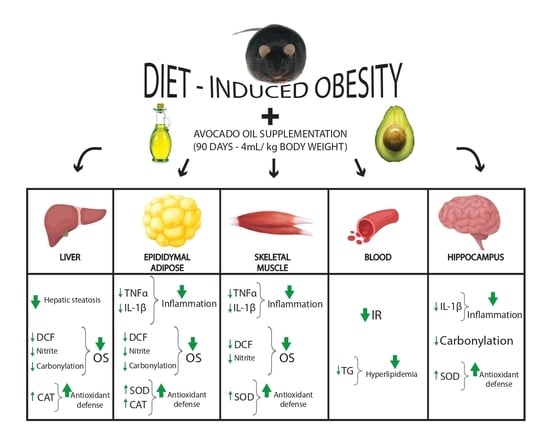
Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet and Characterization of Animals
2.2. Avocado Oil Supplementation
2.3. Characterization of Avocado Oil by Gas Chromatography
2.4. Insulin Tolerance Test
2.5. Behavioral Tasks
2.6. Adipose Fat Pads
2.7. Measurement of Serum Triglycerides Levels
2.8. Measurement of Inflammatory Markers and Neurotrophic Factors Level
2.9. Oxidative Status
2.10. 2′-7′-Dichlorofluorescin Diacetate (DCFH-DA) Oxidation Assay
2.11. Nitric Oxide (NO) Production Levels
2.12. Carbonyl Level in Proteins
2.13. Evaluation of Antioxidant Enzymes
2.14. Protein Content Determination
2.15. Statistical Analysis
3. Results
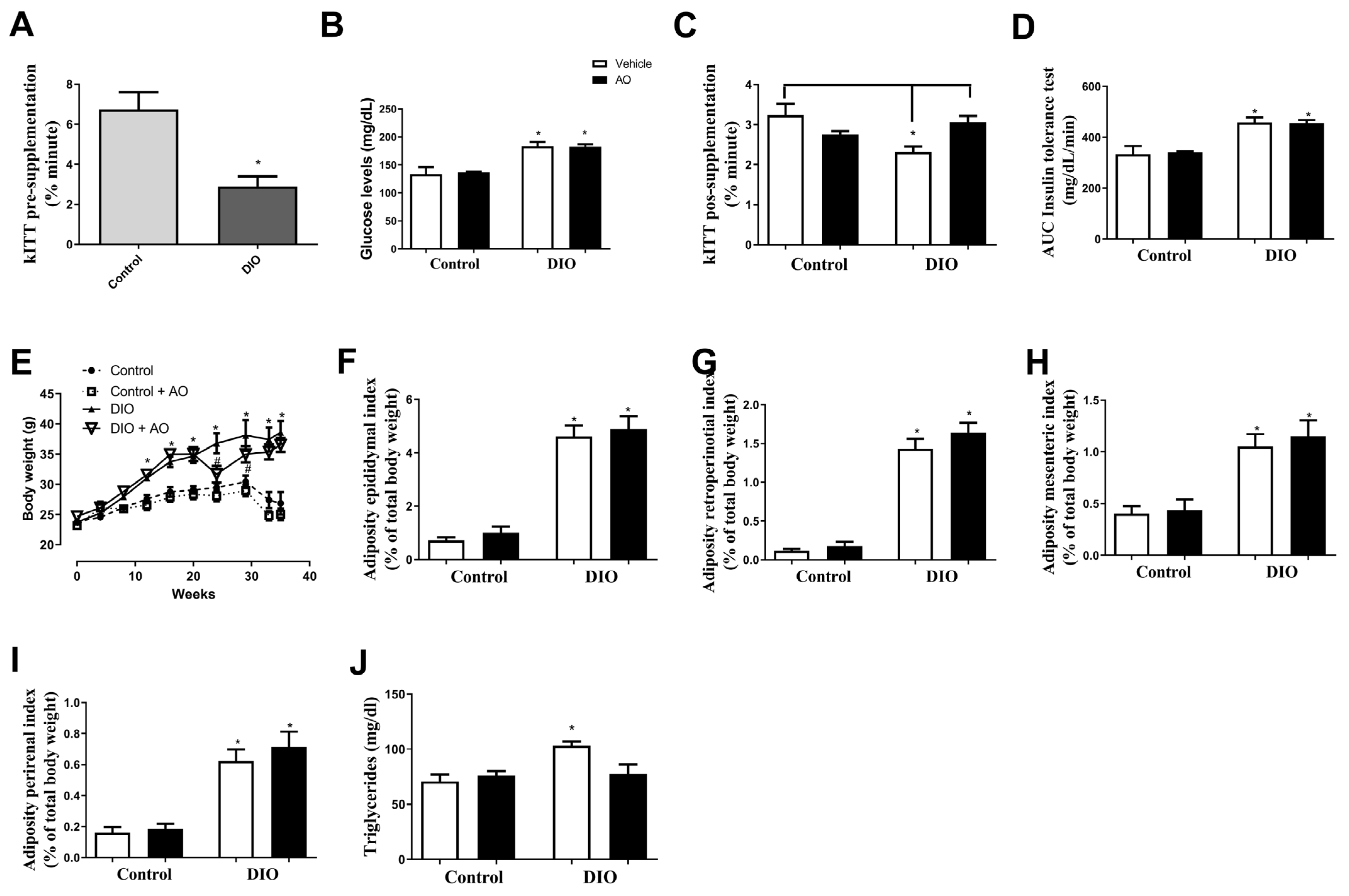
3.1. Effects of DIO and Avocado Oil Supplementation on Glucose Levels, Insulin Sensitivity, Body Weight/Adiposity, and Serum Triglyceride Levels
3.2. Effects of DIO and Avocado Oil Supplementation on Locomotion, Cognition, and Anxiety
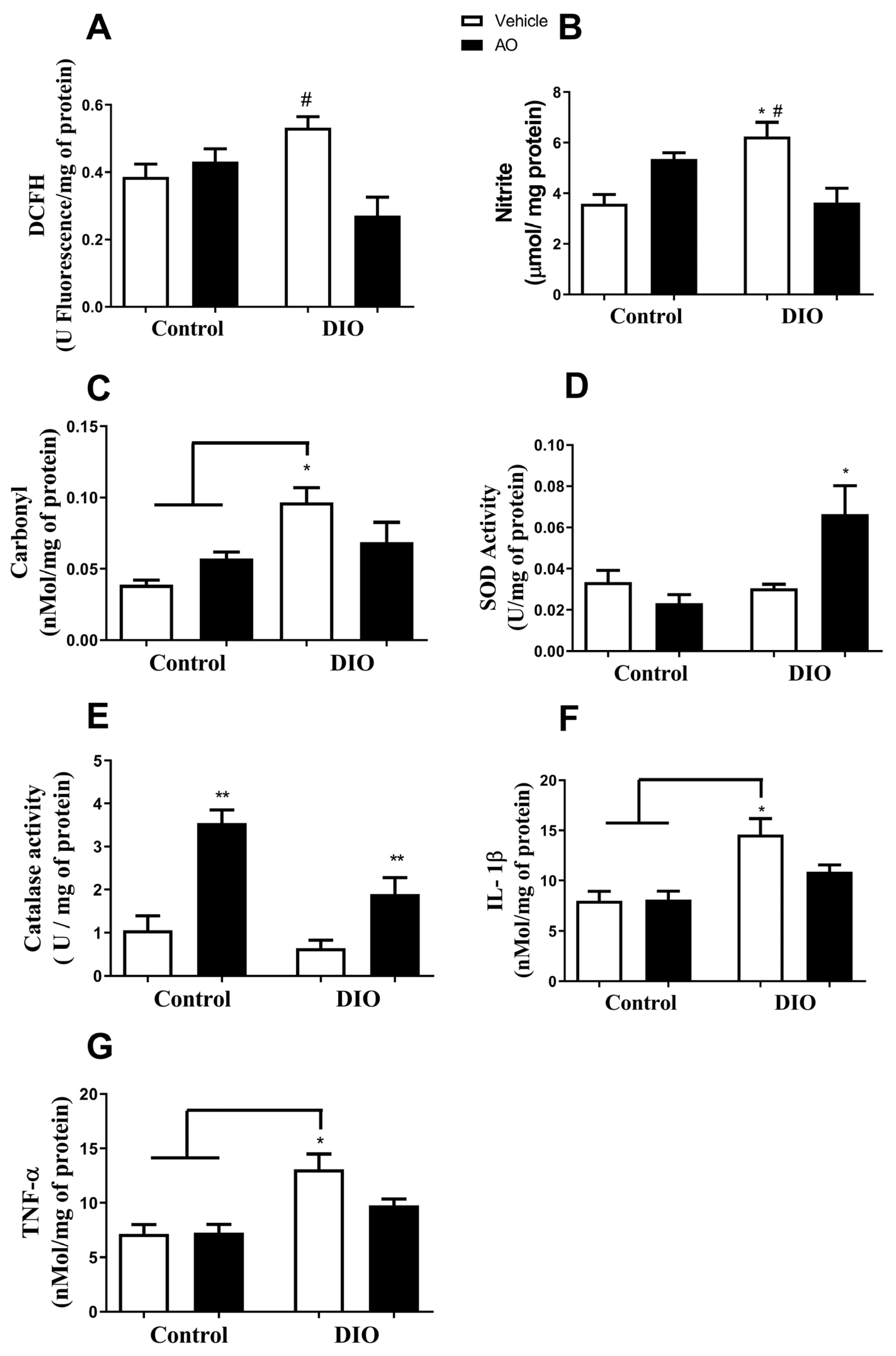
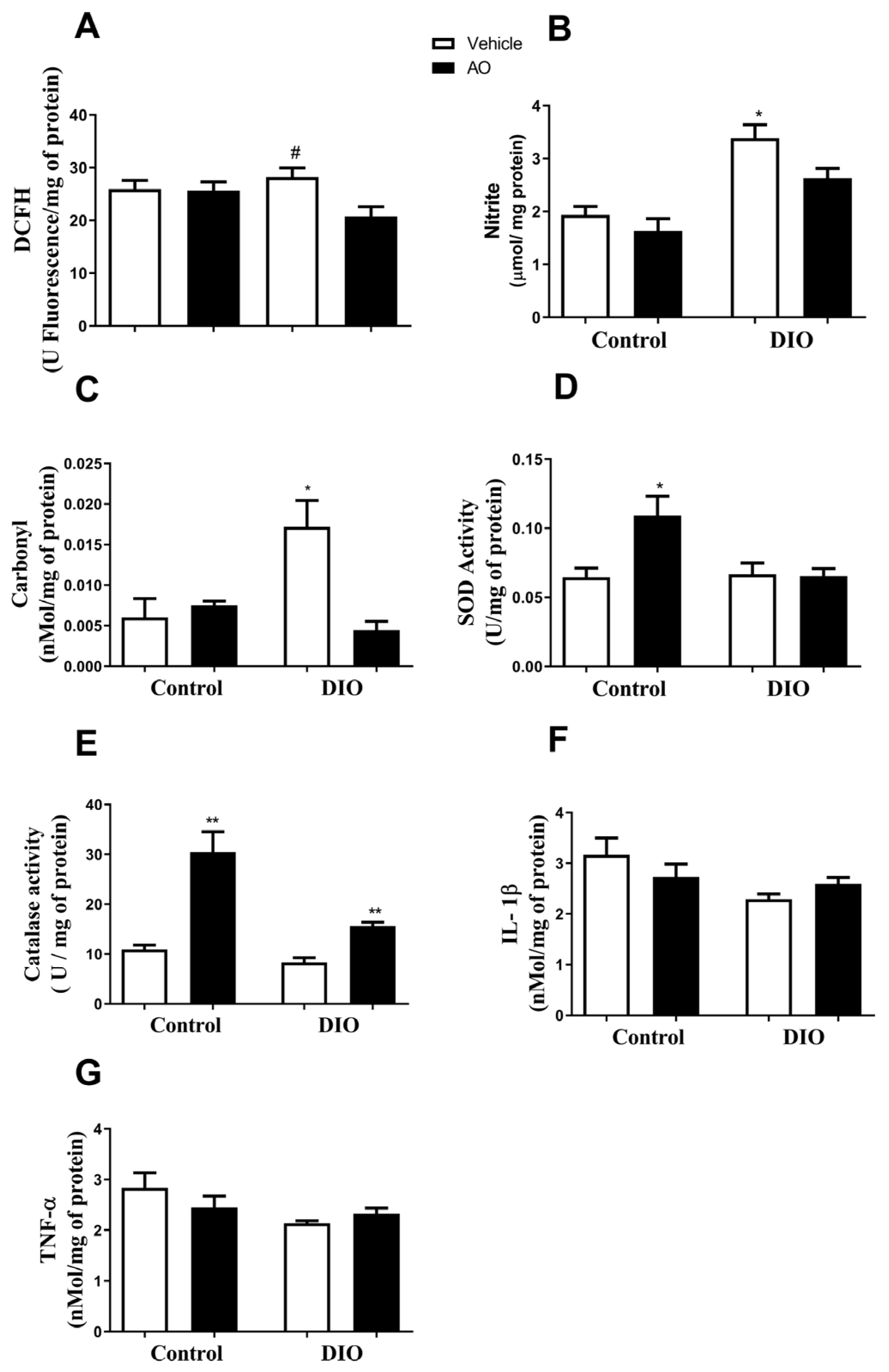
3.3. Effects of DIO and Avocado Oil Supplementation on the Epididymal Adipose Tissue
3.4. Effects of DIO and Avocado Oil Supplementation in the Quadriceps
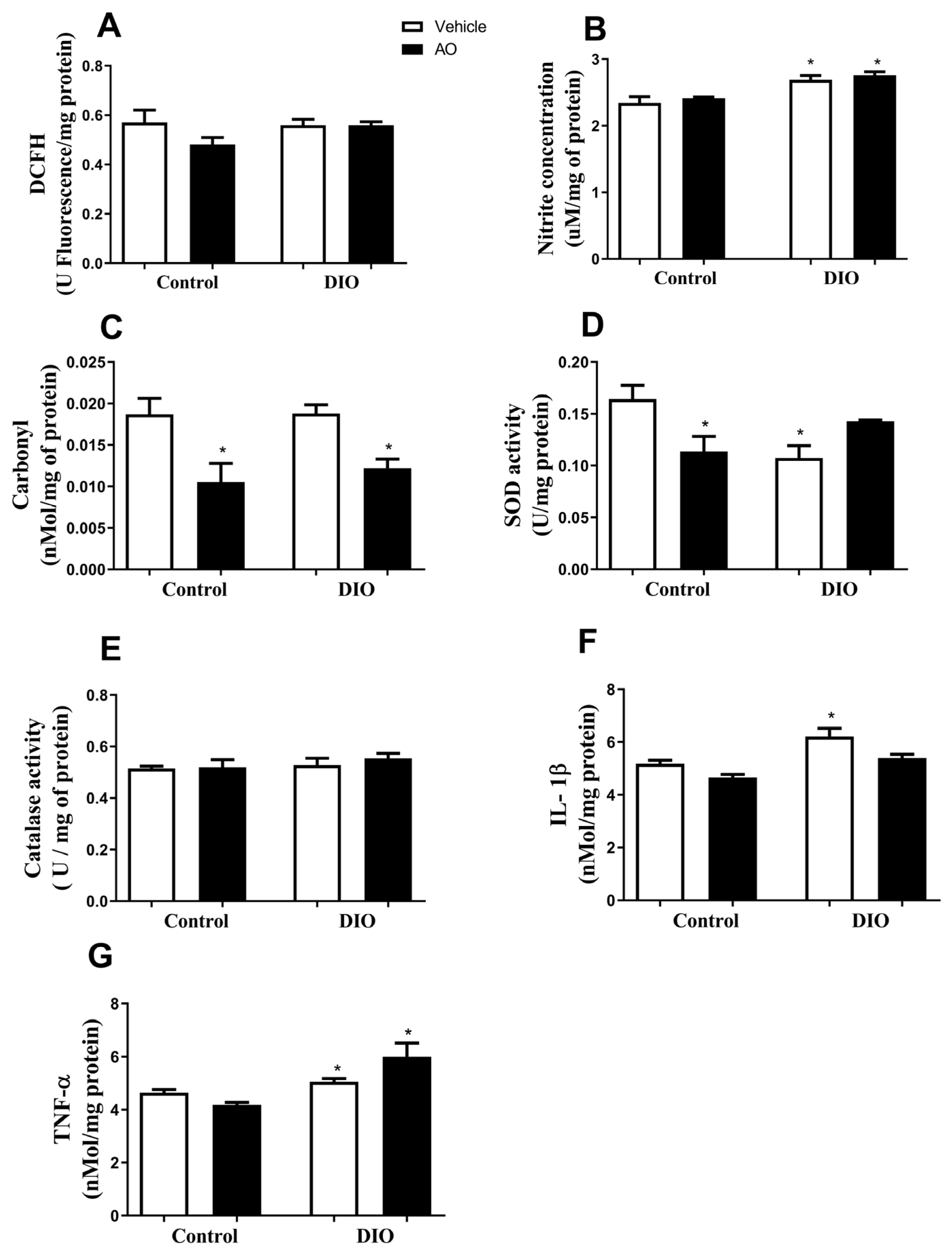
3.5. Effects of DIO and Avocado Oil Supplementation on the Liver
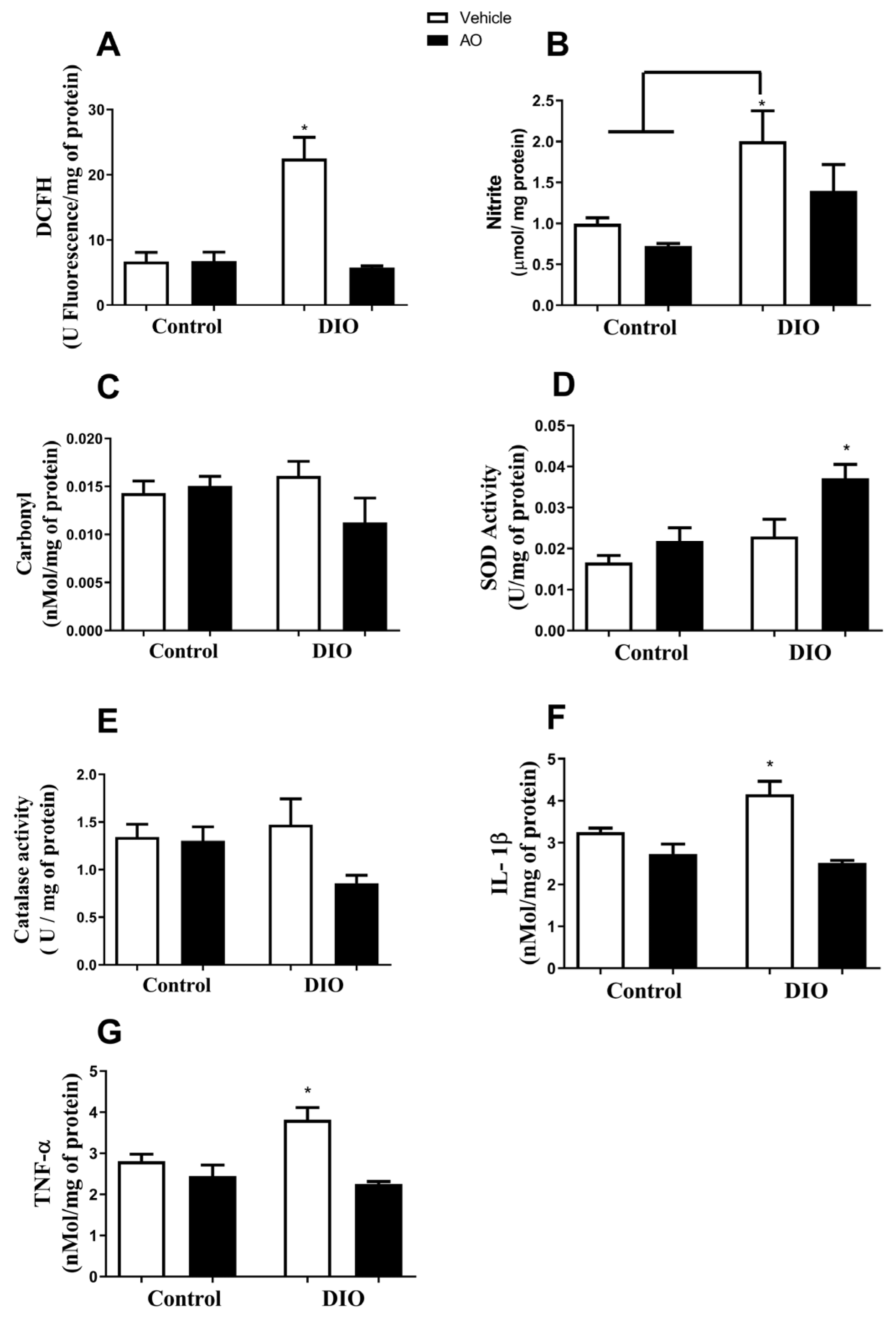
