Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
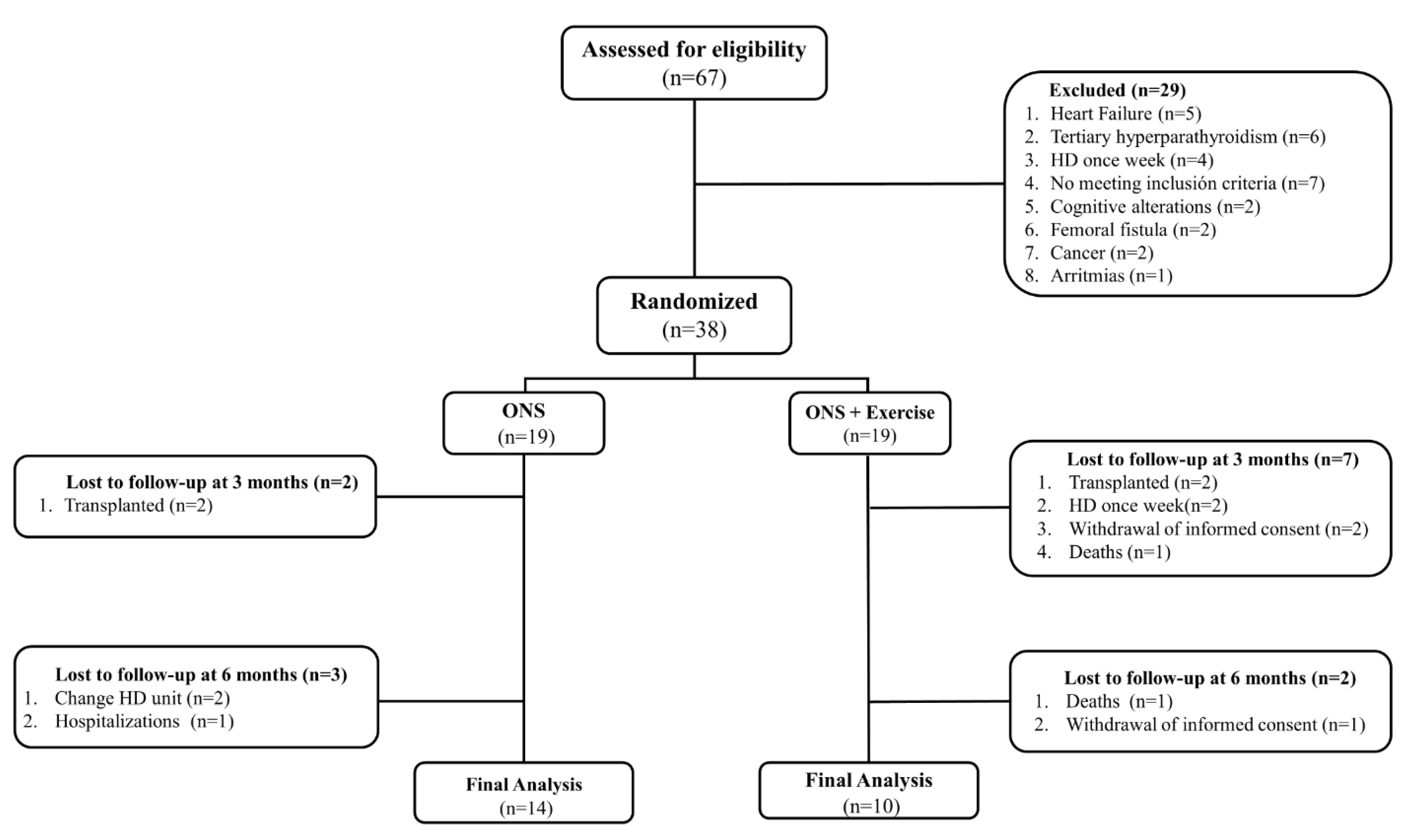
2.1. Study Design and Patients
2.2. Sample Size Calculation
2.3. Intervention
2.3.1. Oral Nutritional Supplementation Group (ONS)
2.3.2. Oral Nutritional Supplementation + Exercise Group (ONS + EX)
2.4. Primary Outcomes
2.4.1. Evaluation of the Quantity and Quality of Muscle Mass with Computed Tomography and Anthropometry
- Mid-arm muscle circumference:
- Mid-arm circumference—(π × triceps skinfold thickness)
- Bone-free arm muscle area:
- Males = [(midarm circumference (cm) − π × triceps (cm)]2/4 π) − 10
- Females = [(midarm circumference (cm) − π × triceps (cm)]2/4 π) − 6.5
2.4.2. Evaluation of Physical Function and Handgrip Strength
2.5. Secondary Outcomes
2.5.1. Body Composition and Nutritional Status Assessment
2.5.2. Laboratory Parameter Assessment
2.5.3. Quality of Life Assessment
2.6. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Changes in the Quality and Quantity of Muscle Mass Measured with Computed Tomography and Anthropometry
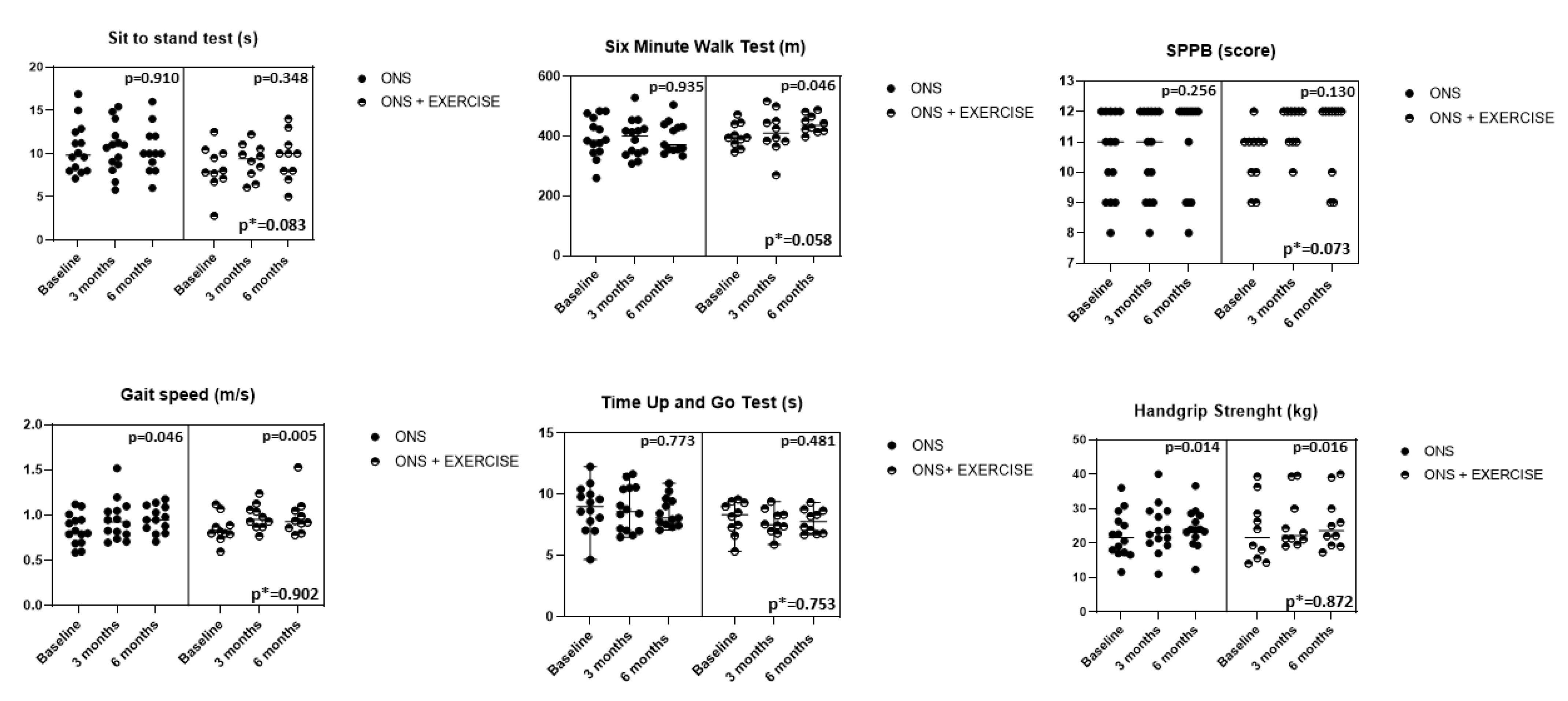
3.3. Changes in the Physical Function Tests and Handgrip Strength
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenvinkel, P.; Carrero, J.J.; Von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle Wasting in End-Stage Renal Disease Promulgates Premature Death: Established, Emerging and Potential Novel Treatment Strategies. Nephrol. Dial. Transpl. 2015, 31, 1070–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahal, I.H.; Bell, G.M.; Bone, J.M.; Edwards, R.H.T. Physiological Abnormalities of Skeletal Muscle in Dialysis Patients. Nephrol. Dial. Transpl. 1997, 12, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, R.L.; LaStayo, P.C.; Ikizler, T.A.; Wei, G.; Giri, A.; Chen, X.; Morrel, G.; Painter, P.; Beddhu, S. Low Physical Function in Maintenance Hemodialysis Patients Is Independent of Muscle Mass and Comorbidity. J. Ren. Nutr. 2015, 25, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.M.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Ba, P.; Cederholm, T.; Stenvinkel, P.; Jesu, J. Comparative Associations of Muscle Mass and Muscle Strength with Mortality in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; De Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between Physical Performance and All-Cause Mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, P.P.; Cappola, A.R.; Shults, J.; Townsend, R.R.; Gadegbeku, C.A.; Anderson, C.; Baker, J.F.; Carlow, D.; Sulik, M.J.; Lo, J.C.; et al. Physical Performance and Frailty in Chronic Kidney Disease. Am. J. Nephrol. 