Vitamin A in Skin and Hair: An Update
Abstract
:1. Introduction
2. Vitamin A Metabolism
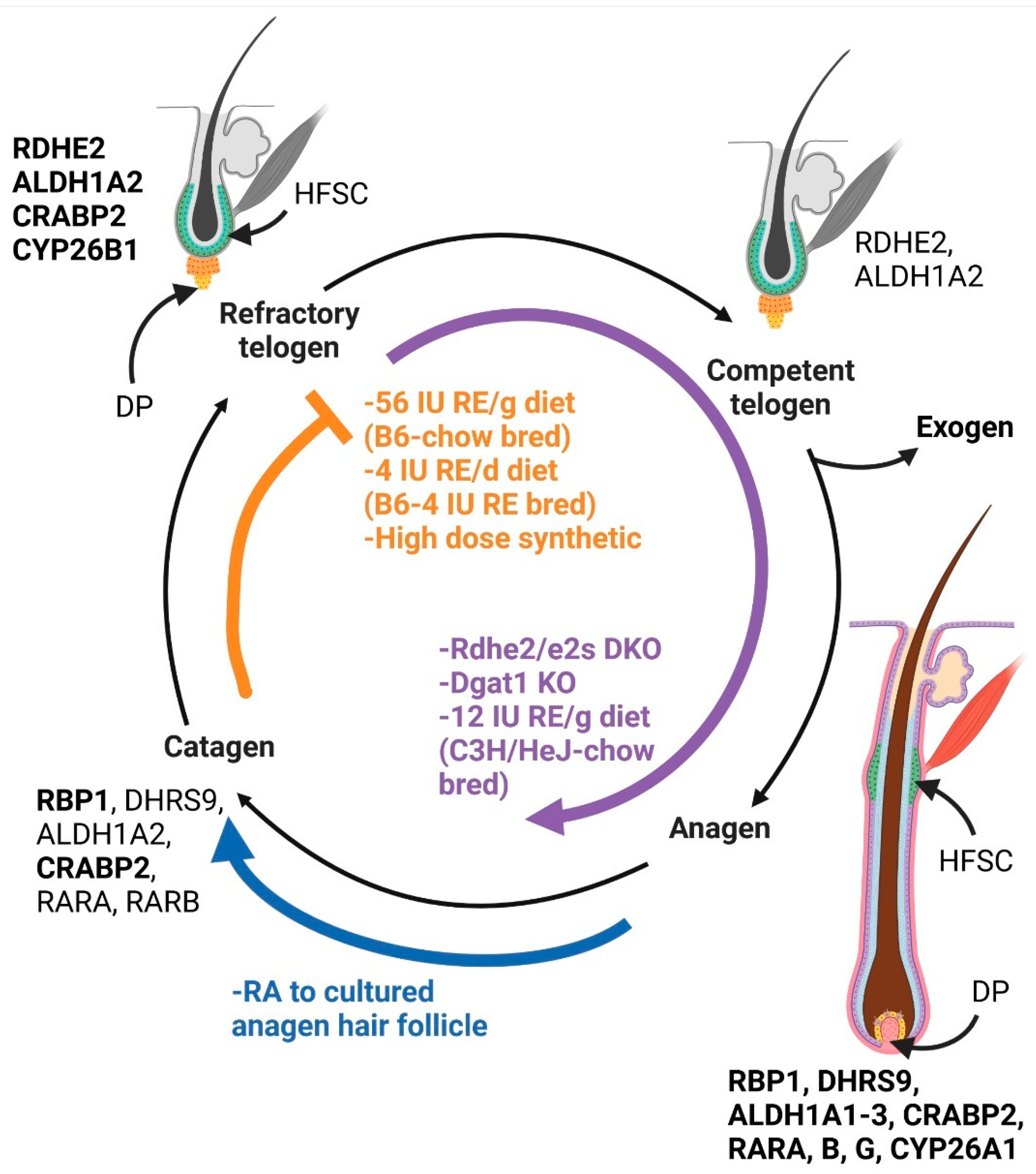
3. Hair Follicle Stem Cells (HFSCs) and the Hair Cycle
4. Wound Induced Hair Follicle Neogenesis (WIHN)
5. Melanocytes
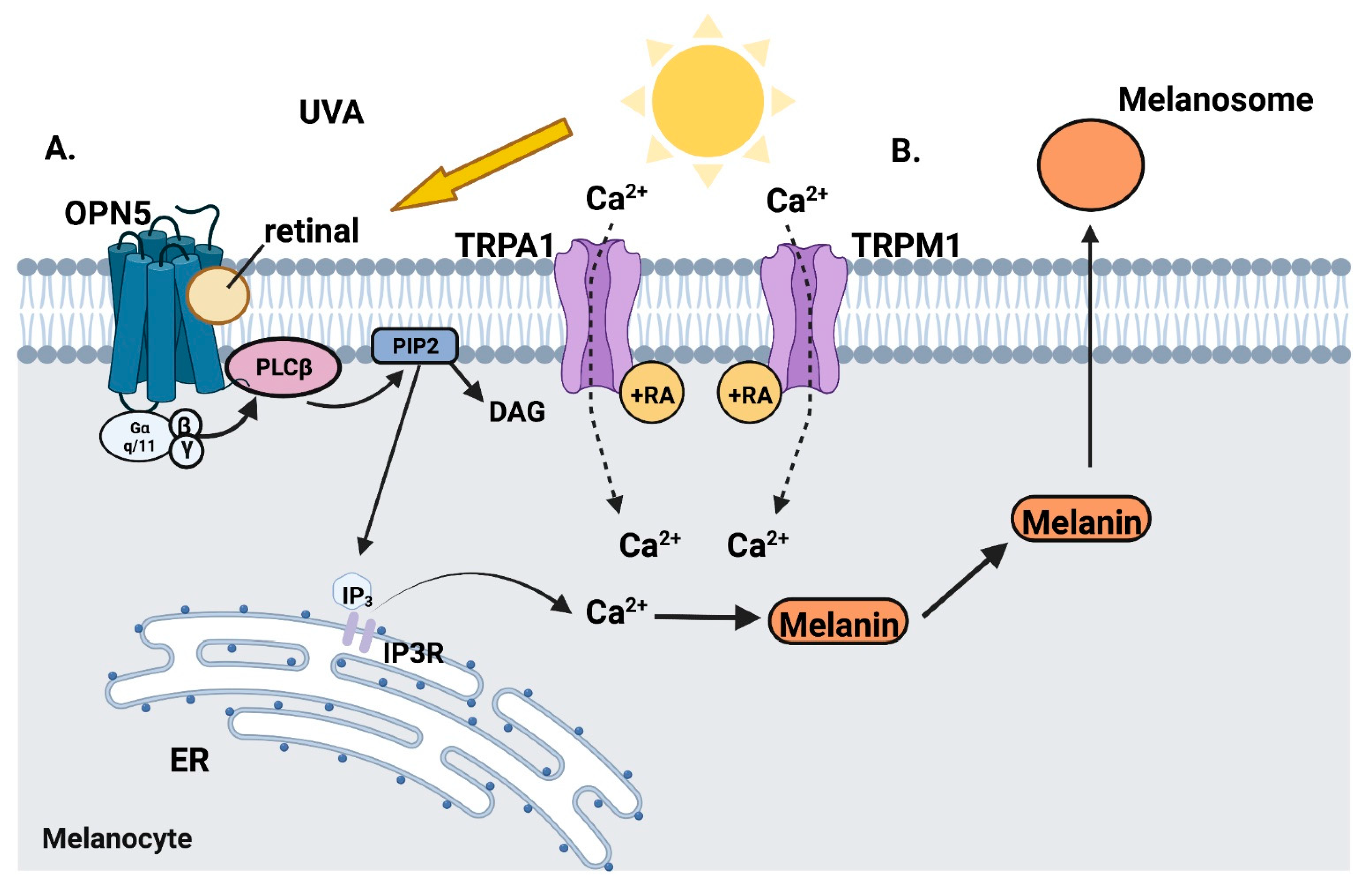
6. The role of Retinal in Epidermal Skin Cell Phototransduction
7. The Effects of UVA and UVB Irradiation on Retinoic Acid in the Epidermis and Epidermal Skin Cells
8. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hardy, M.K. Vitamin A deficiency producing follicular hyperkeratosis. Arch. Dermatol. Syphilol. 1946, 53, 392. [Google Scholar]
- Wolbach, S.B.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, H.B. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim. Biophys. Acta 2012, 1821, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, J.L. Post-natal all-trans-retinoic acid biosynthesis. Methods Enzymol. 2020, 637, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Raz, A.; Goodman, D.S. Retinol-binding protein: The transport protein for vitamin A in human plasma. J. Clin. Investig. 1968, 47, 2025–2044. [Google Scholar] [CrossRef] [Green Version]
- Bouillet, P.; Sapin, V.; Chazaud, C.; Messaddeq, N.; Decimo, D.; Dolle, P.; Chambon, P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 1997, 63, 173–186. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Alapatt, P.; Guo, F.; Komanetsky, S.M.; Wang, S.; Cai, J.; Sargsyan, A.; Rodriguez Diaz, E.; Bacon, B.T.; Aryal, P.; Graham, T.E. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J. Biol. Chem. 2013, 288, 1250–1265. [Google Scholar] [CrossRef] [Green Version]
- Ottonello, S.; Petrucco, S.; Maraini, G. Vitamin A uptake from retinol-binding protein in a cell-free system from pigment epithelial cells of bovine retina. Retinol transfer from plasma retinol-binding protein to cytoplasmic retinol-binding protein with retinyl-ester formation as the intermediate step. J. Biol. Chem. 1987, 262, 3975–3981. [Google Scholar]
- MacDonald, P.N.; Ong, D.E. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem. Biophys. Res. Commun. 1988, 156, 157–163. [Google Scholar] [CrossRef]
- Orland, M.D.; Anwar, K.; Cromley, D.; Chu, C.H.; Chen, L.; Billheimer, J.T.; Hussain, M.M.; Cheng, D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: Diacylglycerol acyltransferase 1. Biochim. Biophys. Acta 2005, 1737, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kurlandsky, S.B.; Duell, E.A.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J. Biol. Chem. 1996, 271, 15346–15352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, J.L. Retinol metabolism in LLC-PK1 Cells. Characterization of retinoic acid synthesis by an established mammalian cell line. J. Biol. Chem. 1986, 261, 13592–13597. [Google Scholar] [CrossRef]