3.6. Effects of DIO and Avocado Oil Supplementation on the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.; Dragun, D.; Skurk, T.; et al. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 19, 5964. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-mediated inflammation and oxidative stress: Relevance to insulin resistance, obesity, and atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants—An update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerman-Garber, I.; Ichazo-Cerro, S.; Zamora-Gonzalez, J.; Cardoso-Saldana, G.; Posadas-Romero, C. Effect of a high-monounsaturated fat diet enriched with avocado in NIDDM patients. Diabetes Care 1994, 17, 311–315. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.G.; Melo-Santiesteban, G.; Hayward-Jones, P.M.; Barradas-Dermitz, D.M. Avocado oil supplementation modifies cardiovascular risk profile markers in a rat model of sucrose-induced metabolic changes. Dis. Markers 2014, 2014, 386425. [Google Scholar] [CrossRef] [PubMed]
- Brai, B.I.; Adisa, R.A.; Odetola, A.A. Hepatoprotective properties of aqueous leaf extract of Persea Americana, Mill (Lauraceae) ‘avocado’ against CCL4-induced damage in rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.Y.; Chung, K.S.; Shin, J.S.; Park, G.; Jang, Y.P.; Lee, K.T. Anti-colitic effects of ethanol extract of persea americana mill. through suppression of pro-inflammatory mediators via NF-kappaB/STAT3 inactivation in dextran sulfate sodium-induced colitis mice. Int. J. Mol. Sci. 2019, 20, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Avila, O.; Esquivel-Martínez, M.; Olmos-Orizaba, B.E.; Saavedra-Molina, A.; Rodriguez-Orozco, A.R.; Cortés-Rojo, C. Avocado