Improvement of Depressed Mood with Green Tea Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
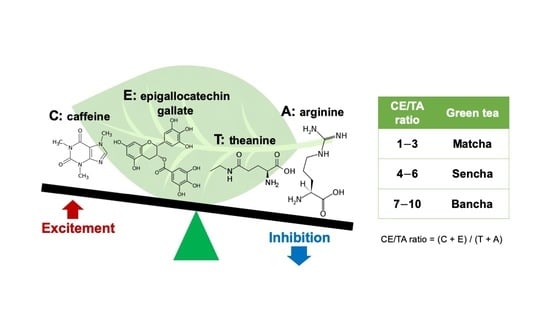
2.1.1. Animals and Green Tea Component Ingestion
2.1.2. Preparation of Depressed Mouse Model and Sucrose Preference Test
2.1.3. Adrenal Hypertrophy and Thymic Atrophy
2.1.4. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.2. Clinical Trials
2.2.1. Participants
2.2.2. Procedure
2.3. Statistical Analysis
3. Results
3.1. Animal Experiment
3.1.1. Effect of Tea Components on Sucrose Preference in LPS-Injected Mice
3.1.2. Effect of Tea Components on the Stress Response
3.1.3. Effect of Ingesting Green Tea Components on Hippocampal Inflammation
3.1.4. Effect of Green Tea Components on the Expression of Transcription Factor Npas4 in the Hippocampus
3.1.5. Summarized Effect of Green Tea Components
3.2. Clinical Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothenberg, D.O.; Zhang, L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasmari, F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J. 2020, 28, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Horie, H.; Nakamura, Y. Stress-reducing function of matcha green tea in animal experiments and clinical trials. Nutrients 2018, 10, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Ingestion of green tea with lowered caffeine improves sleep quality of the elderly via suppression of stress. J. Clin. Biochem. Nutr. 2017, 61, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content. Nutrients 2017, 9, 777. [Google Scholar] [CrossRef]
- Suzuki, M. The chemistry of tea. In Tea Functions and Science; Morita, A., Masuda, S., Nakamura, Y., Sumikawa, O., Suzuki, M., Eds.; Asakura Shoten: Tokyo, Japan, 2013; pp. 98–108. [Google Scholar]
- Monobe, M.; Ema, K.; Tokuda, Y.; Maeda-Yamamoto, M. Effect on the epigallocatechin gallate/epigallocatechin ratio in a green tea (Camellia sinensis L.) extract of different extraction temperatures and its effect on IgA production in mice. Biosci. Biotechnol. Biochem. 2010, 74, 2501–2503. [Google Scholar] [CrossRef] [Green Version]
- Monobe, M. Health functions of compounds extracted in cold-water brewed green tea from Camellia sinensis L. Japan Agric. Res. Q. 2018, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Furushima, D.; Nomura, Y.; Yamada, H.; Iguchi, K.; Taguchi, K.; Suzuki, T.; Ozeki, M.; Nakamura, Y. Antidepressant effect of shaded white leaf tea containing high levels of caffeine and amino acids. Molecules 2020, 25, 3550. [Google Scholar] [CrossRef]
- Seong, K.J.; Lee, H.G.; Kook, M.S.; Ko, H.M.; Jung, J.Y.; Kim, W.J. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J. Physiol. Pharmacol. 2016, 20, 41–51. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Zhao, G.; Kan, Z.; Wang, X.; Wang, H.; Huang, J.; Wang, T.; Qian, F.; Ho, C.T.; et al. Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice. J. Agric. Food Chem. 2018, 66, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine may abrogate LPS-induced oxidative stress and neuroinflammation by regulating Nrf2/TLR4 in adult mouse brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.U.; Ali, T.; Hao, Q.; He, K.; Li, W.; Ullah, N.; Zhang, Z.; Jiang, Y.; Li, S. Xanthohumol attenuates lipopolysaccharide-induced depressive like behavior in mice: Involvement of NF-κB/Nrf2 signaling pathways. Neurochem Res. 2021, 46, 3135–3148. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Tanida, N.; Ishii, N.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; Takeda, A.; Ozawa, H.; Ohkubo, T.; Juneja, L.R.; et al. Anti-stress effect of theanine on students during pharmacy practice: Positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013, 111, 128–135. [Google Scholar] [CrossRef]
