Predicting Sensitivity to Adverse Lifestyle Risk Factors for Cardiometabolic Morbidity and Mortality
Abstract
:1. Introduction
2. Materials and Methods
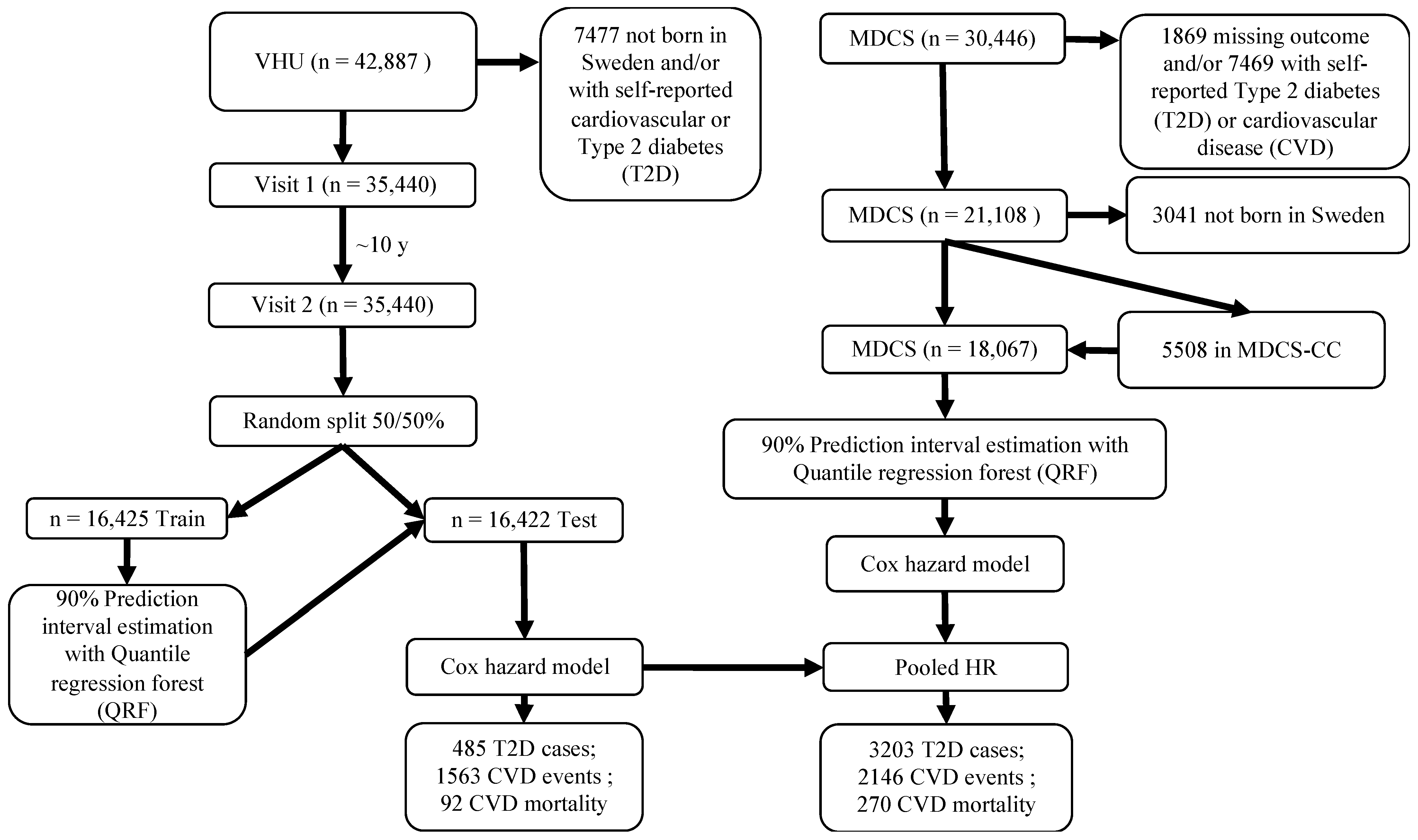
2.1. Study Design and Participants
2.2. MDCS
2.3. Cardiometabolic Risk Markers
2.4. Lifestyle and Dietary Assessments
2.5. Outcome Ascertainment
2.6. Statistical Analysis
2.7. Predictive Performance
2.8. Time-to-Event Analysis
3. Results
3.1. Cardiovascular Events
3.2. T2D Incidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.-F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R. Precision medicine in diabetes: A Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Siegel, K.R.; Ng, B.P.; Jawanda, S.; Proia, K.K.; Zhang, X.; Albright, A.L.; Zhang, P. Cost-effectiveness of diabetes prevention interventions targeting high-risk individuals and whole populations: A systematic review. Diabetes Care 2020, 43, 1593–1616. [Google Scholar] [CrossRef]
- Henley, S.S.; Golden, R.M.; Kashner, T.M. Statistical modeling methods: Challenges and strategies. Biostat. Epidemiol. 2020, 4, 105–139. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.W.; Poveda, A. Lifestyle and precision diabetes medicine: Will genomics help optimise the prediction, prevention and treatment of type 2 diabetes through lifestyle therapy? Diabetologia 2017, 60, 784–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mone, P.; Gambardella, J.; Lombardi, A.; Pansini, A.; De Gennaro, S.; Leo, A.L.; Famiglietti, M.; Marro, A.; Morgante, M.; Frullone, S.; et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc. Diabetol. 2022, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, N. Quantile regression forests. J. Mach. Learn. Res. 2006, 7, 983–999. [Google Scholar]
- Hallmans, G.; Agren, A.; Johansson, G.; Johansson, A.; Stegmayr, B.; Jansson, J.H.; Lindahl, B.; Rolandsson, O.; Soderberg, S.; Nilsson, M.; et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort-evaluation of risk factors and their interactions. Scand. J. Public Health Suppl. 2003, 61, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Vasterbotten Intervention Programme: Background, design and implications. Glob. Health Action 2010, 3, 4643. [Google Scholar] [CrossRef]
- Manjer, J.; Carlsson, S.; Elmstahl, S.; Gullberg, B.; Janzon, L.; Lindstrom, M.; Mattisson, I.; Berglund, G. The Malmo Diet and Cancer Study: Representativity, cancer incidence and mortality in participants and non-participants. Eur. J. Cancer Prev. 2001, 10, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, G.; Elmstahl, S.; Janzon, L.; Larsson, S.A. The Malmo Diet and Cancer Study. Design and feasibility. J. Intern. Med. 1993, 233, 45–51. [Google Scholar] [CrossRef]
- Manjer, J.; Elmståhl, S.; Janzon, L.; Berglund, G. Invitation to a population-based cohort study: Differences between subjects recruited using various strategies. Scand. J. Public Health 2002, 30, 103–112. [Google Scholar] [CrossRef]
