The Relationship between Gut Microbiota and Respiratory Tract Infections in Childhood: A Narrative Review
Abstract
:1. Introduction
2. Preclinical Data on the Gut–Lung Axis in RTIs
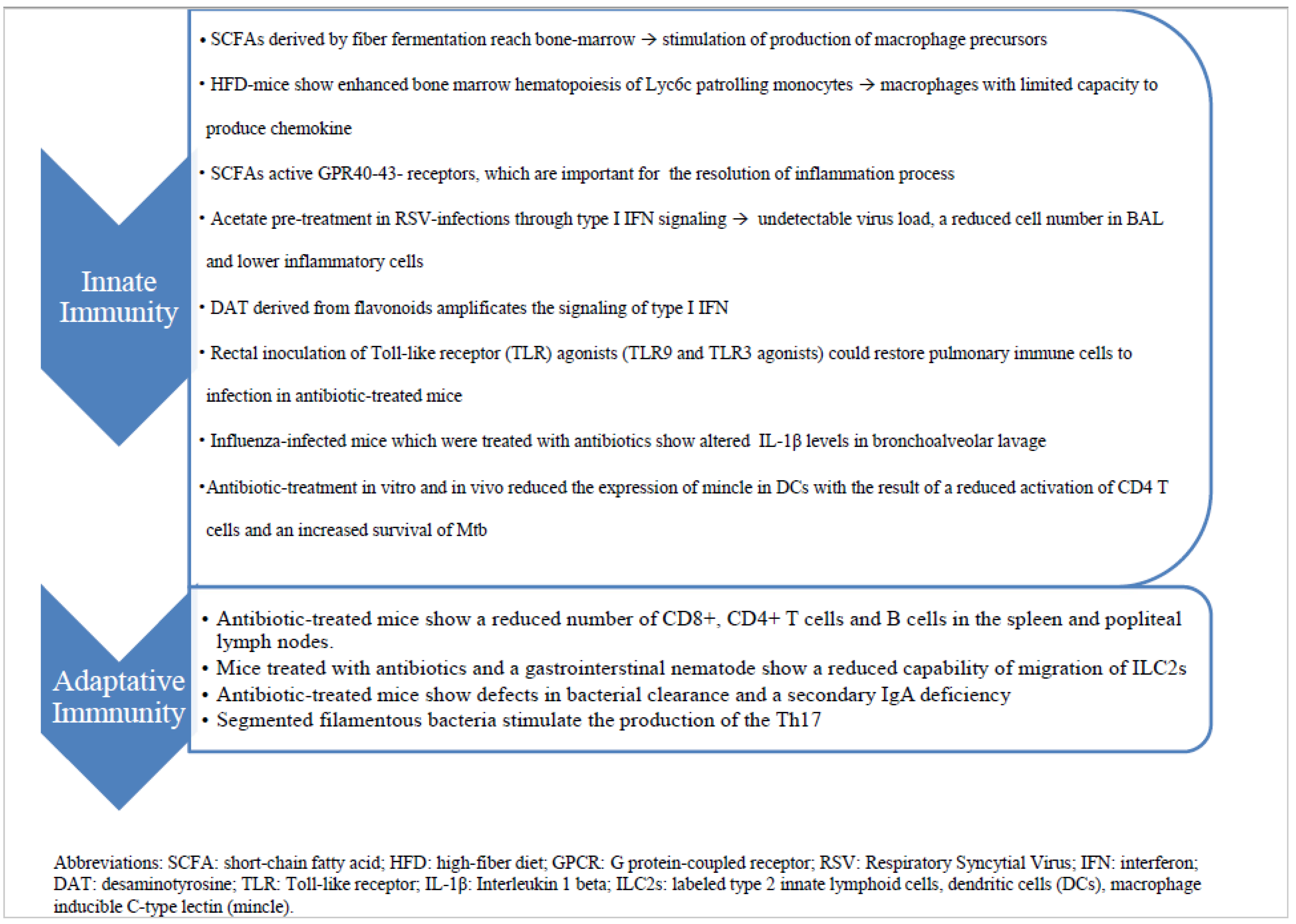
2.1. Innate Immunity
2.2. Adaptative Immunity
3. GM and RTIs in Children
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Wardlaw, T.; Salama, P.; Johansson, E.W.; Mason, E. Pneumonia: The leading killer of children. Lancet 2006, 368, 1048–1050. [Google Scholar] [CrossRef]
- Heiskanen-Kosma, T.; Korppi, M.; Jokinen, C.; Kurki, S.; Heiskanen, L.; Juvonen, H.; Kallinen, S.; Stén, M.; Tarkiainen, A.; Rönnberg, P.-R.; et al. Etiology of childhood pneumonia: Serologic results of a prospective, population-based study. Pediatr. Infect. Dis. J. 1998, 17, 986–991. Available online: https://journals.lww.com/pidj/Fulltext/1998/11000/Etiology_of_childhood_pneumonia__serologic_results.4.aspx (accessed on 24 April 2022). [CrossRef]
- Drummond, P.; Clark, J.; Cant, A.; Wheeler, J.; Galloway, A.; Freeman, R. Community acquired pneumonia—A prospective UK study. Arch. Dis. Child. 2000, 83, 408–412. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Marseglia, G.; Novelli, A.; de Martino, M.; Di Mauro, G.; Gabiano, C.; Galli, L.; De Luca, G.; Leo, G.; Navone, C.; et al. Acute, subacute and recurrent bacterial rhinosinusitis in pediatrics: Guidelines of the Study Group of the Italian Society for Pediatric Infectious Diseases (SITIP). Minerva. Pediatr. 2007, 59, 474–475. [Google Scholar] [PubMed]
- Cutrera, R.; Chiappini, E.; Galli, L.; Marchisio, P.; Marseglia, G.L.; Santamaria, F. La Prevenzione Delle Infezioni Respiratorie Ricorrenti. Consensus Intersocietaria 2020; Societa’ Italiana di Pediatria: Roma, Italy, 2020. [Google Scholar]
- Ciprandi, G.; La Mantia, I.; Damiani, V.; Passali, D. Local Bacteriotherapy–a promising preventive tool in recurrent respiratory infections. Expert Rev. Clin. Immunol. 2020, 16, 1047–1052. [Google Scholar] [CrossRef]
- Pasternak, G.; Lewandowicz-Uszyńska, A.K.-O. Children, Recurrent respiratory tract infections in. Pol. Merkur Lek. 2020, 49, 260–266. [Google Scholar]
- De Martino, M.; Ballotti, S. The child with recurrent respiratory infections: Normal or not? Pediatr. Allergy Immunol. 2007, 18, 13–18. [Google Scholar] [CrossRef]
- de Benedictis, F.M.; Bush, A. Recurrent lower respiratory tract infections in children. BMJ 2018, 362, k2698. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; De Sousa E Melo, F.; Roelofs, J.J.T.H.; De Boer, J.D.; Hoogendijk, A.J.; De Beer, R.; De Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Hickman, H.D.; McCulloch, J.A.; Badger, J.H.; Ajami, N.J.; et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 2017, 171, 1015–1028. [Google Scholar] [CrossRef]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Moratto, D.; Giacomelli, M.; Chiarini, M.; Savarè, L.; Saccani, B.; Motta, M.; Timpano, S.; Poli, P.; Paghera, S.; Imberti, L.; et al. Immune response in children with COVID-19 is characterized by lower levels of T-cell activation than infected adults. Eur. J. Immunol. 2020, 50, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- de Melo, F.F.; Rocha, G.; Rocha, A.M.C.; Teixeira, K.N.; Pedroso, S.H.S.P.; Junior, J.B.P.; de Castro, L.P.F.; Cabral, M.M.D.; Carvalho, S.D.; Bittencourt, P.F.S.; et al. Th1 immune response to H. pylori infection varies according to the age of the patients and influences the gastric inflammatory patterns. Int. J. Med. Microbiol. 