- Keller, S.; Malarski, A.; Reuther, C.; Kertscher, R.; Kiehntopf, M.; Jahreis, G. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1169–1179. [Google Scholar] [CrossRef]
- Dong-Newsom, P.; Powell, N.D.; Bailey, M.T.; Padgett, D.A.; Sheridan, J.F. Repeated social stress enhances the innate immune response to a primary HSV-1 infection in the cornea and trigeminal ganglia of Balb/c mice. Brain Behav. Immun. 2010, 24, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Delépine, C.; Nectoux, J.; Letourneur, F.; Baud, V.; Chelly, J.; Billuart, P.; Bienvenu, T. Astrocyte transcriptome from the Mecp2(308)-truncated mouse model of rett syndrome. Neuromol. Med. 2015, 17, 353–363. [Google Scholar] [CrossRef]
- Ibi, D.; Takuma, K.; Koike, H.; Mizoguchi, H.; Tsuritani, K.; Kuwahara, Y.; Kamei, H.; Nagai, T.; Yoneda, Y.; Nabeshima, T.; et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008, 105, 921–932. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Takai, N.; Mizuno, Y.; Yoshida, H. Performance evaluation of salivary amylase activity monitor. Biosens. Bioelectron. 2004, 20, 491–497. [Google Scholar] [CrossRef]
- Zung, W.W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Suk, K. Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective. Prog. Neurobiol. 2016, 144, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Calabrese, F.; Auletta, F.; Olivier, J.; Racagni, G.; Homberg, J.; Riva, M.A. Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: Modulation by antidepressant treatment. Neuropsychopharmacology 2012, 37, 746–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, C.E.; Coutellier, L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci. Biobehav. Rev. 2019, 105, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, K.; Sumiyoshi, A.; Konishi, T.; Hayashi, M.; Taguchi, K.; Muguruma, Y.; Inoue, K.; Iguchi, K.; Nonaka, H.; Kawashima, R.; et al. Theanine, the main amino acid in tea, prevents stress-induced brain atrophy by modifying early stress responses. Nutrients 2020, 12, 174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Fei, P.; Mu, J.; Li, W.; Song, J. Hippocampal expression of aryl hydrocarbon receptor nuclear translocator 2 and neuronal PAS domain protein 4 in a rat model of depression. Neurol. Sci. 2014, 35, 277–282. [Google Scholar] [CrossRef]
- Drouet, J.B.; Peinnequin, A.; Faure, P.; Denis, J.; Fidier, N.; Maury, R.; Buguet, A.; Cespuglio, R.; Canini, F. Stress-induced hippocampus Npas4 mRNA expression relates to specific psychophysiological patterns of stress response. Brain Res. 2018, 1679, 75–83. [Google Scholar] [CrossRef]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Nakamura, Y. Green tea suppresses brain aging. Molecules 2021, 26, 4897. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Camfield, D.A.; Stough, C.; Farrimond, J.; Scholey, A.B. Acute effects of tea constituents l-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: A systematic review and meta-analysis. Nutr. Rev. 2014, 72, 507–522. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Sequence | Reverse Sequence | Ref. |