2014, 38, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Plantinga, L.C.; Johansen, K.; Crews, D.C.; Vahakn, B.; Robinson, B.M.; Saran, R.; Burrows, N.R. Association of CKD with Disability in the United States. Am. J. Kidney Dis. 2012, 57, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Manuscript, A.; Initiation, D. Frailty, Dialysis Initiation, and Mortality in End-Stage Renal Disease. Arch. Intern. Med. 2014, 172, 1071–1077. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Bortone, I.; Griseta, C.; Sardone, R.; Lampignano, L.; Lozupone, M.; Solfrizzi, V.; Castellana, M.; Giannelli, G.; et al. Nutritional Domains in Frailty Tools: Working towards an Operational Definition of Nutritional Frailty; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 0000000272. [Google Scholar]
- Roshanravan, B.; Gamboa, J. Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and Enteral Supplements for Improving Outcomes in Chronic Kidney Disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and Treatment of Protein Energy Wasting in Chronic Kidney Disease Patients: A Consensus Statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikizler, T.A. Exercise as an Anabolic Intervention in Patients With End-Stage Renal Disease. J. Ren. Nutr. 2011, 21, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilund, K.R.; Perez, L.M.; Sciences, H. A Critical Review of Exercise Training in Hemodialysis Patients: Personalized Activity Prescriptions Are Needed. Exerc. Sport Sci. Rev. 2020, 48, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sundell, M.B.; Cavanaugh, K.L.; Wu, P.; Shintani, A.; Hakim, R.M.; Ikizler, T.A. Oral Protein Supplementation Alone Improves Anabolism in a Dose-Dependent Manner in Chronic Hemodialysis Patients. J. Ren. Nutr. 2010, 19, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Segura-Ortí, E. Ejercicio En Pacientes En Hemodiálisis: Revisión Sistemática de La Literatura. Rev. Nefrol. 2010, 30, 236–246. [Google Scholar]
- Smart, N. The Effect of Exercise Therapy on Physical Function, Biochemistry and Dialysis Adequacy in Haemodialysis Patients: A Systematic Review and Meta-Analysis. Open J. Nephrol. 2013, 3, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, F.; Mallamaci, F.; Arrigo, G.D.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef]
