Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Serum Sample Collection and Measurement
2.3. Fecal Sample Collection and Microbial DNA Extraction and Sequencing
2.4. Quantification of Butyryl-CoA: Acetate CoA-Transferase [BCoAT] Gene
- BCoAT primer:Forward: 5′- GCIGAICATTTCACITGGAAYWSITGGCAYATG-3′Reverse: 5′- CCTGCCTTTGCAATRTCIACRAANGC-3′
- V3-V4 16S gene:Forward: 5′- CCTACGGGNGGCWGCAG-3′Reverse: 5′- CCTGCCTTTGCAATRTCIACRAANGC-3′
2.5. Statistical Analysis
3. Results
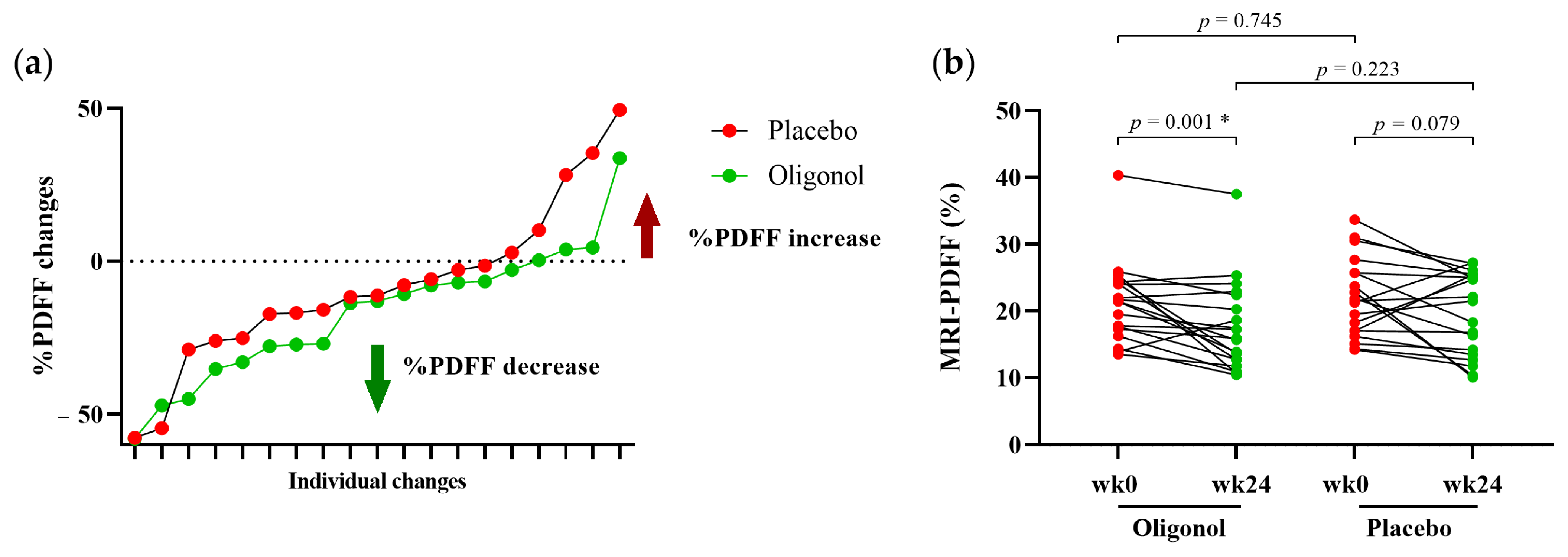
3.1. Clinical Parameters of Participants at Baseline and Week 24
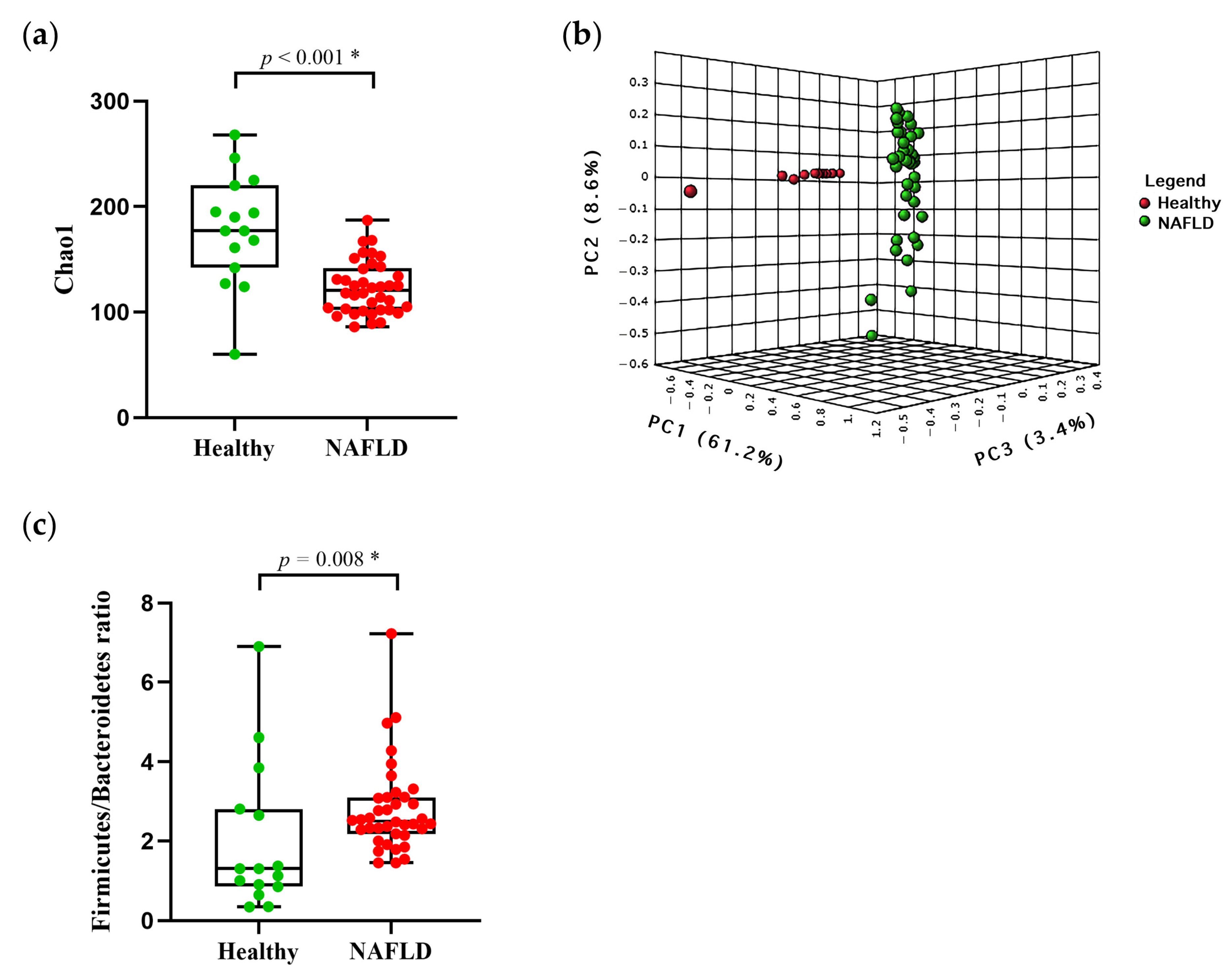
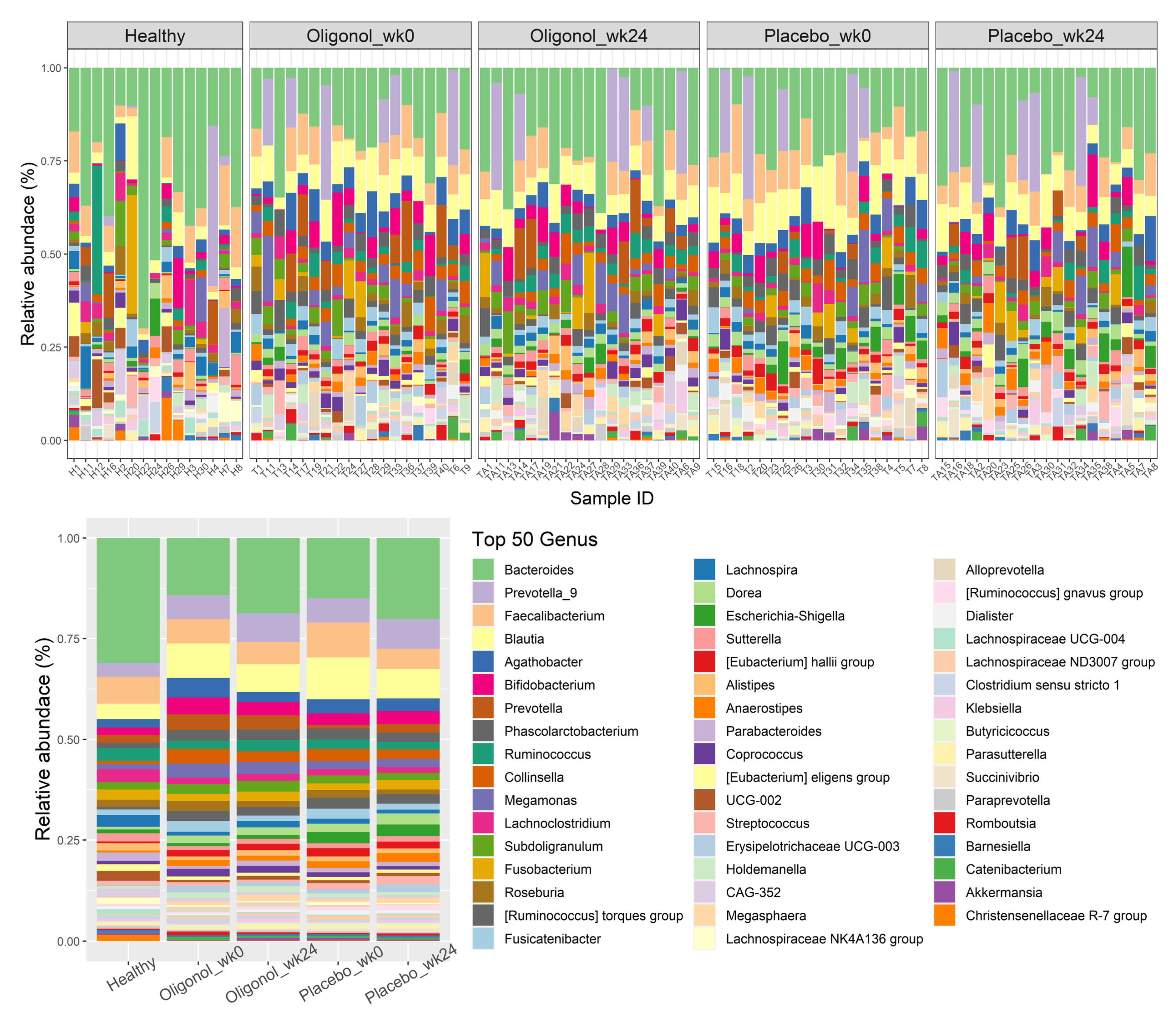
3.2. Baseline of Gut Diversity and Composition
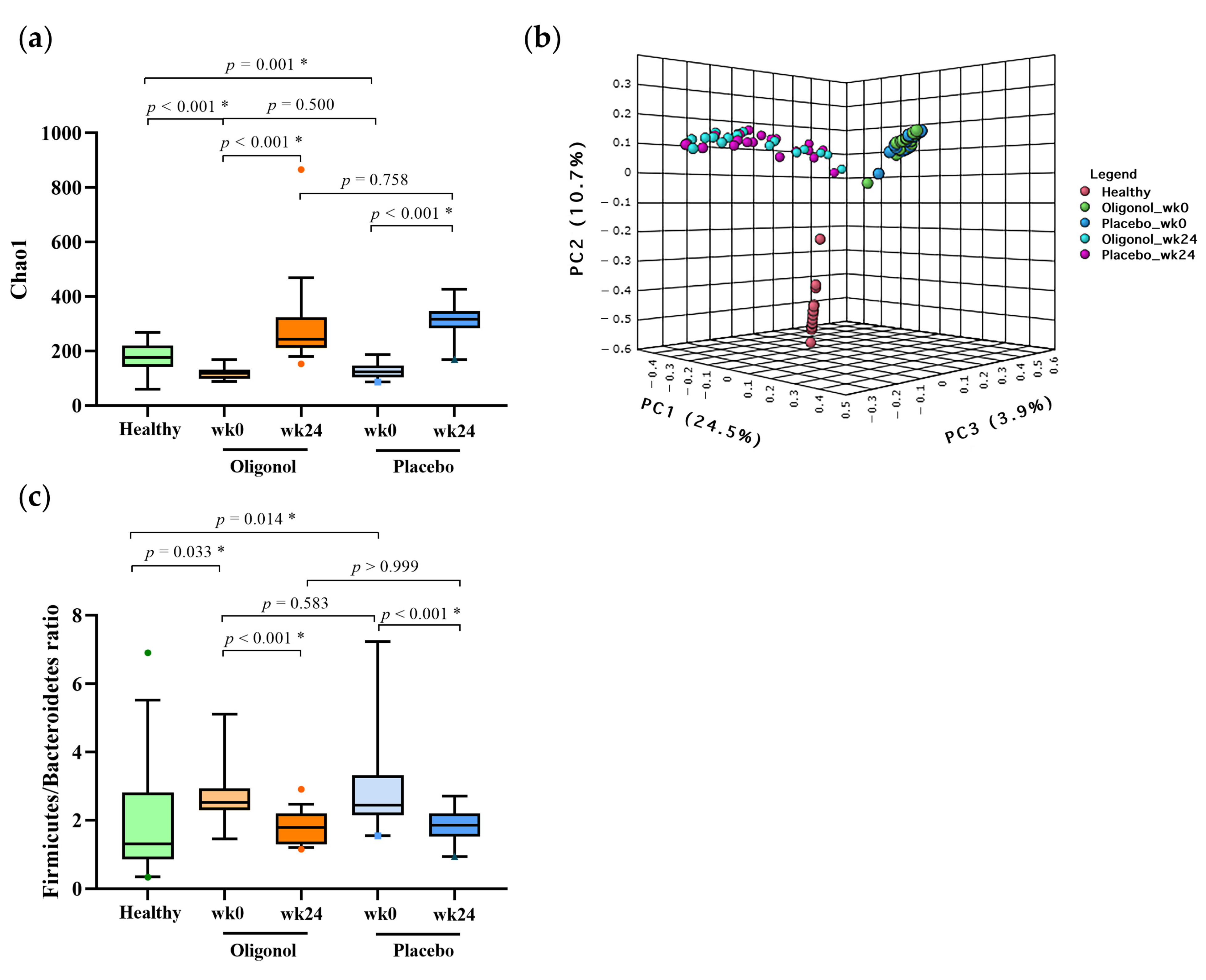
3.3. Changes in Gut Diversities and Composition in the Oligonol and Placebo Groups at Week 24
3.4. Quantification of BCoAT Genes at Week 0 and Week 24
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Hallsworth, K.; Adams, L.A. Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Rep. 2019, 1, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clement, K. Nonalcoholic Fatty Liver Disease: Modulating Gut Microbiota to Improve Severity? Gastroenterology 2020, 158, 1881–1898. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef]
- Panchal, S.K.; Brown, L. Tropical fruits from Australia as potential treatments for metabolic syndrome. Curr. Opin. Pharmacol. 2022, 63, 102182. [Google Scholar] [CrossRef]