- Jurukovski, V.; Markova, N.G.; Karaman-Jurukovska, N.; Randolph, R.K.; Su, J.; Napoli, J.L.; Simon, M. Cloning and characterization of retinol dehydrogenase transcripts expressed in human epidermal keratinocytes. Mol. Genet. Metab. 1999, 67, 62–73. [Google Scholar] [CrossRef]
- Karlsson, T.; Vahlquist, A.; Kedishvili, N.; Torma, H. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: A mechanism for its anti-androgenic effects in sebaceous glands? Biochem. Biophys. Res. Commun. 2003, 303, 273–278. [Google Scholar] [CrossRef]
- Lee, S.A.; Belyaeva, O.V.; Wu, L.; Kedishvili, N.Y. Retinol dehydrogenase 10 but not retinol/sterol dehydrogenase(s) regulates the expression of retinoic acid-responsive genes in human transgenic skin raft culture. J. Biol. Chem. 2011, 286, 13550–13560. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.X.; Chen, Y.; Chen, Y.; Fan, J.; Rohrer, B.; Crouch, R.K.; Ma, J.X. Cloning and characterization of a novel all-trans retinol short-chain dehydrogenase/reductase from the RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3365–3372. [Google Scholar]
- Adams, M.K.; Lee, S.A.; Belyaeva, O.V.; Wu, L.; Kedishvili, N.Y. Characterization of human short chain dehydrogenase/reductase SDR16C family members related to retinol dehydrogenase 10. Chem. Biol. Interact. 2017, 276, 88–94. [Google Scholar] [CrossRef]
- Wu, L.Z.; Belyaeva, O.; Adams, M.K.; Klyuyeva, A.V.; Lee, S.A.; Goggans, K.R.; Kesterson, R.A.; Popov, K.M.; Kedishvili, N.Y. Mice lacking the epidermal retinol dehydrogenases SDR16C5 and SDR16C6 display accelerated hair growth and enlarged meibomian glands. J. Biol. Chem. 2019, 294, 17060–17074. [Google Scholar] [CrossRef]
- Everts, H.B.; Sundberg, J.P.; King, L.E., Jr.; Ong, D.E. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J. Investig. Dermatol. 2007, 127, 1593–1604. [Google Scholar] [CrossRef] [Green Version]
- Markova, N.G.; Pinkas-Sarafova, A.; Karaman-Jurukovska, N.; Jurukovski, V.; Simon, M. Expression pattern and biochemical characteristics of a major epidermal retinol dehydrogenase. Mol. Genet. Metab. 2003, 78, 119–135. [Google Scholar] [CrossRef]
- Rexer, B.N.; Ong, D.E. A novel short-chain alcohol dehydrogenase from rats with retinol dehydrogenase activity, cyclically expressed in uterine epithelium. Biol. Reprod. 2002, 67, 1555–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billings, S.E.; Pierzchalski, K.; Butler Tjaden, N.E.; Pang, X.Y.; Trainor, P.A.; Kane, M.A.; Moise, A.R. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013, 27, 4877–4889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, J.L. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol. Ther. 2017, 173, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessler, R.J.; Noy, N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol. Cell 2005, 18, 343–353. [Google Scholar] [CrossRef]
- Isoherranen, N.; Zhong, G. Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases. Pharmacol. Ther. 2019, 204, 107400. [Google Scholar] [CrossRef]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Petkovich, M.; Brand, N.J.; Krust, A.; Chambon, P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 1987, 330, 444–450. [Google Scholar] [CrossRef]