- Bennett, P.N.; Fraser, S.; Barnard, R.; Haines, T.; Ockerby, C.; Street, M.; Wang, W.C.; Daly, R. Effects of an Intradialytic Resistance Training Programme on Physical Function: A Prospective Stepped-Wedge Randomized Controlled Trial. Nephrol. Dial. Transpl. 2015, 31, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Bohm, C.; Stewart, K.; Onyskie-Marcus, J.; Esliger, D.; Kriellaars, D.; Rigatto, C. Effects of Intradialytic Cycling Compared with Pedometry on Physical Function in Chronic Outpatient Hemodialysis: A Prospective Randomized Trial. Nephrol. Dial. Transpl. 2014, 29, 1947–1955. [Google Scholar] [CrossRef] [Green Version]
- Cheema, B.; Abas, H.; Smith, B.; O’Sullivan, A.; Chan, M.; Patwardhan, A.; Kelly, J.; Gillin, A.; Pang, G.; Lloyd, B.; et al. Progressive Exercise for Anabolism in Kidney Disease (PEAK): A Randomized, Controlled Trial of Resistance Training during Hemodialysis. J. Am. Soc. Nephrol. 2007, 18, 1594–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallamaci, F.; Torino, C.; Tripepi, G. Physical Exercise in Haemodialysis Patients: Time to Start. Nephrol. Dial. Transpl. 2016, 31, 1196–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, K.L.; Painter, P.L.; Sakkas, G.K.; Gordon, P.; Doyle, J.; Shubert, T. Effects of Resistance Exercise Training and Nandrolone Decanoate on Body Composition and Muscle Function among Patients Who Receive Hemodialysis: A Randomized. J. Am. Soc. Nephrol. 2006, 17, 2307–2314. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.P.G.M.; Saris, W.H.M.; van Loon, L.J.C. Protein Supplementation Augments the Adaptive Response of Skeletal Muscle to Resistance-Type Exercise Training: A Meta-Analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [Green Version]
- Martin-Alemañy, G.; Aguire-Esquivel, G.; Miranda-Alatriste, P.; Lopez-Alvarenga, J.C.; Olvera-Soto, G.; Valdez-Ortiz, R.; Espinosa-Cuevas, A.; Gomez-Guerrero, I.; Cantu-Quintanilla, G. The Effects of Resistance Exercise and Oral Nutritional Supplementation during Hemodialysis on Indicators of Nutritional Status and Quality of Life. Nephrol. Dial. Transpl. 2016, 31, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sundell, M.B.; Pupim, L.B.; Wu, P.; Shintani, A.; Ikizler, T.A. The Effect of Resistance Exercise to Augment Long-Term Benefits of Intradialytic Oral Nutritional Supplementation in Chronic Hemodialysis Patients. J. Ren. Nutr. 2011, 21, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Martin-Alemañy, G.; Espinosa-Cuevas, M.D.L.