oil improves mitochondrial function and decreases oxidative stress in brain of diabetic rats. J. Diabetes Res. 2015, 2015, 485759. [Google Scholar] [CrossRef] [Green Version]
- De Melo, M.; Pereira, D.E.; Moura, R.L.; da Silva, E.B.; de Melo, F.; Dias, C.C.Q.; Silva, M.; de Oliveira, M.E.G.; Viera, V.B.; Pintado, M.M.E.; et al. Maternal supplementation with avocado (Persea americana Mill.) pulp and oil alters reflex maturation, physical development, and offspring memory in rats. Front. Neurosci. 2019, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Zaki, S.M.; Fattah, S.A.; Hassan, D.S. The differential effects of high-fat and high- -fructose diets on the liver of male albino rat and the proposed underlying mechanisms. Folia Morphol. 2019, 78, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction Dynamics of Flavonoids and Carotenoids as Antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, K.; Asanuma, M.; Xu, C.; Mizobe, H.; Kojima, K.; Nagai, T.; Beppu, F.; Gotoh, N. Resolution behavior of cis- and trans-octadecenoic acid isomers by AOCS official method using SP-2560 column. J. Oleo Sci. 2013, 62, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Bonora, E.; Moghetti, P.; Zancanaro, C.; Cigolini, M.; Querena, M.; Cacciatori, V.; Corgnati, A.; Muggeo, M. Estimates of in vivo insulin action in man: Comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J. Clin. Endocrinol. Metab. 