- Emanuele, S.; Lauricella, M.; Calvaruso, G.; D’Anneo, A.; Giuliano, M. Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways. Nutrients 2017, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Zhang, R.; Zhou, Q.; Liu, L.; Huang, F.; Deng, Y.; Ma, Y.; Wei, Z.; Tang, X.; Zhang, M. Lychee (Litchi chinensis Sonn.) Pulp Phenolic Extract Provides Protection against Alcoholic Liver Injury in Mice by Alleviating Intestinal Microbiota Dysbiosis, Intestinal Barrier Dysfunction, and Liver Inflammation. J. Agric. Food Chem. 2017, 65, 9675–9684. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Chi, Y.Y.; Han, J.X.; Kong, P.; Liang, Z.; Wang, D.; Xiang, H.; Xie, Q. Litchi chinensis seed prevents obesity and modulates the gut microbiota and mycobiota compositions in high-fat diet-induced obese zebrafish. Food Funct. 2022, 13, 2832–2845. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease than Transient Elastography. Gastroenterology 2016, 150, 626–637.E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, H.; Sun, B.; Nishioka, H.; Hirose, A.; Aruoma, O.I. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem. Toxicol. 2007, 45, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Bettencourt, R.; Cui, J.; Salotti, J.; Hooker, J.; Bhatt, A.; Hernandez, C.; Nguyen, P.; Aryafar, H.; Valasek, M.; et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 2016, 9, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Costea, P.I.; Zeller, G.; Sunagawa, S.; Pelletier, E.; Alberti, A.; Levenez, F.; Tramontano, M.; Driessen, M.; Hercog, R.; Jung, F.E.; et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017, 35, 1069–1076. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Development of a Semiquantitative Degenerate Real-Time PCR-Based Assay for Estimation of Numbers of Butyryl-Coenzyme A (CoA) CoA Transferase Genes in Complex Bacterial Samples. Appl. Environ. Microbiol. 