Á.; Pérez-Navarro, M.; Wilund, K.R.; Miranda-Alatriste, P.; Cortés-Pérez, M.; García-Villalobos, G.; Gómez-Guerrero, I.; Cantú-Quintanilla, G.; Ramírez-Mendoza, M.; et al. Effect of Oral Nutritional Supplementation with and without Exercise on Nutritional Status and Physical Function of Adult Hemodialysis Patients: A Parallel Controlled Clinical Trial (AVANTE-HEMO Study). J. Ren. Nutr. 2020, 30, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.H.; Biruete, A.; Tomayko, E.J.; Wu, P.T.; Fitschen, P.; Chung, H.R.; Ali, M.; McAuley, E.; Fernhall, B.; Phillips, S.A.; et al. Results from the Randomized Controlled IHOPE Trial Suggest No Effects of Oral Protein Supplementation and Exercise Training on Physical Function in Hemodialysis Patients. Kidney Int. 2019, 96, 777–786. [Google Scholar] [CrossRef]
- Hristea, D.; Deschamps, T.; Paris, A.; Lefrançois, G.; Collet, V.; Savoiu, C.; Ozenne, S.; Coupel, S.; Testa, A.; Magnard, J. Combining Intra-Dialytic Exercise and Nutritional Supplementation in Malnourished Older Haemodialysis Patients: Towards Better Quality of Life and Autonomy. Nephrology 2016, 21, 785–790. [Google Scholar] [CrossRef]
- López-Alvarenga, J.C.; Reyes-Díaz, S.; Castillo-Martínez, L.; Dávalos-Ibáñez, A.; González-Barranco, J. Reproducibility and Sensitivity of a Questionnaire on Physical Activity in a Mexican Population. Salud Publica Mex. 2001, 43, 306–312. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbonity in Longitudinal Studies: Development and Validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cocks, K.; Torgerson, D.J. Sample Size Calculations for Pilot Randomized Trials: A Confidence Interval Approach. J. Clin. Epidemiol. 2013, 66, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-gray, L.D.; Campbell, K.L.; Carrero, J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-fuchs, D.J.; et al. Kdoqi Clinical Practice Guideline for Nutrition in Ckd: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, B. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar]
- Painter, P. Exercise: A Guide for the People on Dialysis; Medical Education Institute: Madison, WI, USA, 2000; p. 6. Available online: http://lifeoptions.org/catalog/pdfs/booklets/exercise.pdf (accessed on 7 June 2022).