2014, 304, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, F.; Liu, Y.; Gu, F. Intestinal microbiota dysbiosis in children with recurrent respiratory tract infections. Microb. Pathog. 2019, 136, 103709. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota-Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2018, 61, 47–61. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung–gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Huang, Y.J.; Charlson, E.S.; Collman, R.G.; Colombini-Hatch, S.; Martinez, F.D.; Senior, R.M. The role of the lung microbiome in health and disease: A national heart, lung, and blood institute workshop report. Am. J. Respir. Crit. Care Med. 2013, 187, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Kim, K.-H.; Hwang, H.S.; Lee, Y.; Kwon, Y.-M.; Ko, E.-J.; Jung, Y.-J.; Lee, Y.-N.; Kim, M.-C.; Kang, S.-M. Innate and adaptive cellular phenotypes contributing to pulmonary disease in mice after respiratory syncytial virus immunization and infection. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Antunes, K.H.; Stein, R.T.; Franceschina, C.; da Silva, E.F.; de Freitas, D.N.; Silveira, J.; Mocellin, M.; Leitão, L.; Fachi, J.L.; Pral, L.P.; et al. Short-chain fatty acid acetate triggers antiviral response mediated by RIG-I in cells from infants with respiratory syncytial virus bronchiolitis. EBioMedicine 2022, 77, 103891. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005. [Google Scholar] [CrossRef]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; MacKay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Thackray, L.B.; Handley, S.A.; Gorman, M.J.; Poddar, S.; Briseño, C.G.; Theisen, D.J.; Tan, Q.; Hykes, B.L., Jr.; Lucas, T.M.; Desai, C.; et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep. 2018, 22, 3440–3453. [Google Scholar] [CrossRef]
- Brown, R.L.; Sequeira, R.P.; Clarke, T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017, 8, 1512. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Hara, T.; Katsuma, S.; Adachi, T.; Tsujimoto, G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 2008, 31, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; Dos Santos, A.Á.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef]
- Ock, K.C.; Sang, J.C.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Negi, S.; Pahari, S.; Bashir, H.; Agrewala, J.N. Gut microbiota regulates mincle mediated activation of lung dendritic cells to protect against mycobacterium tuberculosis. Front. Immunol. 2019, 10, 1142. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Herman, C.E.; MacIver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef]
- Yagi, K.; Asai, N.; Huffnagle, G.B.; Lukacs, N.W.; Fonseca, W. Early-Life Lung and Gut Microbiota Development and Respiratory Syncytial Virus Infection. Front. Immunol. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Chen, X.; Sun, M.-A.; Kawabe, T.; Li, W.; Usher, N.; Zhu, J.; Urban, J.F., Jr.; Paul, W.E.; et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 2020, 359, 114–119. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef]