1989, 68, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.L.; Buettner, G.R.; O’Malley, Y.Q.; Wessels, D.A.; Flaherty, D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2’,7’-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, M.; Kim, S.W.; Bae, Y.H. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 2004, 25, 843–850. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Del Toro-Equihua, M.; Velasco-Rodriguez, R.; Lopez-Ascencio, R.; Vasquez, C. Effect of an avocado oil-enhanced diet (Persea americana) on sucrose-induced insulin resistance in Wistar rats. J. Food Drug Anal. 2016, 24, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Soriguer, F.; Morcillo, S.; Cardona, F.; Rojo-Martinez, G.; de la Cruz Almaraz, M.; de la Soledad Ruiz de Adana, M.; Olveira, G.; Tinahones, F.; Esteva, I. Pro12Ala polymorphism of the PPARG2 gene is associated with type 2 diabetes mellitus and peripheral insulin sensitivity in a population with a high intake of oleic acid. J. Nutr. 2016, 136, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and oleic acid: The yin and yang of fatty acids in type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, e29, 178–190. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. N-acetylcysteine protects mice from high fat diet-induced metabolic disorders. Pharm. Res. 2016, 33, 2033–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, M.; Arumugam, G. Effect of Persea americana (avocado) fruit extract on the level of expression of adiponectin and PPAR-gamma in rats subjected to experimental hyperlipidemia and obesity. J. Complement. Integr Med. 2014, 11, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Lee, H.Y. Non-Alcoholic fatty liver disease: The emerging burden in cardiometabolic and renal diseases. Diabetes Metab. J. 2017, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castejon, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Bessesen, D.H.; Vensor, S.H.; Jackman, M.R. Trafficking of dietary oleic, linolenic, and stearic acids in fasted or fed lean rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1124–E1132. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2016, 116, 1793–1801. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; de Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Antonioli, L.; Moriconi, D.; Mais, S.; Bottazzo, D.; Pellegrini, C.; Fornai, M.; Anselmino, M.; Ferrannini, E.; Blandizzi, C.; Taddei, S.; et al. Differential Impact of Weight Loss and Glycemic Control on Inflammasome Signaling. Obesity 2020, 28, 609–615. [Google Scholar] [CrossRef]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Avila, O.; Samano-Garcia, C.A.; Calderon-Cortes, E.; Perez-Hernandez, I.H.; Mejia-Zepeda, R.; Rodriguez-Orozco, A.R.; Saavedra-Molina, A.; Cortes-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Griesser, E.; Vemula, V.; Raulien, N.; Wagner, U.; Reeg, S.; Grune, T.; Fedorova, M. Cross-talk between lipid and protein carbonylation in a dynamic cardiomyocyte