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.D. Quantitative Analysis of Skeletal Muscle by Computed Tomography Imaging—State of the Art. J. Orthop. Transl. 2018, 15, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, B. Anthropometric Measurement of Muscle Mass: Revised Equations for Calculating Arm Muscle Area. Am. J. Clin. Nutr. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission Energetic Cost of Walking in Older Adults View Project IOM Committee on Cognitive Agi. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J.; Bittner, V.; et al. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- González-Ortiz, A.J.; Arce-Santander, C.V.; Vega-Vega, O.; Correa-Rotter, R.; Espinosa-Cuevas, M.D.L.A. Assessment of the Reliability and Consistency of the “Malnutrition Inflammation Score” (MIS) in Mexican Adults with Chronic Kidney Disease for Diagnosis of Protein-Energy Wasting Syndrome (PEW). Nutr. Hosp. 2014, 31, 1352–1358. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Hacker, E.; Lora, C.M.; Ackerson, L.; De Salvo, K.B.; Go, A.; Kusek, J.W.; Nessel, L.; Ojo, A.; Townsend, R.R.; et al. Validation of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36TM) US Spanish and English Versions in a Cohort of Hispanics with Chronic Kidney Disease. Ethn. Dis. 2013, 23, 202–209. [Google Scholar]
- Ramos-Acevedo, S.; González-Ortiz, A.; Serralde-Zúñiga, A.E.; Colín-Ramírez, E.; Miranda-Alatriste, P.; López-Cisneros, S.; Rodríguez-González, N.; Correa-Rotter, R.; Atilano-Carsi, X.; Espinosa-Cuevas, Á. Frequency of Intradialytic Hypotension Events Do Not Increase with Oral Nutritional Supplementation during Hemodialysis Treatment: A Randomized Controlled Trial. J. Ren. Nutr. 2021, 31, 669–678. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Skinner, S.K.; Beals, J.W.; Pagni, B.A.; Fang, H.Y.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; Mazzulla, M.; West, D.W.D.; et al. Dysregulated Handling of Dietary Protein and Muscle Protein Synthesis After Mixed-Meal Ingestion in Maintenance Hemodialysis Patients. Kidney Int. Rep. 2018, 3, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Molsted, S.; Harrison, A.P.; Eidemak, I.; Andersen, J.L. The Effects of High-Load Strength Training With Protein- or Nonprotein-Containing Nutritional Supplementation in Patients Undergoing Dialysis. J. Ren. Nutr. 2013, 23, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Cafarelli, E.; Gary, A.; Dooly, C.; Matthew, S.; Fleck, S.J.; Fry, A.C.; Hoffman, J.R.; Newton, R.U.; Potteiger, J.; et al. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2002, 34, 364–380. [Google Scholar]
