Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioactive Components of the Food Supplements
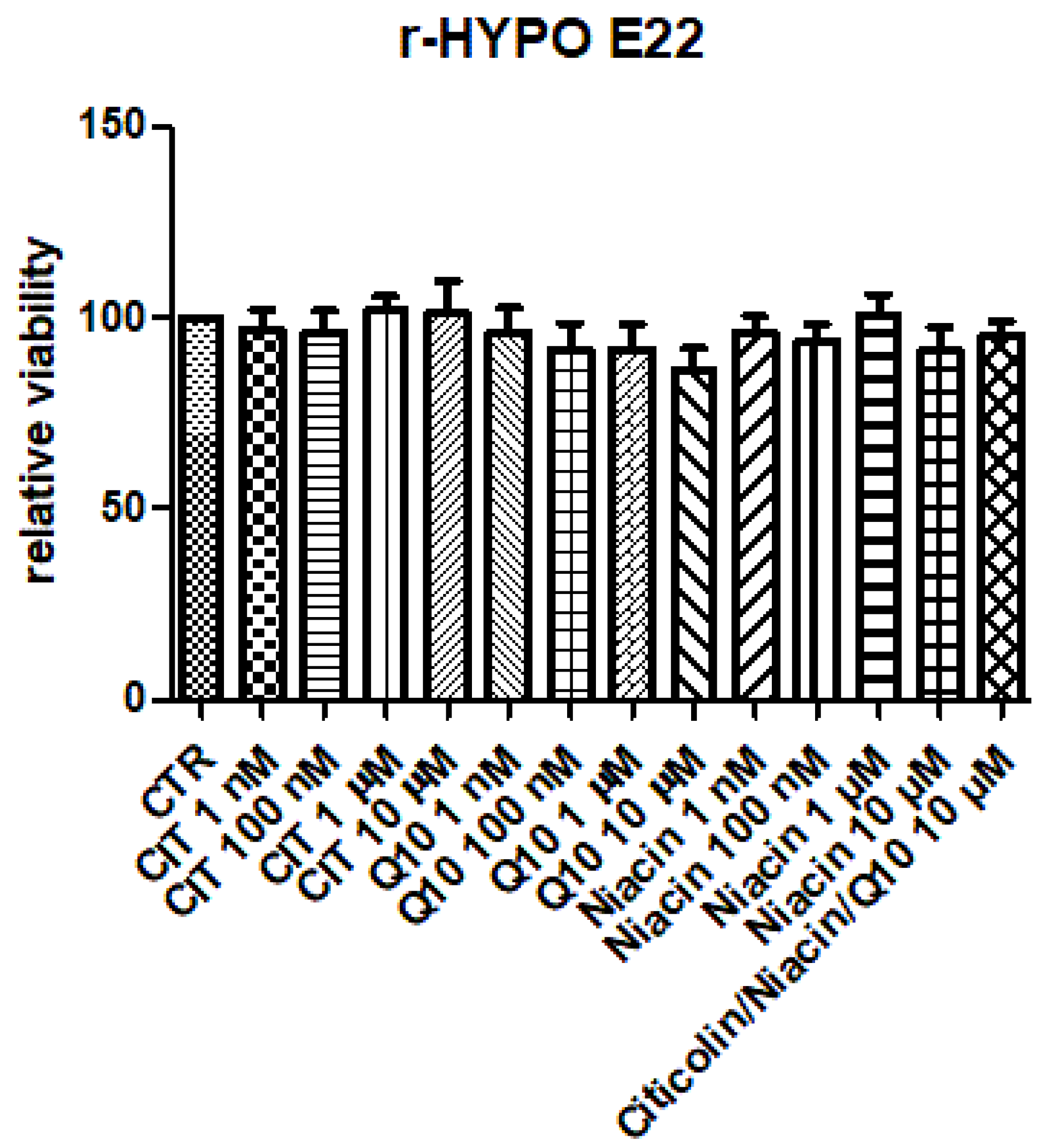
2.2. Hypothalamic HypoE22 Cells: Evaluation of Biocompatibility
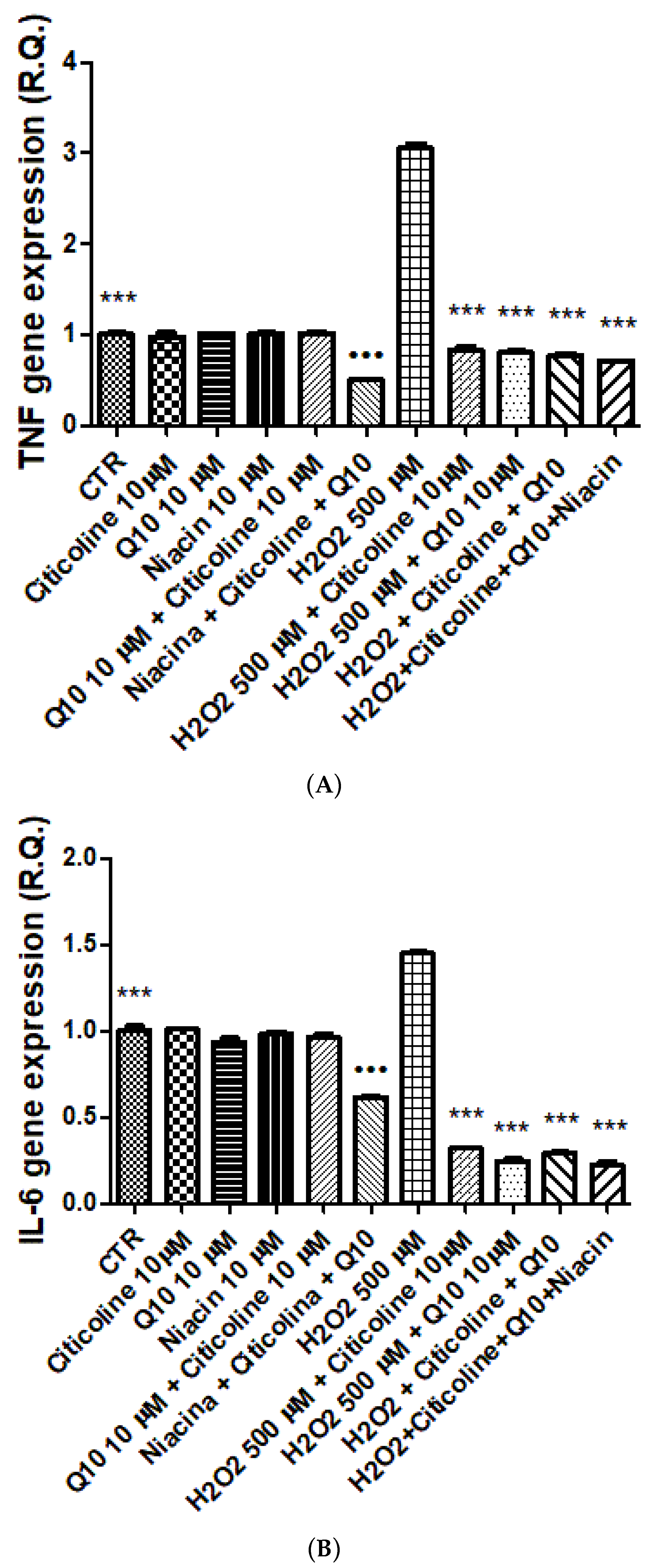
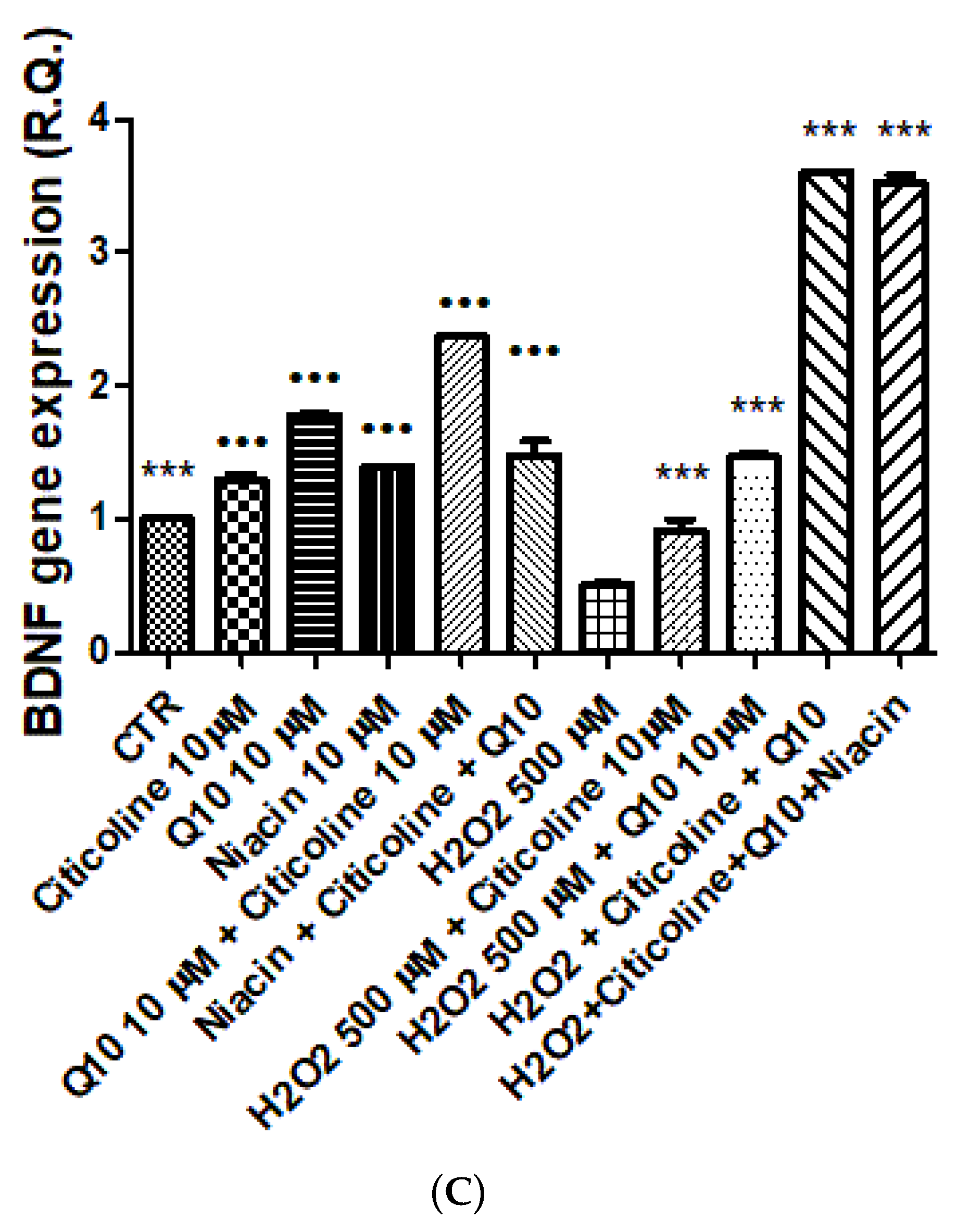
2.3. Isolated Hypothalamic Specimens Exposed to Oxidative Stress
2.4. Gene Expression Analysis
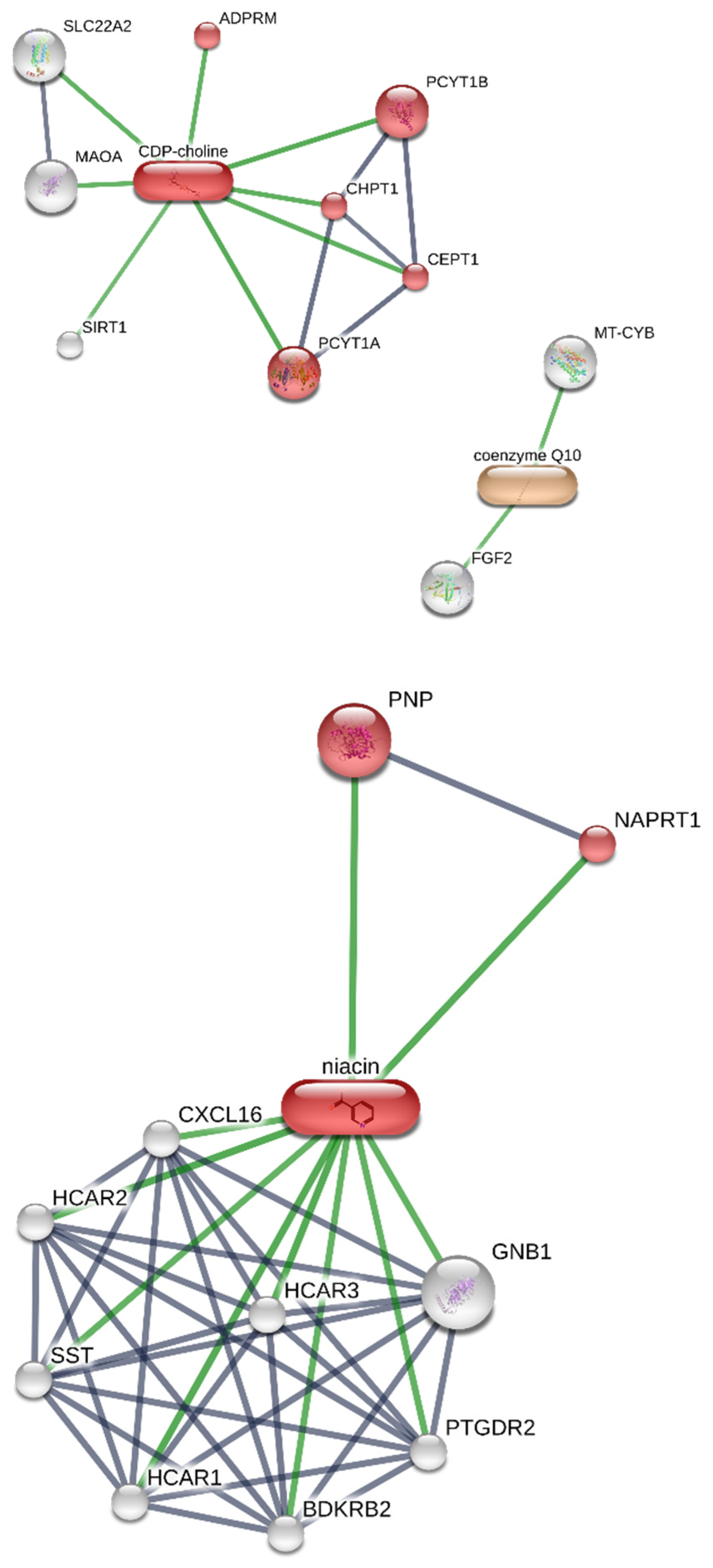
2.5. In Silico
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kritsilis, M.; Rizou, V.S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [Green Version]
- Vajda, F.J. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 2002, 9, 4–8. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog. Retin. Eye Res. 2019, 72, 1007–1067. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Nucci, C. Evidence on neuroprotective properties of coenzyme Q10 in the treatment of glaucoma. Neural Regen Res. 2019, 14, 197–200. [Google Scholar] [CrossRef]
- Saligaut, S.; Daoust, M.; Moore, N.; Boismare, F. Effects of hypoxia and cytidine (5′) diphosphocholine on the concentrations of dopamine, norepinephrine and metabolites in rat hypothalamus and striatum. Arch. Int. Pharm. Ther. 1987, 285, 25–33. [Google Scholar]
- Petkov, V.D.; Stancheva, S.L.; Tocuschieva, L.; Petkov, V.V. Changes in brain biogenic monoamines induced by the nootropic drugs adafenoxate and meclofenoxate and by citicholine (experiments on rats). Gen. Pharmacol. 1990, 21, 71–75. [Google Scholar] [CrossRef]