model of mild nitroxidative stress. Redox Biol. 2017, 11, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, E.; Artemenko, K.; Manukyan, L.; Bergquist, J.; Bergsten, P. Oleate protects beta-cells from the toxic effect of palmitate by activating pro-survival pathways of the ER stress response. Biochim. Biophys. Acta 2016, 1861, 1151–1160. [Google Scholar] [CrossRef]
- Curtis, J.M.; Grimsrud, P.A.; Wright, W.S.; Xu, X.; Foncea, R.E.; Graham, D.W.; Brestoff, J.R.; Wiczer, B.M.; Ilkayeva, O.; Cianflone, K.; et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes 2010, 59, 1132–1142. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Vargas, R.; Martinez, A. Carotenoids can act as antioxidants by oxidizing the superoxide radical anion. Phys. Chem. Chem. Phys. 2010, 12, 193–200. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Figueroa-Garcia, M.D.; Garcia-Berumen, C.I.; Calderon-Cortes, E.; Mejia-Barajas, J.A.; Rodriguez-Orozco, A.R.; Mejia-Zepeda, R.; Saavedra-Molina, A.; Cortes-Rojo, C. Avocado oil induces long-term alleviation of oxidative damage in kidney mitochondria from type 2 diabetic rats by improving glutathione status. J. Bioenerg. Biomembr. 2017, 49, 205–214. [Google Scholar] [CrossRef]
- Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation. Mol. Nutr. Food Res. 2019, 63, e1800978. [Google Scholar] [CrossRef]
- Denver, P.; Gault, V.A.; McClean, P.L. Sustained high-fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high-fat model. Diabetes Obes. Metab. 2018, 20, 1166–1175. [Google Scholar] [CrossRef]