- Berry, D.C.; Jin, H.; Majumdar, A.; Noy, N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA 2011, 108, 4340–4345. [Google Scholar] [CrossRef] [Green Version]
- Carrera, S.; Cuadrado-Castano, S.; Samuel, J.; Jones, G.D.; Villar, E.; Lee, S.W.; Macip, S. Stra6, a retinoic acid-responsive gene, participates in p53-induced apoptosis after DNA damage. Cell Death Differ. 2013, 20, 910–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, F.J.; Silva, K.A.; Johnson, C.; King, B.; Szatkiewicz, J.P.; Kamdar, S.; Ong, D.E.; Napoli, J.L.; Wang, J.; King, L.E., Jr.; et al. Endogenous retinoids in the pathogenesis of alopecia areata. J. Investig. Dermatol. 2013, 133, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.M.; Wu, C.; Jiang, Y.Y.; Wang, W.M.; Jin, H.Z. Retinol and vitamin A metabolites accumulate through RBP4 and STRA6 changes in a psoriasis murine model. Nutr. Metab. 2020, 17, 5. [Google Scholar] [CrossRef] [Green Version]
- Skazik, C.; Amann, P.M.; Heise, R.; Marquardt, Y.; Czaja, K.; Kim, A.; Ruhl, R.; Kurschat, P.; Merk, H.F.; Bickers, D.R.; et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J. Investig. Dermatol. 2014, 134, 1579–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, N.; Elholm, M.; Noy, N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J. Biol. Chem. 2003, 278, 41589–41592. [Google Scholar] [CrossRef] [Green Version]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Ito, M.; Liu, Y.P.; Yang, Z.X.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef]
- White, A.C.; Tran, K.; Khuu, J.; Dang, C.; Cui, Y.Y.; Binder, S.W.; Lowry, W.E. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc. Nat. Acad. Sci. USA 2011, 108, 7425–7430. [Google Scholar] [CrossRef] [Green Version]
- Chase, H.B.; Rauch, H.; Smith, V.W. Critical stages of hair development and pigmentation in the mouse. Physiol. Zool. 1951, 24, 1–7. [Google Scholar] [CrossRef]
- Milner, Y.; Sudnik, J.; Filippi, M.; Kizoulis, M.; Kashgarian, M.; Stenn, K. Exogen, shedding phase of the hair growth cycle: Characterization of a mouse model. J. Investig. Dermatol. 2002, 119, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Chuong, C.M. Multi-layered environmental regulation on the homeostasis of stem cells: The saga of hair growth and alopecia. J. Dermatol. Sci. 2012, 66, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Yi, R. Concise review: Mechanisms of quiescent hair follicle stem cell regulation. Stem Cells 2017, 35, 2323–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura-Ueki, M.; Oda, Y.; Oki, J.; Komi-Kuramochi, A.; Honda, E.; Asada, M.; Suzuki, M.; Imamura, T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Investig. Dermatol. 2012, 132, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Siegenthaler, J.A.; Dowell, R.D.; Yi, R. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 2016, 351, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Oshimori, N.; Fuchs, E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Botchkarev, V.A.; Botchkareva, N.V.; Roth, W.; Nakamura, M.; Chen, L.H.; Herzog, W.; Lindner, G.; McMahon, J.A.; Peters, C.; Lauster, R.; et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1999, 1, 158–164. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Botchkareva, N.V.; Nakamura, M.; Huber, O.; Funa, K.; Lauster, R.; Paus, R.; Gilchrest, B.A. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001, 15, 2205–2214. [Google Scholar] [CrossRef]
- Kandyba, E.; Leung, Y.; Chen, Y.B.; Widelitz, R.; Chuong, C.M.; Kobielak, K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc. Nat. Acad. Sci. USA 2013, 110, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Kandyba, E.; Kobielak, K. Wnt7b Is an Important Intrinsic Regulator of Hair Follicle Stem Cell Homeostasis and Hair Follicle Cycling. Stem Cells 2014, 32, 886–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Plikus, M.V.; Tang, P.C.; Widelitz, R.B.; Chuong, C.M. The Modulatable Stem Cell Niche: Tissue Interactions during Hair and Feather Follicle Regeneration. J. Mol. Biol. 2016, 428, 1423–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, H.B.; King, L.E., Jr.; Sundberg, J.P.; Ong, D.E. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J. Investig. Dermatol. 2004, 123, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, L.Y.; VanBuren, C.; Hovland, E.D.; Kedishvili, N.Y.; Sundberg, J.P.; Everts, H.B. Dietary vitamin A impacts refractory telogen. Front. Cell Dev. Biol. 2021, 9, 571474. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.Y.S.; Kane, M.A.; Zhou, P.; Yen, C.L.E.; Streeper, R.S.; Napoli, J.L.; Farese, R.V. Retinol esterification by DGAT1 is essential for retinoid homeostasis in murine skin. J. Biol. Chem. 2009, 284, 4292–4299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, L.; Sundberg, J.P.; Everts, H.B. Dietary vitamin A regulates wingless-related MMTV integration site signaling to alter the hair cycle. Exp. Biol. Med. 2015, 240, 618–623. [Google Scholar] [CrossRef]
- Everts, H.B.; Silva, K.A.; Montgomery, S.; Suo, L.; Menser, M.; Valet, A.; King, L.E.; Ong, D.E.; Sundberg, J.P. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J. Investig. Dermatol. 2013, 133, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, J.P.; Taylor, D.; Lorch, G.; Miller, J.; Silva, K.A.; Sundberg, B.A.; Roopenian, D.; Sperling, L.; Ong, D.; King, L.E.; et al. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet. Pathol. 2011, 48, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Berth-Jones, J.; Hutchinson, P.E. Novel cycle changes in scalp hair are caused by etretinate therapy. Br. J. Dermatol. 1995, 132, 367–375. [Google Scholar] [CrossRef]