2007, 73, 2009–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Munaganuru, N.; Barnard, A.; Wang, J.L.; Kaulback, K.; Argo, C.K.; Singh, S.; Fowler, K.J.; Sirlin, C.B.; Loomba, R. Change in MRI-PDFF and Histologic Response in Patients with Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 2274–2283.e5. [Google Scholar] [CrossRef]
- Jayakumar, S.; Middleton, M.S.; Lawitz, E.J.; Mantry, P.S.; Caldwell, S.H.; Arnold, H.; Mae Diehl, A.; Ghalib, R.; Elkhashab, M.; Abdelmalek, M.F.; et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J. Hepatol. 2019, 70, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.S.; Park, C.H.; Yokozawa, T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br. J. Nutr. 2011, 106, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, J.; Kitadate, K.; Nishioka, H.; Fujii, H.; Sakurai, T.; Kizaki, T.; Izawa, T.; Ishida, H.; Ohno, H. Oligonol, a new lychee fruit-derived low-molecular form of polyphenol, enhances lipolysis in primary rat adipocytes through activation of the ERK1/2 pathway. Phytother. Res. 2009, 23, 1626–1633. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.; Im, J.A.; You, S.; Lee, H. Inhibition of Adipogenesis by Oligonol through Akt-mTOR Inhibition in 3T3-L1 Adipocytes. Evid.-Based Complement. Altern. Med. 2014, 2014, 895272. [Google Scholar] [CrossRef]