- Hryhorczuk, C.; Décarie-Spain, L.; Sharma, S.; Daneault, C.; Rosiers, C.D.; Alquier, T.; Fulton, S. Saturated high-fat feeding independent of obesity alters hypothalamus-pituitary-adrenal axis function but not anxiety-like behaviour. Psychoneuroendocrinology 2017, 83, 142–149. [Google Scholar] [CrossRef]
- Scott, T.M.; Rasmussen, H.M.; Chen, O.; Johnson, E.J. Avocado consumption increases macular pigment density in older adults: A randomized, controlled trial. Nutrients 2017, 23, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef] [PubMed]


| High-Fat Diet | Standard Diet | ||||
|---|---|---|---|---|---|
| g/1000 g | kcal/1000 g | g/1000 g | kcal/1000 g | ||
| Corn starch | 147.5 | 590 | Carbohydrates | 530 | 2120 |
| Casein | 200 | 800 | Protein | 220 | 880 |
| Dextrinized starch | 100 | 400 | Lipids | 40 | 360 |
| Sucrose | 100 | 400 | Phosphorus | 8 | - |
| Soy oil | 40 | 360 | Mineral mix | 80 | - |
| Microcrystallized cellulose | 50 | - | Calcium | 10 | - |
| Mineral mix AIN93G | 35 | - | Fiber | 70 | - |
| Vitamin mix AIN93 | 10 | - | |||
| L-cystine | 3 | - | |||
| Choline bitartrate | 2.5 | - | |||
| Lard | 312 | 2808 | |||
| Identification | Concentration of Fatty Acids (%/) |
|---|---|
| C16:0 Methyl palmitate | 7.68 |
| C18:0 Methyl stearate | 2.79 |
| C16:1 (cis-9) Methyl palmitoleate | 2.00 |
| C18:1 (cis-9) Methyl oleate (omega 9) | 48.73 |
| C18:2 (cis-9,12) Methyl linoleate (omega 6) | 37.10 |