- Okano, J.; Levy, C.; Lichti, U.; Sun, H.W.; Yuspa, S.H.; Sakai, Y.; Morasso, M.I. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. J. Biol. Chem. 2012, 287, 39304–39315. [Google Scholar] [CrossRef] [Green Version]
- Metallo, C.M.; Ji, L.; De Pablo, J.J.; Palecek, S.P. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells 2008, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bilousova, G.; Chen, J.A.; Roop, D.R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J. Investig. Dermatol. 2011, 131, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foitzik, K.; Spexard, T.; Nakamura, M.; Halsner, U.; Paus, R. Towards dissecting the pathogenesis of retinoid-induced hair loss: All-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta 2 in the dermal papilla. J. Investig. Dermatol. 2005, 124, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Li, G.; Si, H.; Lou, Y.; Wang, D.; Guo, R.; Zhang, H. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-beta2/Smad2/3 pathway. Acta Histochem. 2020, 122, 151603. [Google Scholar] [CrossRef] [PubMed]
- Bhoopalam, M.; Garza, L.A.; Reddy, S.K. Wound Induced Hair Neogenesis—A Novel Paradigm for Studying Regeneration and Aging. Front. Cell Dev. Biol. 2020, 8, 582346. [Google Scholar] [CrossRef]
- Kim, D.; Chen, R.; Sheu, M.; Kim, N.; Kim, S.; Islam, N.; Wier, E.M.; Wang, G.; Li, A.; Park, A.; et al. Noncoding dsRNA induces retinoic acid synthesis to stimulate hair follicle regeneration via TLR3. Nat. Commun. 2019, 10, 2811. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, S.; Sinha, S.; Labit, E.; Rosin, N.L.; Yoon, G.; Rahmani, W.; Jaffer, A.; Sharma, N.; Hagner, A.; Shah, P.; et al. Distinct Regulatory Programs Control the Latent Regenerative Potential of Dermal Fibroblasts during Wound Healing. Cell Stem Cell 2021, 28, 581–583. [Google Scholar] [CrossRef]
- Phan, Q.M.; Sinha, S.; Biernaskie, J.; Driskell, R.R. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp. Dermatol. 2021, 30, 92–101. [Google Scholar] [CrossRef]
- Thomas, A.J.; Erickson, C.A. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008, 21, 598–610. [Google Scholar] [CrossRef]
- Nishimura, E.; Jordan, S.; Oshima, H.; Yoshida, H.; Osawa, M.; Moriyama, M.; Jackson, I.; Barrandon, Y.; Miyachi, Y.; Nishikawa, S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 2002, 416, 854–860. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, Y.; Huang, H.; Jiang, K.; Zhou, B.; Wang, F.; Chen, T. Hair follicle stem cells regulate retinoid metabolism to maintain the self-renewal niche for melanocyte stem cells. eLife 2020, 9, e52712. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Costin, G.E.; Shellman, Y.; Kechris, K.; Olteanu, E.D.; Filip, A.; Cosgarea, M.R.; Norris, D.A.; Birlea, S.A. Biphasic pro-melanogenic and pro-apoptotic effects of all-trans-retinoic acid (ATRA) on human melanocytes: Time-course study. J. Dermatol. Sci. 2013, 72, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fligiel, S.E.; Inman, D.R.; Talwar, H.S.; Fisher, G.J.; Voorhees, J.J.; Varani, J. Modulation of growth in normal and malignant melanocytic cells by all-trans retinoic acid. J. Cutan. Pathol. 1992, 19, 27–33. [Google Scholar] [CrossRef]