- Hedblad, B.; Nilsson, P.; Janzon, L.; Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet. Med. 2000, 17, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Carlberg, B.; Weinehall, L.; Norberg, M. Trends of blood pressure levels and management in Vasterbotten County, Sweden, during 1990–2010. Glob. Health Action 2012, 5, 18195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Province, M.A.; Coon, H.; Hunt, S.C.; Eckfeldt, J.H.; Arnett, D.K.; Heiss, G.; Lewis, C.E.; Ellison, R.C.; Rao, D.C.; et al. An investigation of the effects of lipid-lowering medications: Genome-wide linkage analysis of lipids in the HyperGEN study. BMC Genet. 2007, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.D.; Sheehan, N.A.; Scurrah, K.J.; Burton, P.R. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat. Med. 2005, 24, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Johansson, I.; Hallmans, G.; Wikman, A.; Biessy, C.; Riboli, E.; Kaaks, R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002, 5, 487–496. [Google Scholar] [CrossRef]
- Winkvist, A.; Hornell, A.; Hallmans, G.; Lindahl, B.; Weinehall, L.; Johansson, I. More distinct food intake patterns among women than men in northern Sweden: A population-based survey. Nutr. J. 2009, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, C.J.; Bhattacharya, J.; Butte, A.J. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS ONE 2010, 5, e10746. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Pomares-Millan, H.; Chen, Y.; Kurbasic, A.; Patel, C.J.; Renstrom, F.; Hallmans, G.; Johansson, I.; Franks, P.W. Exposome-wide ranking of modifiable risk factors for cardiometabolic disease traits. Sci. Rep. 2022, 12, 4088. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: Berlin/Heidelberg, Germany, 2013; Volume 26. [Google Scholar]
- Zhang, Z. Missing data exploration: Highlighting graphical presentation of missing pattern. Ann. Transl. Med. 2015, 3, 356. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Buhlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’donnell, C.J. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino Sr, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norberg, M.; Lundqvist, G.; Nilsson, M.; Gilljam, H.; Weinehall, L. Changing patterns of tobacco use in a middle-aged population: The role of snus, gender, age, and education. Glob. Health Action 2011, 4, 5613. [Google Scholar] [CrossRef] [PubMed]
- Padyab, M.; Blomstedt, Y.; Norberg, M. No association found between cardiovascular mortality, and job demands and decision latitude: Experience from the Vasterbotten Intervention Programme in Sweden. Soc. Sci. Med. 2014, 117, 58–66. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Borenstein, M.; Higgins, J.P. Meta-analysis and subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Volume 888, Available online: https://www.R-project.org/ (accessed on 16 February 2022).