- Krzysztofik, M.; Wilk, M.; Wojdała, G.; Gołaś, A. Maximizing Muscle Hypertrophy: A Systematic Review of Advanced Resistance Training Techniques and Methods. Int. J. Environ. Res. Public Health 2019, 16, 4897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahal, I.H. Uraemic Sarcopenia: Aetiology and Implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological Inhibition of Myostatin Suppresses Systemic Inflammation and Muscle Atrophy in Mice with Chronic Kidney Disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [Green Version]
- Taaffe, D.R.; Henwood, T.R.; Nalls, M.A.; Walker, D.G.; Lang, T.F.; Harris, T.B. Alterations in Muscle Attenuation Following Detraining and Retrainingin Resistance Trained Older Adults. Gerontology 2009, 55, 217–223. [Google Scholar] [CrossRef]
- Paulussen, K.J.M.; Mckenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849. [Google Scholar] [CrossRef]
| Variables | ONS (n = 14) | ONS + EXERCISE (n = 10) | p |
|---|---|---|---|
| Age (years) mean ± SD | 38.14 ± 12 | 28.5 ± 9.5 | 0.047 |
| Male (n/%) | 5 (35.7) | 5 (50) | 0.484 |
| Etiology (n/%) | 0.318 | ||
| Unknown | 9 (64.3) | 7 (70) | |
| Diabetes mellitus | 2 (14.3) | 0 (0) | |
| Glomerulopathy | 1 (7.1) | 0 (0) | |
| Hypertension | 1 (7.1) | 3 (30) | |
| Other | 1 (7.1) | 0 (0) | |
| Frequency of dialysis (%) | 0.550 | ||
| 2 times per week | 13 (92.9) | 8 (80) | |
| 3 times per week | 1 (7.1) | 2 (20) | |
| Dialysis vintage, months | 61 ± 43 | 33 ± 19 | 0.078 |
| Comorbidities (%) | |||
| Diabetes | 2 (14.3) | 0 (0) | 0.493 |
| Hypertension | 14 (100) | 10 (100) | 0.05 |
| Vascular access (%) | 0.188 | ||
| Catheter | 8 (57.1) | 7 (70) | |
| AV fistula | 6 (42.9) | 3 (30) | |
| Residual uresis (ml) | 0 (0.0–162) | 0 (0.0–0.0) | 0.546 |
| Charlson Index Comorbidity | 2 (2–4) | 2 (2–2) | 0.259 |
| Variables | ONS (n = 14) | ONS + EXERCISE (n = 10) | |||||
|---|---|---|---|---|---|---|---|
| BASELINE (n = 14) | 6 MONTHS (n = 14) | p* | BASELINE (n = 10) | 6 MONTHS (n = 10) | p* | p+ | |
| Anthropometrics Weight (kg) Mid-arm circumference (cm) Arm muscle circumference (mm) Arm muscle area (cm2) Fat mass (%) Triceps skin-fold thickness (mm) | 54.7 ± 7.4 27 ± 3.1 230 (213–249) 37 ± 8.8 23 ± 8.4 12.8 ± 4.6 | 55.8 ± 6.7 26 ± 3 220 (207–238) 33.9 ± 9.1 23.8 ± 8.2 13.1 ± 5.2 | 0.014 0.151 0.084 0.097 0.311 0.537 | 56.2 ± 8.8 27.1 ± 3.5 228 (209–257) 36 ± 9.8 21.1 ± 7 13 ± 5.1 | 58.2 ± 9.2 26.9 ± 3.1 226 (207–246) 34.7 ± 9.2 22.9 ± 7.9 13.7 ± 5.2 | 0.001 0.778 0.508 0.544 0.046 0.066 | 0.462 0.770 0.886 0.838 0.793 0.798 |