- Inoue, Y.; Hasegawa, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Matsunaga, K.; Akamatsu, H. Bimodal effect of retinoic acid on melanocyte differentiation identified by time-dependent analysis. Pigment Cell Melanoma Res. 2012, 25, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ohgushi, A.; Hirobe, T.; Soma, Y. Analysis of the effects of all-trans retinoic acid on human melanocytes and melanoblasts in vitro. J. Dermatol. 2017, 44, 93–94. [Google Scholar] [CrossRef]
- Yoshimura, K.; Tsukamoto, K.; Okazaki, M.; Virador, V.M.; Lei, T.C.; Suzuki, Y.; Uchida, G.; Kitano, Y.; Harii, K. Effects of all-trans retinoic acid on melanogenesis in pigmented skin equivalents and monolayer culture of melanocytes. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S68–S75. [Google Scholar] [CrossRef]
- Romero, C.; Aberdam, E.; Larnier, C.; Ortonne, J.P. Retinoic acid as modulator of UVB-induced melanocyte differentiation. Involvement of the melanogenic enzymes expression. J. Cell Sci. 1994, 107 Pt 4, 1095–1103. [Google Scholar] [CrossRef]
- Paterson, E.K.; Ho, H.; Kapadia, R.; Ganesan, A.K. 9-cis retinoic acid is the ALDH1A1 product that stimulates melanogenesis. Exp. Dermatol. 2013, 22, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Amann, P.M.; Luo, C.; Owen, R.W.; Hofmann, C.; Freudenberger, M.; Schadendorf, D.; Eichmuller, S.B.; Bazhin, A.V. Vitamin A metabolism in benign and malignant melanocytic skin cells: Importance of lecithin/retinol acyltransferase and RPE65. J. Cell Physiol. 2012, 227, 718–728. [Google Scholar] [CrossRef]
- Haltaufderhyde, K.; Ozdeslik, R.N.; Wicks, N.L.; Najera, J.A.; Oancea, E. Opsin expression in human epidermal skin. Photochem. Photobiol. 2015, 91, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zeng, W.; Dong, X.; Lu, H. Opsin 5 is a key regulator of ultraviolet radiation-induced melanogenesis in human epidermal melanocytes. Br. J. Dermatol. 2021, 185, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Wicks, N.L.; Chan, J.W.; Najera, J.A.; Ciriello, J.M.; Oancea, E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr. Biol. 2011, 21, 1906–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellono, N.W.; Najera, J.A.; Oancea, E. UV light activates a Galphaq/11-coupled phototransduction pathway in human melanocytes. J. Gen. Physiol. 2014, 143, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef] [Green Version]
- Zhiqi, S.; Soltani, M.H.; Bhat, K.M.; Sangha, N.; Fang, D.; Hunter, J.J.; Setaluri, V. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res. 2004, 14, 509–516. [Google Scholar] [CrossRef]
- Miller, A.J.; Du, J.; Rowan, S.; Hershey, C.L.; Widlund, H.R.; Fisher, D.E. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004, 64, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.M.; Yi, W.J.; Su, M.Y.; Jiang, S.; Xu, S.Z.; Lei, T.C. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 2017, 50, e12372. [Google Scholar] [CrossRef]