- Nishihira, J.; Sato-Ueshima, M.; Kitadate, K.; Wakame, K. Amelioration of abdominal obesity by low-molecular-weight polyphenol (Oligonol) from lychee. J. Funct. Foods 2009, 1, 341–348. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and Meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Z.; Wu, G.; Zhang, R.; Dong, L.; Huang, F.; Zhang, M.; Su, D. Lychee (Litchi chinensis Sonn.) Pulp Phenolics Activate the Short-Chain Fatty Acid-Free Fatty Acid Receptor Anti-inflammatory Pathway by Regulating Microbiota and Mitigate Intestinal Barrier Damage in Dextran Sulfate Sodium-Induced Colitis in Mice. J. Agric. Food Chem. 2021, 69, 3326–3339. [Google Scholar] [CrossRef]
- Boisseau, N.; Barnich, N.; Koechlin-Ramonatxo, C. The Nutrition-Microbiota-Physical Activity Triad: An Inspiring New Concept for Health and Sports Performance. Nutrients 2022, 14, 924. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandona, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef]
- Reid, D.T.; McDonald, B.; Khalid, T.; Vo, T.; Schenck, L.P.; Surette, M.G.; Beck, P.L.; Reimer, R.A.; Probert, C.S.; Rioux, K.P.; et al. Unique microbial-derived volatile organic compounds in portal venous circulation in murine non-alcoholic fatty liver disease. Biochim. Biophys. Acta 2016, 1862, 1337–1344. [Google Scholar] [CrossRef]
- Si, J.; Lee, G.; You, H.J.; Joo, S.K.; Lee, D.H.; Ku, B.J.; Park, S.; Kim, W.; Ko, G. Gut microbiome signatures distinguish type 2 diabetes mellitus from non-alcoholic fatty liver disease. Comput. Struct. Biotechnol. J. 2021, 19, 5920–5930. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hou, H.; Zhang, W.; Liu, T.; Li, Y.; Wang, S.; Wang, B.; Cao, H. Microbial Metabolites: Critical Regulators in NAFLD. Front. Microbiol. 2020, 11, 567654. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Endo, T.; Mikami, K.; Hasegawa, T.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: A large BMI- and sex-matched population study. Hepatol. Int. 2019, 13, 748–756. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia muciniphila: Is it the Holy Grail for ameliorating metabolic diseases? Gut Microbes 2021, 13, 1984104. [Google Scholar] [CrossRef]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Ebrahimzadeh Leylabadlo, H. Akkermansia muciniphila: From its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit. Rev. Food Sci. Nutr. 2022, Mar 3, 1–21. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]


| Healthy (n = 15) | Oligonol (n = 19) | Placebo (n = 19) | p-Value | |
|---|---|---|---|---|
| Age (years) | 50.7 ± 7.9 | 50.8 ± 10.1 | 49.5 ± 13.8 | 0.993 |
| Male gender, n (%) | 3(20%) | 12 (63.2%) | 11 (57.9%) | 0.012 *a,0.026 *b,0.740 c |
| BMI (kg/m2) | 23.4 ± 2.3 | 27.9 ± 2.6 | 27.9 ± 3.3 | <0.001 *a,b, 0.968 c |
| Waist circumference (cm) | 95.9 ± 6.8 | 95.1 ± 7.1 | 0.728 | |
| MetS, No n (%) | 4 (21.1%) | 3 (15.8%) | 0.676 | |
| AST (IU/L) | 20.3 ± 7.9 | 25.9 ± 5.1 | 34.4 ± 17.3 | 0.014 *a, 0.001 *b, 0.059 c |
| ALT (IU/L) | 17.3 ± 8.4 | 42.8 ± 17.5 | 53.6 ± 26.0 | <0.001 *a,b, 0.280 c |
| Fasting blood sugar (mg/dL) | 111.0 ± 41.4 | 96.5 ± 17.4 | 0.293 | |
| Total cholesterol (mg/dL) | 192.0 ± 31.7 | 190.1 ± 36.8 | 0.737 | |
| Triglyceride (mg/dL) | 140.1 ± 70.8 | 133.3 ± 76.4 | 0.737 | |
| HDL (mg/dL) | 50.0 ± 11.8 | 46.5 ± 11.1 | 0.357 | |
| LDL (mg/dL) | 119.9 ± 27.1 | 126.2 ± 31.6 | 0.516 | |
| HOMA-IR (median ± IQR) | 2.6 ± 3.0 | 2.4 ± 0.9 | 0.293 | |