- Oshitari, T.; Yoshida-Hata, N.; Yamamoto, S. Effect of neurotrophic factors on neuronal apoptosis and neurite regeneration in cultured rat retinas exposed to high glucose. Brain Res. 2010, 1346, 43–51. [Google Scholar] [CrossRef]
- Barrachina, M.; Domínguez, I.; Ambrosio, S.; Secades, J.; Lozano, R.; Ferrer, I. Neuroprotective effect of citicoline in 6-hydroxydopamine-lesioned rats and in 6-hydroxydopamine-treated SH-SY5Y human neuroblastoma cells. J. Neurol. Sci. 2003, 215, 105–110. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Xiaojing, J.; Durany, N.; Rausch, W.D. CDP-choline reduces dopaminergic cell loss induced by MPP(+) and glutamate in primary mesencephalic cell culture. Int. J. Neurosci. 2007, 117, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Combination of Neuroprotective and Regenerative Agents for AGE-Induced Retinal Degeneration: In Vitro Study. Biomed. Res. Int. 2017, 2017, 8604723. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Barbano, L.; Giorno, P.; Varano, M.; Parravano, M. Citicoline and Vitamin B(12) Eye Drops in Type 1 Diabetes: Results of a 36-Month Pilot Study Evaluating Macular Electrophysiological Changes. Adv. Ther. 2021, 38, 3924–3936. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5’-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in Glaucoma: Old and New Promising Treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Young, A.J.; Johnson, S.; Steffens, D.C.; Doraiswamy, P.M. Coenzyme Q10: A review of its promise as a neuroprotectant. CNS Spectr. 2007, 12, 62–68. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef]
- Ahmad, S.S. Coenzyme Q and its role in glaucoma. Saudi J. Ophthalmol. 2020, 34, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Salamon, A.; Zádori, D.; Szpisjak, L.; Klivényi, P.; Vécsei, L. Fixed-dose combination therapy for Parkinson’s disease with a spotlight on entacapone in the past 20 years: A reduced pill burden and a simplified dosing regimen. Expert Opin. Pharmacother. 2020, 21, 2265–2278. [Google Scholar] [CrossRef]
- di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.V.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P.; et al. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules 2021, 26, 4422. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5’-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef] [Green Version]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrient 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vega, B.; Nicieza, J.; Álvarez-Barrios, A.; Álvarez, L.; García, M.; Fernández-Vega, C.; Vega, J.A.; González-Iglesias, H. The Use of Vitamins and Coenzyme Q10 for the Treatment of Vascular Occlusion Diseases Affecting the Retina. Nutrients 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Recinella, L.; Chiavaroli, A.; di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules 2021, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.L.; Yu, X.J.; Qi, J.; Yi, Q.Y.; Jing, W.H.; Sun, W.Y.; Cui, W.; Mu, J.J.; Yuan, Z.Y.; Zhao, X.F.; et al. Oral CoQ10 attenuates high salt-induced hypertension by restoring neurotransmitters and cytokines in the hypothalamic paraventricular nucleus. Sci. Rep. 2016, 6, 30301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Weber, A.J. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Investig. Ophthalmol. Vis. Sci. 2001, 42, 966–974. [Google Scholar]
- Mastropasqua, R.; Fasanella, V.; Agnifili, L.; Fresina, M.; Di Staso, S.; Di Gregorio, A.; Marchini, G.; Ciancaglini, M. Advance in the pathogenesis and treatment of normal-tension glaucoma. Prog. Brain Res. 2015, 221, 213–232. [Google Scholar] [CrossRef]
- Igarashi, T.; Nakamoto, K.; Kobayashi, M.; Suzuki, H.; Arima, T.; Tobita, Y.; Takao, K.; Igarashi, T.; Okuda, T.; Okada, T.; et al. Brain-derived Neurotrophic Factor in the Aqueous Humor of Glaucoma Patients. J. Nippon Med. Sch. 2021, 88, 128–132. [Google Scholar] [CrossRef]