- Gauguet, S.; D’Ortona, S.; Ahnger-Pier, K.; Duan, B.; Surana, N.K.; Lu, R.; Cywes-Bentley, C.; Gadjeva, M.; Shan, Q.; Priebe, G.P.; et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect. Immun. 2015, 83, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Linnemann, R.W.; Mansbach, J.M.; Ajami, N.J.; Espinola, J.A.; Petrosino, J.F.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Thompson, A.D.; et al. The fecal microbiota profile and bronchiolitis in infants. Pediatrics 2016, 138, e20160218. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.N.; Siefker, D.; Vu, L.; You, D.; Devincenzo, J.; Pierre, J.F.; Cormier, S.A. Altered gut microbiota in infants is associated with respiratory syncytial virus disease severity. BMC Microbiol. 2020, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Alba, C.; Aparicio, M.; González-Martínez, F.; González-Sánchez, M.I.; Pérez-Moreno, J.; Toledo del Castillo, B.; Rodríguez, J.M.; Rodríguez-Fernández, R.; Fernández, L. Nasal and Fecal Microbiota and Immunoprofiling of Infants With and Without RSV Bronchiolitis. Front. Microbiol. 2021, 12, 667832. [Google Scholar] [CrossRef]
- Li, K.-L.; Wang, B.-Z.; Li, Z.-P.; Li, Y.-L.; Liang, J.-J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef]
- Xu, R.; Liu, P.; Zhang, T.; Wu, Q.; Zeng, M.; Ma, Y.; Jin, X.; Xu, J.; Zhang, Z.; Zhang, C. Progressive deterioration of the upper respiratory tract and the gut microbiomes in children during the early infection stages of COVID-19. J. Genet. Genom. 2021, 48, 803–814. [Google Scholar] [CrossRef]
- Gottschick, C.; Raupach-Rosin, H.; Langer, S.; Hassan, L.; Horn, J.; Dorendorf, E.; Caputo, M.; Bittner, M.; Beier, L.; Rübsamen, N.; et al. Cohort Profile: The LoewenKIDS Study—Life-course perspective on infections, the microbiome and the development of the immune system in early childhood. Int. J. Epidemiol. 2019, 48, 1042–1043H. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Aloisio, I.; Mazzola, G.; Corvaglia, L.T.; Tonti, G.; Faldella, G.; Biavati, B.; Di Gioia, D.; Quagliariello, A.; De Fanti, S.; Luiselli, D.; et al. Building a Bene fi cial Microbiome from Birth 1, 2. Appl. Microbiol. Biotechnol. 2016, 7, 323–330. [Google Scholar] [CrossRef]
| Study, Year | Aim | Results |
|---|---|---|
| Trompette et al., 2018 [28] | Effect of an HFD, through SCFA modulation in influenza-infected mice. |
|
| McAleer et al., 2017 [29] | Effects of GM composition on lung immunity. |
|
| Maslowski et al., 2009 [30] | The role of SCFAs in the regulation of the immune response by GPR43 activation. |
|
| Antunes et al., 2019 [27] | The role of SCFAs in RSV infection. |
|
| Steed et al., 2017, [15] | Evaluation of DAT in protecting from influenza through type I IFN. |
|
| Ichinohe et al., 2011 [16] | The function of GM in influenza A-infected mice. |
|
| Thackray et al., 2018 [31] | Effects of oral antibiotics in flaviviridae-infected mice. |
|
| Wypich et al., 2019 [24] | Analysis of the gut–lung axis and its communication pathways. |
|
| Brown et al., 2017 [32] | GM signaling that protects against respiratory infections. |
|
| Author, Year of Publication, Country | Study Design | No. of Subjects and Population | Age Range | Aim | Main Results |
|---|---|---|---|---|---|
| Hasegawa et al. [43], 2016, Massachusetts | Case-control | 40 hospitalized infants with bronchiolitis vs. 115 healthy controls. | <12 months | To identify faecal microbiota profiles and compare the likelihood of bronchiolitis. | The highest likelihood of bronchiolitis in the Bacteroides-dominant profile compared to the Enterobacter/Veillonella-, Escherichia- and Bifidobacterium-dominant profiles. |
