Diet on Gut Microbiota Alterations and Symptom Severity in Iranian Patients with Irritable Bowel Syndrome. BMC Gastroenterol. 2021, 21, 292. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet Compared with Traditional Dietary Advice for Diarrhea-Predominant Irritable Bowel Syndrome: A Parallel-Group, Randomized Controlled Trial with Analysis of Clinical and Microbiological Factors Associated with Patient Outcomes. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef]
- Eetemadi, A.; Tagkopoulos, I. Methane and Fatty Acid Metabolism Pathways Are Predictive of Low-FODMAP Diet Efficacy for Patients with Irritable Bowel Syndrome. Clin. Nutr. Edinb. Scotl. 2021, 40, 4414–4421. [Google Scholar] [CrossRef]
- Valdez-Palomares, F.; Nambo-Venegas, R.; Uribe-García, J.; Mendoza-Vargas, A.; Granados-Portillo, O.; Meraz-Cruz, N.; Palacios-González, B. Intestinal Microbiota Fingerprint in Subjects with Irritable Bowel Syndrome Responders to a Low FODMAP Diet. Food Funct. 2021, 12, 3206–3218. [Google Scholar] [CrossRef]
- Thomassen, R.A.; Luque, V.; Assa, A.; Borrelli, O.; Broekaert, I.; Dolinsek, J.; Martin-de-Carpi, J.; Mas, E.; Miele, E.; Norsa, L.; et al. An ESPGHAN Position Paper on the Use of Low-FODMAP Diet in Pediatric Gastroenterology. J. Pediatr. Gastroenterol. Nutr. 2022. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Hollister, E.B.; Oezguen, N.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Savidge, T.C.; Versalovic, J.; Shulman, R.J. Gut Microbiota Influences Low Fermentable Substrate Diet Efficacy in Children with Irritable Bowel Syndrome. Gut Microbes 2014, 5, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised Clinical Trial: Gut Microbiome Biomarkers Are Associated with Clinical Response to a Low FODMAP Diet in Children with the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Alfadhel, M.; Benmeakel, M.; Hossain, M.A.; Al Mutairi, F.; Al Othaim, A.; Alfares, A.A.; Al Balwi, M.; Alzaben, A.; Eyaid, W. Thirteen Year Retrospective Review of the Spectrum of Inborn Errors of Metabolism Presenting in a Tertiary Center in Saudi Arabia. Orphanet J. Rare Dis. 2016, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, E.R.B. Metabolite Flux: A Dynamic Concept for Inherited Metabolic Disorders as Complex Traits. Mol. Genet. Metab. 2019, 128, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lanpher, B.; Brunetti-Pierri, N.; Lee, B. Inborn Errors of Metabolism: The Flux from Mendelian to Complex Diseases. Nat. Rev. Genet. 2006, 7, 449–459. [Google Scholar] [CrossRef]
- Putignani, L.; Del Chierico, F.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The Human Gut Microbiota: A Dynamic Interplay with the Host from Birth to Senescence Settled during Childhood. Pediatr. Res. 2014, 76, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Colonetti, K.; Roesch, L.F.; Schwartz, I.V.D. The Microbiome and Inborn Errors of Metabolism: Why We Should Look Carefully at Their Interplay? Genet. Mol. Biol. 2018, 41, 515–532. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Montanari, C.; Parolisi, S.; Borghi, E.; Putignani, L.; Bassanini, G.; Zuvadelli, J.; Bonfanti, C.; Tummolo, A.; Dionisi Vici, C.; Biasucci, G.; et al. Dysbiosis, Host Metabolism, and Non-Communicable Diseases: Trialogue in the Inborn Errors of Metabolism. Front. Physiol. 2021, 12, 716520. [Google Scholar] [CrossRef]
- MacDonald, A.; van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU Dietary Handbook to Accompany PKU Guidelines. Orphanet J. Rare Dis. 2020, 15, 171. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primer 2021, 7, 36. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European Guidelines for the Diagnosis and Management of Patients with Phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, M.; Verduci, E.; Salvatici, E.; Paci, S.; Riva, E. Phenylketonuria: Nutritional Advances and Challenges. Nutr. Metab. 2012, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Verduci, E.; Moretti, F.; Bassanini, G.; Banderali, G.; Rovelli, V.; Casiraghi, M.C.; Morace, G.; Borgo, F.; Borghi, E. Phenylketonuric Diet Negatively Impacts on Butyrate Production. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 385–392. [Google Scholar] [CrossRef]
- Moretti, F.; Pellegrini, N.; Salvatici, E.; Rovelli, V.; Banderali, G.; Radaelli, G.; Scazzina, F.; Giovannini, M.; Verduci, E. Dietary Glycemic Index, Glycemic Load and Metabolic Profile in Children with Phenylketonuria. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 176–182. [Google Scholar] [CrossRef]
- Pinheiro de Oliveira, F.; Mendes, R.H.; Dobbler, P.T.; Mai, V.; Pylro, V.S.; Waugh, S.G.; Vairo, F.; Refosco, L.F.; Roesch, L.F.W.; Schwartz, I.V.D. Phenylketonuria and Gut Microbiota: A Controlled Study Based on Next-Generation Sequencing. PLoS ONE 2016, 11, e0157513. [Google Scholar] [CrossRef] [Green Version]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Veeriah, S.; Will, F.; Dietrich, H.; Pool-Zobel, B.L.; Schrenk, D. Histone-Deacetylase Inhibition and Butyrate Formation: Fecal Slurry Incubations with Apple Pectin and Apple Juice Extracts. Nutrition 2008, 24, 366–374. [Google Scholar] [CrossRef]