|---|---|---|---|
| TNF-α | CTGTCTACTGAACTTCGGGGTGAT | GGTCTGGGCCATAGAACTGATG | [16] |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT | [17] |
| Lcn2 | TACAATGTCACCTCCATCCTGG | TGCACATTGTAGCTCTGTACCT | [18] |
| Npas4 | AGCATTCCAGGCTCATCTGAA | GGCGAAGTAAGTCTTGGTAGGATT | [19] |
| β-actin | TGACAGGATGCAGAAGGAGA | GCTGGAAGGTGGACAGTGAG |
| Item | Group A (CE/TA = 4.7) | Group B (CE/TA = 3.9) |
|---|---|---|
| Number of participants | 41 | 40 |
| Gender (male/female) | 6/35 | 12/28 |
| Age | 52.3 (±15.7) | 54.5 (±15.4) |
| Green tea drinking habit (%) | 39 (0.95) | 36 (0.90) |
| CE/TA (Mole Ratio) | Theanine (µmole) | Arginine (µmole) | Caffeine (µmole) | EGCG (µmole) |
|---|---|---|---|---|
| 1.0 | 80 | 40 | 60 | 60 |
| 2.0 | 80 | 40 | 120 | 120 |
| 4.0 | 40 | 20 | 120 | 120 |
| 8.0 | 40 | 20 | 240 | 240 |
| 12.0 | 40 | 20 | 360 | 360 |
| Depression-Related Items | Optimum CE/TA | |
|---|---|---|
| 1-Day | 2-Day | |
| Sucrose preference | 4–12 | (No depressive behavior) |
| Adrenal hypertrophy | 2 | 4–8 |
| Thymic atrophy | 1–2 | No protective effect |
| Inflammatory gene expression | 1–2 | 4–12 |
| Npas4 suppression | 4 | 4 |
| Powdered Green Tea | Theanine (µmole) | Arginine (µmole) | Caffeine (µmole) | EGCG (µmole) | CE/TA (Mole Ratio) |
|---|---|---|---|---|---|
| A | 48 ± 1 | 7 ± 0 | 120 ± 2 | 139 ± 2 | 4.71 ± 0.09 |
| B | 53 ± 0 | 5 ± 0 | 93 ± 1 | 136 ± 2 | 3.94 ± 0.11 |
| Item | Comparison before and after Intervention (p-Value) | |
|---|---|---|
| Group A (CE/TA = 4.7) | Group B (CE/TA = 3.9) | |
| State-Trait Anxiety Inventory (STAI) | 0.0614 | 0.0045 |
| Self-rated depression scale (SDS) | 0.0410 | 0.0219 |
| Stress (salivary amylase activity) | 0.3307 | 0.1227 |
| Physical condition | 0.0241 | 0.2262 |
| Subjective sleep sensation | 0.6351 | 0.9070 |
| Number of awakenings | 0.7801 | 0.0631 |
| Energy consumption | 0.7382 | 0.0868 |
| Resting heart rate | 0.5200 | 0.8725 |
| Rest time | 0.6305 | 0.7118 |
| Number of steps | 0.6694 | 0.0963 |
| Sleep time (A) | 0.0357 | 0.0839 |
| Bedtime (B) | 0.0734 | 0.1212 |
| Sleep efficiency (A/B) | 0.3503 | 0.3842 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unno, K.; Furushima, D.; Tanaka, Y.; Tominaga, T.; Nakamura, H.; Yamada, H.; Taguchi, K.; Goda, T.; Nakamura, Y. Improvement of Depressed Mood with Green Tea Intake. Nutrients 2022, 14, 2949. https://doi.org/10.3390/nu14142949
Unno K, Furushima D, Tanaka Y, Tominaga T, Nakamura H, Yamada H, Taguchi K, Goda T, Nakamura Y. Improvement of Depressed Mood with Green Tea Intake. Nutrients. 2022; 14(14):2949. https://doi.org/10.3390/nu14142949
Chicago/Turabian StyleUnno, Keiko, Daisuke Furushima, Yuya Tanaka, Takeichiro Tominaga, Hirotomo Nakamura, Hiroshi Yamada, Kyoko Taguchi, Toshinao Goda, and Yoriyuki Nakamura. 2022. "Improvement of Depressed Mood with Green Tea Intake" Nutrients 14, no. 14: 2949. https://doi.org/10.3390/nu14142949
APA StyleUnno, K., Furushima, D., Tanaka, Y., Tominaga, T., Nakamura, H., Yamada, H., Taguchi, K., Goda, T., & Nakamura, Y. (2022). Improvement of Depressed Mood with Green Tea Intake. Nutrients, 14(14), 2949. https://doi.org/10.3390/nu14142949