| Harding et al. [44], 2020, Louisiana | Case-control | 53 hospitalized infants with RSV- bronchiolitis vs. 37 healthy controls. | <7 months | To compare GM in children with different bronchiolitis severity vs. controls. | Increase in S24_7, Clostridiales, Odoribacteraceae, Lactobacillaceae, and Actinomyces in patients with bronchiolitis compared to controls. Increase in S24_7 in severe patients compared to moderate patients and controls. |
| Alba et al. [45], 2021, Spain | Case-control | 58 infants with RSV-bronchiolitis vs. 17 healthy controls. | <24 months | To compare GM in children with bronchiolitis vs. controls. | No significant differences regarding the most abundant genera (Bifidobacterium, Streptococcus, and Escherichia) between infants with bronchiolitis and controls. |
| Reyman et al. [12], 2019, Netherlands | Prospective single centre | 74 VD children, 46 born by CS. | First year of life | Differences in GM between VD and CS-born children. Correlation between GM and RTIs in the first year of life. | Prevalence of Bifidobacterium in the first week of life was significantly associated with fewer RTI events. Klebsiella and Enterococcus prevalence were negatively associated with fewer RTI events. |
| Li et al. [19], 2019, China | Case-control | 26 children with RRTIs vs. 23 healthy controls. | >5 years | To compare GM in children with RRTIs vs. controls. To identify GM biomarkers that could discriminate RRTI status. | Alpha diversity in the RRTI patients’ GM was significantly lower. Reduction of Verrucomicrobia and Tenericutes phyla with increase in Enterococcus and decrease in Eubacterium in the RRTI group. Enterococcus, taken as a biomarker, showed the highest accuracy in identifying RRTIs. |
| Li et al. [46], 2019, China | Case-control | 90 children with RRTIs vs. 30 heathy controls. | <11 years | To compare GM in children with RRTIs vs. controls. Effects of probiotics on GM and RRTIs. | Significant reduction of lactobacilli and bifidobacteria in children with RRTIs. |
| Xu et al. [47], 2021, China | Case-control | 9 children with COVID-19 vs. 14 healthy controls. | <12 years | To compare GM in children with COVID-19 vs. controls. | Increased representation of Bacteroidetes and Firmicutes, and decrease in Proteobacteria, with significant increase in opportunistic pathogenic and environmental bacteria in COVID-19 children. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zama, D.; Totaro, C.; Biscardi, L.; Rocca, A.; Turroni, S.; Brigidi, P.; Lanari, M. The Relationship between Gut Microbiota and Respiratory Tract Infections in Childhood: A Narrative Review. Nutrients 2022, 14, 2992. https://doi.org/10.3390/nu14142992
Zama D, Totaro C, Biscardi L, Rocca A, Turroni S, Brigidi P, Lanari M. The Relationship between Gut Microbiota and Respiratory Tract Infections in Childhood: A Narrative Review. Nutrients. 2022; 14(14):2992. https://doi.org/10.3390/nu14142992
Chicago/Turabian StyleZama, Daniele, Camilla Totaro, Lorenzo Biscardi, Alessandro Rocca, Silvia Turroni, Patrizia Brigidi, and Marcello Lanari. 2022. "The Relationship between Gut Microbiota and Respiratory Tract Infections in Childhood: A Narrative Review" Nutrients 14, no. 14: 2992. https://doi.org/10.3390/nu14142992
APA StyleZama, D., Totaro, C., Biscardi, L., Rocca, A., Turroni, S., Brigidi, P., & Lanari, M. (2022). The Relationship between Gut Microbiota and Respiratory Tract Infections in Childhood: A Narrative Review. Nutrients, 14(14), 2992. https://doi.org/10.3390/nu14142992