- Liang, S.P.; Chen, Q.; Cheng, Y.B.; Xue, Y.Y.; Wang, H.J. Comparative Effects of Monosialoganglioside versus Citicoline on Apoptotic Factor, Neurological Function and Oxidative Stress in Newborns with Hypoxic-Ischemic Encephalopathy. J. Coll Physicians Surg. Pak. 2019, 29, 324–327. [Google Scholar] [CrossRef]
- Nagib, M.M.; Tadros, M.G.; Rahmo, R.M.; Sabri, N.A.; Khalifa, A.E.; Masoud, S.I. Ameliorative Effects of α-Tocopherol and/or Coenzyme Q10 on Phenytoin-Induced Cognitive Impairment in Rats: Role of VEGF and BDNF-TrkB-CREB Pathway. Neurotox Res. 2019, 35, 451–462. [Google Scholar] [CrossRef]
- Zengin, G.; Ak, G.; Ceylan, R.; Uysal, S.; Llorent-Martínez, E.; Di Simone, S.C.; Rapino, M.; Acquaviva, A.; Libero, M.L.; Chiavaroli, A.; et al. Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle. Plants 2022, 11, 233. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Castillo, G.; Sullivan, P.; Sharabi, Y. Differential Susceptibilities of Catecholamines to Metabolism by Mono-443 amine Oxidases. J. Pharmacol. Exp. Ther. 2021, 379, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Park, S.Y.; Yi, E.Y.; Kim, Y.J.; Jeong, J.W. Coenzyme Q10 decreases basic fibroblast growth factor (bFGF)-induced 445 angiogenesis by blocking ERK activation. Oncol. Res. 2011, 19, 455–461. [Google Scholar] [CrossRef]