| MRI-PDFF (%) | 21.4 ± 6.1 | 22.0 ± 5.9 | 0.378 |
| Oligonol (n = 19) | Placebo (n = 19) | p-Value ϯ | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | p-Value | Week 0 | Week 24 | p-Value | ||
| Body weight (kg) | 78.1 ± 9.7 | 76.6 ± 10.1 | 0.004 * | 76.3 ± 13.0 | 75.2 ± 12.4 | 0.024 * | 0.697 |
| Waist circumference (cm) | 95.9 ± 6.8 | 94.4 ± 6.8 | 0.074 | 95.1 ± 7.1 | 93.7 ± 6.9 | 0.089 | 0.761 |
| BMI (kg/m2) | 27.9 ± 2.6 | 27.3 ± 2.6 | 0.007 * | 27.9 ± 3.3 | 27.5 ± 3.3 | 0.027 * | 0.942 |
| AST (IU/L) | 25.9 ± 5.1 | 25.4 ± 7.1 | 0.569 | 34.4 ± 17.3 | 31.1 ± 11.8 | 0.294 | 0.065 |
| ALT (IU/L) | 42.8 ± 17.5 | 37.5 ± 14.0 | 0.036 * | 53.6 ± 26.0 | 46.7 ± 23.5 | 0.144 | 0.422 |
| Total bilirubin (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.494 | 0.7 ± 0.3 | 0.9 ± 0.3 | 0.006 * | 0.002 * |
| Fasting blood sugar (mg/dL) | 111.0 ± 41.4 | 102.4 ± 33.5 | 0.005 * | 96.5 ± 17.4 | 93.8 ± 12.2 | 0.163 | 0.919 |
| Total cholesterol (mg/dL) | 192.0 ± 31.7 | 194.3 ± 42.7 | 0.499 | 190.1 ± 36.8 | 185.0 ± 35.8 | 0.251 | 0.549 |
| Triglyceride (mg/dL, median ± IQR) | 121.0 ± 90.0 | 146.0 ± 121.0 | 0.026 * | 108.0 ± 38.0 | 149.0 ± 90.0 | 0.020 * | 0.770 |
| HDL (mg/dL) | 50.0 ± 11.8 | 48.5 ± 10.6 | 0.137 | 46.5 ± 11.1 | 44.7 ± 9.6 | 0.080 * | 0.219 |
| LDL (mg/dL) | 119.9 ± 27.1 | 116.4 ± 29.2 | 0.446 | 126.2 ± 31.6 | 122.3 ± 36.4 | 0.365 | 0.582 |
| HOMA-IR (median ± IQR) | 2.6 ± 3.0 | 3.1 ± 2.2 | 0.546 | 2.4 ± 0.9 | 3.1 ± 2.6 | 0.010 * | 0.804 |
| MRI-PDFF (%) | 21.4 ± 6.1 | 17.6 ± 6.8 | 0.030 * | 22.0 ± 5.9 | 19.7 ± 6.2 | 0.077 | 0.215 |