- Bassanini, G.; Ceccarani, C.; Borgo, F.; Severgnini, M.; Rovelli, V.; Morace, G.; Verduci, E.; Borghi, E. Phenylketonuria Diet Promotes Shifts in Firmicutes Populations. Front. Cell. Infect. Microbiol. 2019, 9, 101. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Chen, T.; Li, C.; Fu, X.; Huang, Q. Chemical Cross-Linking Controls in Vitro Fecal Fermentation Rate of High-Amylose Maize Starches and Regulates Gut Microbiota Composition. J. Agric. Food Chem. 2019, 67, 13728–13736. [Google Scholar] [CrossRef]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Re-Print of Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A Review of the Relationship between the Gut Microbiota and Amino Acid Metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, S.F. Physiological Effects of Dietary Amino Acids on Gut Health and Functions of Swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The Complete European Guidelines on Phenylketonuria: Diagnosis and Treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide Is a Prebiotic That Reduces Desulfovibrio Bacteria, Increases Cecal Short-Chain Fatty Acids, and Is Anti-Inflammatory in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef] [Green Version]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Dei Cas, M.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Rizowy, G.M.; Poloni, S.; Colonetti, K.; Donis, K.C.; Dobbler, P.T.; Leistner-Segal, S.; Roesch, L.F.W.; Schwartz, I.V.D. Is the Gut Microbiota Dysbiotic in Patients with Classical Homocystinuria? Biochimie 2020, 173, 3–11. [Google Scholar] [CrossRef]
- Heller, S.; Worona, L.; Consuelo, A. Nutritional Therapy for Glycogen Storage Diseases. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S15–S21. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Austin, S.L.; Abdenur, J.E.; Arn, P.; Bali, D.S.; Boney, A.; Chung, W.K.; Dagli, A.I.; Dale, D.; Koeberl, D.; et al. Diagnosis and Management of Glycogen Storage Disease Type I: A Practice Guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2014, 16, e1–e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarani, C.; Bassanini, G.; Montanari, C.; Casiraghi, M.C.; Ottaviano, E.; Morace, G.; Biasucci, G.; Paci, S.; Borghi, E.; Verduci, E. Proteobacteria Overgrowth and Butyrate-Producing Taxa Depletion in the Gut Microbiota of Glycogen Storage Disease Type 1 Patients. Metabolites 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colonetti, K.; Bento dos Santos, B.; Nalin, T.; Moura de Souza, C.F.; Triplett, E.W.; Dobbler, P.T.; Schwartz, I.V.D.; Roesch, L.F.W. Hepatic Glycogen Storage Diseases Are Associated to Microbial Dysbiosis. PLoS ONE 2019, 14, e0214582. [Google Scholar] [CrossRef]
- Gertsman, I.; Gangoiti, J.A.; Nyhan, W.L.; Barshop, B.A. Perturbations of Tyrosine Metabolism Promote the Indolepyruvate Pathway via Tryptophan in Host and Microbiome. Mol. Genet. Metab. 2015, 114, 431–437. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic Diet for Human Diseases: The Underlying Mechanisms and Potential for Clinical Implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the Ketogenic Diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef]
- Laux, L.; Blackford, R. The Ketogenic Diet in Dravet Syndrome. J. Child Neurol. 2013, 28, 1041–1044. [Google Scholar] [CrossRef]
- Kelley, S.A.; Kossoff, E.H. Doose Syndrome (Myoclonic-Astatic Epilepsy): 40 Years of Progress: Doose Syndrome. Dev. Med. Child Neurol. 2010, 52, 988–993. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic Diet Poses a Significant Effect on Imbalanced Gut Microbiota in Infants with Refractory Epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef]
- Pittman, Q.J. A Gut Feeling about the Ketogenic Diet in Epilepsy. Epilepsy Res. 2020, 166, 106409. [Google Scholar] [CrossRef]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-Term Impact of a Classical Ketogenic Diet on Gut Microbiota in GLUT1 Deficiency Syndrome: A 3-Month Prospective Observational Study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered Gut Microbiome Composition in Children with Refractory Epilepsy after Ketogenic Diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The Ketogenic Diet Influences Taxonomic and Functional Composition of the Gut Microbiota in Children with Severe Epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; Liu, X.; Zhang, J.; Liu, G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediat. Inflamm. 2019, 2019, 8373060. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in Mediating Very Low-Calorie Ketogenic Diet-Infant Gut Microbiota Relationships and Its Therapeutic Potential in Obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in the Interplay between a Very Low-Calorie Ketogenic Diet and the Infant Gut Microbiota and Its Therapeutic Implications for Reducing Asthma. Int. J. Mol. Sci. 2020, 21, 9580. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’Auria, E.; et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients 2022, 14, 3198. https://doi.org/10.3390/nu14153198
Di Profio E, Magenes VC, Fiore G, Agostinelli M, La Mendola A, Acunzo M, Francavilla R, Indrio F, Bosetti A, D’Auria E, et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients. 2022; 14(15):3198. https://doi.org/10.3390/nu14153198
Chicago/Turabian StyleDi Profio, Elisabetta, Vittoria Carlotta Magenes, Giulia Fiore, Marta Agostinelli, Alice La Mendola, Miriam Acunzo, Ruggiero Francavilla, Flavia Indrio, Alessandra Bosetti, Enza D’Auria, and et al. 2022. "Special Diets in Infants and Children and Impact on Gut Microbioma" Nutrients 14, no. 15: 3198. https://doi.org/10.3390/nu14153198
APA StyleDi Profio, E., Magenes, V. C., Fiore, G., Agostinelli, M., La Mendola, A., Acunzo, M., Francavilla, R., Indrio, F., Bosetti, A., D’Auria, E., Borghi, E., Zuccotti, G., & Verduci, E. (2022). Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients, 14(15), 3198. https://doi.org/10.3390/nu14153198