| MIS | 5.5 (3.7–8.0) | 5 (3.5–8) | 0.063 | 4 (3–6.5) | 3.5 (1.7–6) | 0.086 | 0.259 |
| Bioimpedance analysis Resistance (ohm) Reactance (ohm) Phase angle (°) | 593 ± 96 57 ± 12 5.5 ± 0.98 | 599 ± 118 59 ± 21 5.5 ± 1.5 | 0.750 0.651 0.896 | 631 ± 109 64 ± 14 5.8 ± 0.68 | 622 ± 109 60 ± 13 5.5 ± 1.1 | 0.586 0.443 0.515 | 0.633 0.876 0.992 |
| Computed tomography Muscle attenuation (HU) Thigh muscle area (cm2) | 52 ± 5.3 96.2 ± 24 | 53 ± 3.7 98 ± 20 | 0.592 0.138 | 54.6 ± 3.4 100 ± 14 | 56 ± 3.3 97 ± 12 | 0.280 0.205 | 0.054 0.895 |
| Biochemical parameters Hemoglobin (g/dL) Total lymphocytes count (cells/mm3) Creatinine (mg/dL) Albumin (g/dL) Phosphorus (mg/dL) Potassium (mmol/L) CRP (mg/L) | 9.8 ± 1.8 1013 (850–1313) 13.3 ± 2.8 4.3 ± 0.41 5.9 ± 2.3 5.7 (5–6.1) 5.6 (2.8–8.9) | 9.9 ± 1.4 886 (795–1263) 11.4 ± 4.4 4.3 ± 0.47 5.2 ± 2.2 5.4 (5–5.8) 4.1 (2–7.3) | 0.834 0.551 0.049 0.390 0.128 0.115 0.638 | 10.9 ± 2 1065 (932–1556) 13.3 ± 3.5 4.2 ± 0.53 6.1 ± 2.1 5.1 (4.6–6.1) 4.5 (1.2–12.8) | 10.6 ± 1.8 1038 (864–1240) 13.5 ± 2.2 4.2 ± 0.29 5.5 ± 1.5 4.9 (4.7–5.6) 3.3 (2.9–9) | 0.740 0.594 0.873 0.849 0.242 0.212 0.594 | 0.306 0.477 0.207 0.396 0.770 0.336 0.781 |
| Variables | Cohen’s-d |
|---|---|
| Six-minute walk test (m) | 1.02 |
| Gait speed (m/s) | 0.17 |
| 5-Sit to stand test (s) | 0.33 |
| Timed up and go test (s) | 0.63 |
| Handgrip strength (kg) | 0.30 |
| SPPB (score) | 0.07 |
| ONS (n = 14) | ONS + EXERCISE (n = 10) | |||||
|---|---|---|---|---|---|---|
| Specific part | Pre | Post | p* | Pre | Post | p* |
| Symptoms | 74.1 ± 11.9 | 82.4 ± 9.8 | 0.04 | 83.5 ± 6.1 | 86.1 ± 7.9 | 0.25 |
| Effects of Kidney disease | 61.7 ± 21.3 | 73 ± 25 | 0.15 | 74.1 ± 12.3 | 74.4 ± 22 | 0.94 |
| Burden of kidney disease | 47.3 ± 15.6 | 59.3 ± 18.7 | 0.00 | 63 ± 14.7 | 57.8 ± 16.8 | 0.28 |
| Work status | 41.6 ± 41.7 | 50 ± 42.6 | 0.50 | 66.6 ± 38.9 | 62.5 ± 48.2 | 0.80 |
| Cognitive function | 25.5 ± 17.2 | 26.6 ± 17.9 | 0.85 | 15.5 ± 11.8 | 12.2 ± 9.7 | 0.35 |
| Quality of social interaction | 33.3 ± 15.8 | 27.7 ± 13.5 | 0.31 | 14.4 ± 9.7 | 19 ± 18 | 0.47 |
| Sexual function | 83.3 ± 28.8 | 75 ± 43.3 | 0.42 | 78.1 ± 31.1 | 65.6 ± 37.6 | 0.22 |
| Sleep | 66.8 ± 21.1 | 71.6 ± 13.7 | 0.42 | 78.7 ± 8.8 | 83.3 ± 13 | 0.17 |
| Social Support | 62.4 ± 18.9 | 70.8 ± 16 | 0.13 | 66.6 ± 14.2 | 66.6 ± 25.6 | 1.00 |