- Berne, B.; Nilsson, M.; Vahlquist, A. UV irradiation and cutaneous vitamin A: An experimental study in rabbit and human skin. J. Investig. Dermatol. 1984, 83, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Sorg, O.; Tran, C.; Carraux, P.; Didierjean, L.; Falson, F.; Saurat, J.H. Oxidative stress-independent depletion of epidermal vitamin A by UVA. J. Investig. Dermatol. 2002, 118, 513–518. [Google Scholar] [CrossRef]
- Sorg, O.; Tran, C.; Carraux, P.; Didierjean, L.; Saurat, J. Retinol and retinyl ester epidermal pools are not identically sensitive to UVB irradiation and anti-oxidant protective effect. Dermatology 1999, 199, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Morinobu, T.; Takitani, K.; Kimura, M.; Tamai, H. Measurement of retinoids and beta-carotene 15,15′-dioxygenase activity in HR-1 hairless mouse skin with UV exposure. J. Nutr. Sci. Vitaminol. 2003, 49, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.; Sorg, O.; Carraux, P.; Didierjean, L.; Saurat, J.H. Topical delivery of retinoids counteracts the UVB-induced epidermal vitamin A depletion in hairless mouse. Photochem. Photobiol. 2001, 73, 425–431. [Google Scholar] [CrossRef]
- Gressel, K.L.; Duncan, F.J.; Oberyszyn, T.M.; La Perle, K.M.; Everts, H.B. Endogenous retinoic acid required to maintain the epidermis following ultraviolet light exposure in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, E.; Rosdahl, I.; Torma, H.; Vahlquist, A. Ultraviolet irradiation depletes cellular retinol and alters the metabolism of retinoic acid in cultured human keratinocytes and melanocytes. Melanoma Res. 1999, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Boudjelal, M.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat. Med. 1999, 5, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Talwar, H.S.; Xiao, J.H.; Datta, S.C.; Reddy, A.P.; Gaub, M.P.; Rochette-Egly, C.; Chambon, P.; Voorhees, J.J. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J. Biol. Chem. 1994, 269, 20629–20635. [Google Scholar] [CrossRef]
- Andersson, E.; Rosdahl, I.; Torma, H.; Vahlquist, A. Differential effects of UV irradiation on nuclear retinoid receptor levels in cultured keratinocytes and melanocytes. Exp. Dermatol. 2003, 12, 563–571. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. https://doi.org/10.3390/nu14142952
VanBuren CA, Everts HB. Vitamin A in Skin and Hair: An Update. Nutrients. 2022; 14(14):2952. https://doi.org/10.3390/nu14142952
Chicago/Turabian StyleVanBuren, Christine A., and Helen B. Everts. 2022. "Vitamin A in Skin and Hair: An Update" Nutrients 14, no. 14: 2952. https://doi.org/10.3390/nu14142952
APA StyleVanBuren, C. A., & Everts, H. B. (2022). Vitamin A in Skin and Hair: An Update. Nutrients, 14(14), 2952. https://doi.org/10.3390/nu14142952