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J.; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, S.; Cai, X.; Mai, W.; Hu, Y.; Tang, H.; Xu, D. Prehypertension and incidence of cardiovascular disease: A meta-analysis. BMC Med. 2013, 11, 177. [Google Scholar] [CrossRef] [Green Version]
- Gewandter, J.S.; McDermott, M.P.; He, H.; Gao, S.; Cai, X.; Farrar, J.T.; Katz, N.P.; Markman, J.D.; Senn, S.; Turk, D.C. Demonstrating heterogeneity of treatment effects among patients: An overlooked but important step toward precision medicine. Clin. Pharmacol. Ther. 2019, 106, 204–210. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S211–S220. [Google Scholar] [CrossRef]
- Bruins, M.J.; Van Dael, P.; Eggersdorfer, M. The Role of Nutrients in Reducing the Risk for Noncommunicable Diseases during Aging. Nutrients 2019, 11, 85. [Google Scholar] [CrossRef]
- Braunwald, E. SGLT2 inhibitors: The statins of the 21st century. Eur. Heart J. 2022, 43, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef] [PubMed]

| VHU | MDCS | |
|---|---|---|
| n | 35,440 | 18,067 |
| Male (%) | 15,599 (46.8) | 6772 (37.5) |
| Age | 42.96 (7.02) | 57.72 (7.71) |
| BMI (kg/m2) | 25.10 (3.71) | 25.30 (3.78) |
| Total cholesterol (mmol/L) | 5.47 (1.14) | 6.20 (1.11) |
| HDL-C (mmol/L) | 1.32 (0.57) | 1.40 (0.37) |
| LDL-C (mmol/L) | 3.92 (1.16) | 4.19 (1.02) |
| Triglycerides (mmol/L) | 1.32 (0.76) | 1.47 (0.75) |
| Fasting glucose (mmol/L) | 5.31 (0.63) | 5.02 (0.83) |
| 2 h glucose (mmol/L) | 6.39 (1.30) | - |
| HbA1c (mmol/mol) a | - | 31.4 (5.05) |
| Systolic blood pressure (mm Hg) | 123.27 (15.77) | 138.58 (18.97) |
| Diastolic blood pressure (mm Hg) | 77.25 (10.86) | 84.02 (9.53) |
| CVD | Test between Groups ª | T2D | Test between Groups ª | CVD Mortality | Test between Groups ª | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | HR | 95% (CIs) | Q | p | HR | 95% (CIs) | Q | p | HR | 95% (CIs) | Q | p | |||
| Fasting glucose | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 0.77 | 0.31 | 1.90 | 0.25 | 0.62 | 0.73 | 0.46 | 1.16 | 0.75 | 0.39 | 1.04 | 0.61 | 1.75 | 0.12 | 0.73 |
| Pooled sensitive | 1.01 | 0.55 | 1.86 | 1.69 | 0.26 | 10.87 | 1.18 | 0.69 | 2.03 | ||||||
| b 2 h Glucose/HbA1c | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 0.77 | 0.54 | 1.12 | 5.41 | 0.02 | 0.62 | 0.08 | 4.55 | 0.36 | 0.55 | 0.75 | 0.39 | 1.47 | 0.74 | 0.39 |
| Pooled sensitive | 1.46 | 0.99 | 2.17 | 1.23 | 0.46 | 3.31 | 1.11 | 0.62 | 2.00 | ||||||
| Diastolic blood pressure | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 0.72 | 0.38 | 1.38 | 3.88 | 0.05 | 0.64 | 0.26 | 1.55 | 0.15 | 0.70 | 1.05 | 0.81 | 1.37 | 3.45 | 0.06 |
| Pooled sensitive | 1.61 | 1.01 | 2.55 | 0.81 | 0.36 | 1.82 | 1.47 | 1.16 | 1.85 | ||||||