| C18:3 (cis-9,12,15) Methyl linolenate (omega 3) | 3.70 |
| Identification | Concentration of most important phytosterols (%) |
| α-Tocopherol | 75.00 |
| β-Sitosterol | 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Marques, S.; Muller, A.P.; Luciano, T.F.; dos Santos Tramontin, N.; da Silva Caetano, M.; Luis da Silva Pieri, B.; Amorim, T.L.; de Oliveira, M.A.L.; de Souza, C.T. Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients 2022, 14, 2906. https://doi.org/10.3390/nu14142906
de Oliveira Marques S, Muller AP, Luciano TF, dos Santos Tramontin N, da Silva Caetano M, Luis da Silva Pieri B, Amorim TL, de Oliveira MAL, de Souza CT. Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients. 2022; 14(14):2906. https://doi.org/10.3390/nu14142906
Chicago/Turabian Stylede Oliveira Marques, Schérolin, Alexandre Pastoris Muller, Thais Fernandes Luciano, Natália dos Santos Tramontin, Mateus da Silva Caetano, Bruno Luis da Silva Pieri, Tatiane Lima Amorim, Marcone Augusto Leal de Oliveira, and Cláudio Teodoro de Souza. 2022. "Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice" Nutrients 14, no. 14: 2906. https://doi.org/10.3390/nu14142906
APA Stylede Oliveira Marques, S., Muller, A. P., Luciano, T. F., dos Santos Tramontin, N., da Silva Caetano, M., Luis da Silva Pieri, B., Amorim, T. L., de Oliveira, M. A. L., & de Souza, C. T. (2022). Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients, 14(14), 2906. https://doi.org/10.3390/nu14142906