- De Moraes, C.G.; Liebmann, J.M. Combined Use of Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glau- 447 coma-Reply. JAMA Ophthalmol. 2022, 140, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Quah Qin Xian, N.; Wong, F.Y.K. Combined Use of Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glau- 449 coma. JAMA Ophthalmol. 2022, 140, 440. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastropasqua, L.; Agnifili, L.; Ferrante, C.; Sacchi, M.; Figus, M.; Rossi, G.C.M.; Brescia, L.; Aloia, R.; Orlando, G. Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress. Nutrients 2022, 14, 2963. https://doi.org/10.3390/nu14142963
Mastropasqua L, Agnifili L, Ferrante C, Sacchi M, Figus M, Rossi GCM, Brescia L, Aloia R, Orlando G. Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress. Nutrients. 2022; 14(14):2963. https://doi.org/10.3390/nu14142963
Chicago/Turabian StyleMastropasqua, Leonardo, Luca Agnifili, Claudio Ferrante, Matteo Sacchi, Michele Figus, Gemma Caterina Maria Rossi, Lorenza Brescia, Raffaella Aloia, and Giustino Orlando. 2022. "Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress" Nutrients 14, no. 14: 2963. https://doi.org/10.3390/nu14142963
APA StyleMastropasqua, L., Agnifili, L., Ferrante, C., Sacchi, M., Figus, M., Rossi, G. C. M., Brescia, L., Aloia, R., & Orlando, G. (2022). Citicoline/Coenzyme Q10/Vitamin B3 Fixed Combination Exerts Synergistic Protective Effects on Neuronal Cells Exposed to Oxidative Stress. Nutrients, 14(14), 2963. https://doi.org/10.3390/nu14142963