| Enriched in the Oligonol Group | ΔOligonol | ΔPlacebo | p−Value | ||
| Mean | SD | Mean | SD | ||
| g_Lachnospira | 0.005 | 0.011 | −0.002 | 0.009 | 0.047 |
| g_Feacalibacterium | −0.005 | 0.015 | −0.032 | 0.043 | 0.019 |
| g_Dialister | 0.002 | 0.005 | −0.002 | 0.006 | 0.044 |
| g_Akkermansia | 0.005 | 0.016 | −0.004 | 0.011 | 0.043 |
| Enriched in the Placebo Group | ΔOligonol | ΔPlacebo | p−Value | ||
| Mean | SD | Mean | SD | ||
| g_Dorea | −0.001 | 0.011 | 0.007 | 0.011 | 0.049 |
| g_Agathobacter | −0.022 | 0.030 | −0.002 | 0.017 | 0.009 |
| g_Erysipelotrichaceae UCG−003 | −0.006 | 0.013 | 0.009 | 0.017 | 0.011 |
| g_Romboutsia | −0.005 | 0.009 | 0.002 | 0.009 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jinato, T.; Chayanupatkul, M.; Dissayabutra, T.; Chutaputti, A.; Tangkijvanich, P.; Chuaypen, N. Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients 2022, 14, 2921. https://doi.org/10.3390/nu14142921
Jinato T, Chayanupatkul M, Dissayabutra T, Chutaputti A, Tangkijvanich P, Chuaypen N. Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients. 2022; 14(14):2921. https://doi.org/10.3390/nu14142921
Chicago/Turabian StyleJinato, Thananya, Maneerat Chayanupatkul, Thasinas Dissayabutra, Anuchit Chutaputti, Pisit Tangkijvanich, and Natthaya Chuaypen. 2022. "Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study" Nutrients 14, no. 14: 2921. https://doi.org/10.3390/nu14142921
APA StyleJinato, T., Chayanupatkul, M., Dissayabutra, T., Chutaputti, A., Tangkijvanich, P., & Chuaypen, N. (2022). Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients, 14(14), 2921. https://doi.org/10.3390/nu14142921