| HDL-C | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 1.21 | 0.50 | 2.98 | 0.03 | 0.87 | 2.22 | 0.96 | 5.12 | 1.12 | 0.29 | 1.39 | 0.79 | 2.44 | 0.01 | 0.94 |
| Pooled sensitive | 1.12 | 0.67 | 1.84 | 0.69 | 0.10 | 5.03 | 1.47 | 0.38 | 5.62 | ||||||
| BMI | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 1.07 | 0.84 | 1.37 | 0.59 | 0.44 | 1.37 | 0.30 | 6.24 | 0.98 | 0.32 | 1.57 | 1.20 | 2.06 | 1.70 | 0.19 |
| Pooled sensitive | 0.86 | 0.51 | 1.44 | 0.59 | 0.31 | 1.13 | 1.22 | 0.93 | 1.60 | ||||||
| LDL-C | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 1.34 | 0.91 | 1.98 | 0.99 | 0.32 | 0.59 | 0.24 | 1.44 | 0.03 | 0.87 | 1.31 | 0.80 | 2.15 | 0.21 | 0.65 |
| Pooled sensitive | 1.75 | 1.24 | 2.46 | 0.65 | 0.29 | 1.48 | 1.72 | 0.60 | 4.97 | ||||||
| Total Cholesterol | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 1.17 | 0.55 | 2.51 | 0.32 | 0.57 | 1.07 | 0.62 | 1.85 | 0.20 | 0.66 | 1.58 | 0.99 | 2.53 | 0.23 | 0.63 |
| Pooled sensitive | 1.58 | 0.78 | 3.19 | 1.30 | 0.67 | 2.53 | 1.25 | 0.53 | 2.92 | ||||||
| Triglycerides | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 1.09 | 0.66 | 1.78 | 0.01 | 0.94 | - | - | - | - | - | 0.84 | 0.44 | 1.59 | 1.53 | 0.22 |
| Pooled sensitive | 1.06 | 0.74 | 1.52 | 1.04 | 0.48 | 2.25 | 1.39 | 0.85 | 2.29 | ||||||
| Systolic blood pressure | |||||||||||||||
| Pooled neutrality | 1.00 | 1.00 | 1.00 | ||||||||||||
| Pooled resilient | 0.72 | 0.40 | 1.28 | 6.55 | 0.01 | 0.74 | 0.38 | 1.47 | 3.17 | 0.07 | 1.01 | 0.77 | 1.32 | 5.74 | 0.02 |
| Pooled sensitive | 1.58 | 1.32 | 1.88 | 1.65 | 0.95 | 2.84 | 1.53 | 1.25 | 1.89 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomares-Millan, H.; Poveda, A.; Atabaki-Pasdar, N.; Johansson, I.; Björk, J.; Ohlsson, M.; Giordano, G.N.; Franks, P.W. Predicting Sensitivity to Adverse Lifestyle Risk Factors for Cardiometabolic Morbidity and Mortality. Nutrients 2022, 14, 3171. https://doi.org/10.3390/nu14153171
Pomares-Millan H, Poveda A, Atabaki-Pasdar N, Johansson I, Björk J, Ohlsson M, Giordano GN, Franks PW. Predicting Sensitivity to Adverse Lifestyle Risk Factors for Cardiometabolic Morbidity and Mortality. Nutrients. 2022; 14(15):3171. https://doi.org/10.3390/nu14153171
Chicago/Turabian StylePomares-Millan, Hugo, Alaitz Poveda, Naemieh Atabaki-Pasdar, Ingegerd Johansson, Jonas Björk, Mattias Ohlsson, Giuseppe N. Giordano, and Paul W. Franks. 2022. "Predicting Sensitivity to Adverse Lifestyle Risk Factors for Cardiometabolic Morbidity and Mortality" Nutrients 14, no. 15: 3171. https://doi.org/10.3390/nu14153171
APA StylePomares-Millan, H., Poveda, A., Atabaki-Pasdar, N., Johansson, I., Björk, J., Ohlsson, M., Giordano, G. N., & Franks, P. W. (2022). Predicting Sensitivity to Adverse Lifestyle Risk Factors for Cardiometabolic Morbidity and Mortality. Nutrients, 14(15), 3171. https://doi.org/10.3390/nu14153171