| Dialysis staff encouragement | 77 ± 11.7 | 73.9 ± 8.3 | 0.38 | 73.9 ± 11.2 | 77 ± 4.8 | 0.33 |
| Patient satisfaction | 74.2 ± 17.2 | 68.1 ± 26.3 | 0.22 | 72.2 ± 16.4 | 68 ± 22.9 | 0.51 |
| Generic part | Pre | Post | p* | Pre | Post | p* |
| Physical function | 74.1 ± 15.6 | 75.4 ± 20.6 | 0.78 | 88.3 ± 8.3 | 86.2 ± 7.4 | 0.21 |
| Physical role | 56.2 ± 44.1 | 56.2 ± 44.1 | 1.00 | 87.5 ± 31 | 85.4 ± 34.4 | 0.79 |
| Pain | 85.6 ± 16.1 | 85.2 ± 21.8 | 0.94 | 74.7 ± 28.3 | 79.1 ± 30.4 | 0.61 |
| General Health perceptions | 40.4 ± 13.8 | 46.6 ± 13.4 | 0.20 | 61.2 ± 9.5 | 58.7 ± 15.9 | 0.54 |
| Emotional well-being | 68.3 ± 18 | 73 ± 22.1 | 0.48 | 75.3 ± 16.2 | 82 ± 13.9 | 0.08 |
| Emotional role | 66.6 ± 34.8 | 66.6 ± 34.8 | 1.00 | 69.4 ± 36.1 | 97.2 ± 9.6 | 0.02 |
| Social function | 75 ± 25.5 | 94.7 ± 14.5 | 0.01 | 88.5 ± 13.5 | 86.4 ± 20.9 | 0.74 |
| Energy/fatigue | 61.6 ± 16.2 | 64.5 ± 18.6 | 0.58 | 70.8 ± 14.5 | 70.4 ± 18.1 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Alemañy, G.; Perez-Navarro, M.; Wilund, K.R.; García-Villalobos, G.; Gómez-Guerrero, I.; Cantú-Quintanilla, G.; Reyes-Caldelas, M.A.; Espinosa-Cuevas, A.; Escobedo, G.; Medeiros, M.; et al. Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study. Nutrients 2022, 14, 2946. https://doi.org/10.3390/nu14142946
Martin-Alemañy G, Perez-Navarro M, Wilund KR, García-Villalobos G, Gómez-Guerrero I, Cantú-Quintanilla G, Reyes-Caldelas MA, Espinosa-Cuevas A, Escobedo G, Medeiros M, et al. Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study. Nutrients. 2022; 14(14):2946. https://doi.org/10.3390/nu14142946
Chicago/Turabian StyleMartin-Alemañy, Geovana, Monserrat Perez-Navarro, Kenneth R. Wilund, Gloria García-Villalobos, Irma Gómez-Guerrero, Guillermo Cantú-Quintanilla, Miguel Angel Reyes-Caldelas, Angeles Espinosa-Cuevas, Galileo Escobedo, Mara Medeiros, and et al. 2022. "Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study" Nutrients 14, no. 14: 2946. https://doi.org/10.3390/nu14142946
APA StyleMartin-Alemañy, G., Perez-Navarro, M., Wilund, K. R., García-Villalobos, G., Gómez-Guerrero, I., Cantú-Quintanilla, G., Reyes-Caldelas, M. A., Espinosa-Cuevas, A., Escobedo, G., Medeiros, M., Bennett, P. N., & Valdez-Ortiz, R. (2022). Effect of Intradialytic Oral Nutritional Supplementation with or without Exercise Improves Muscle Mass Quality and Physical Function in Hemodialysis Patients: A Pilot Study. Nutrients, 14(14), 2946. https://doi.org/10.3390/nu14142946







