Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Anthropometry and Body Composition
2.4. Biochemical Measurements and Sexual Development
2.5. Physical Activity
2.6. Dietary Assessment
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Magnesium Intake
3.3. Associations of Magnesium Intake with Cardiometabolic Risks
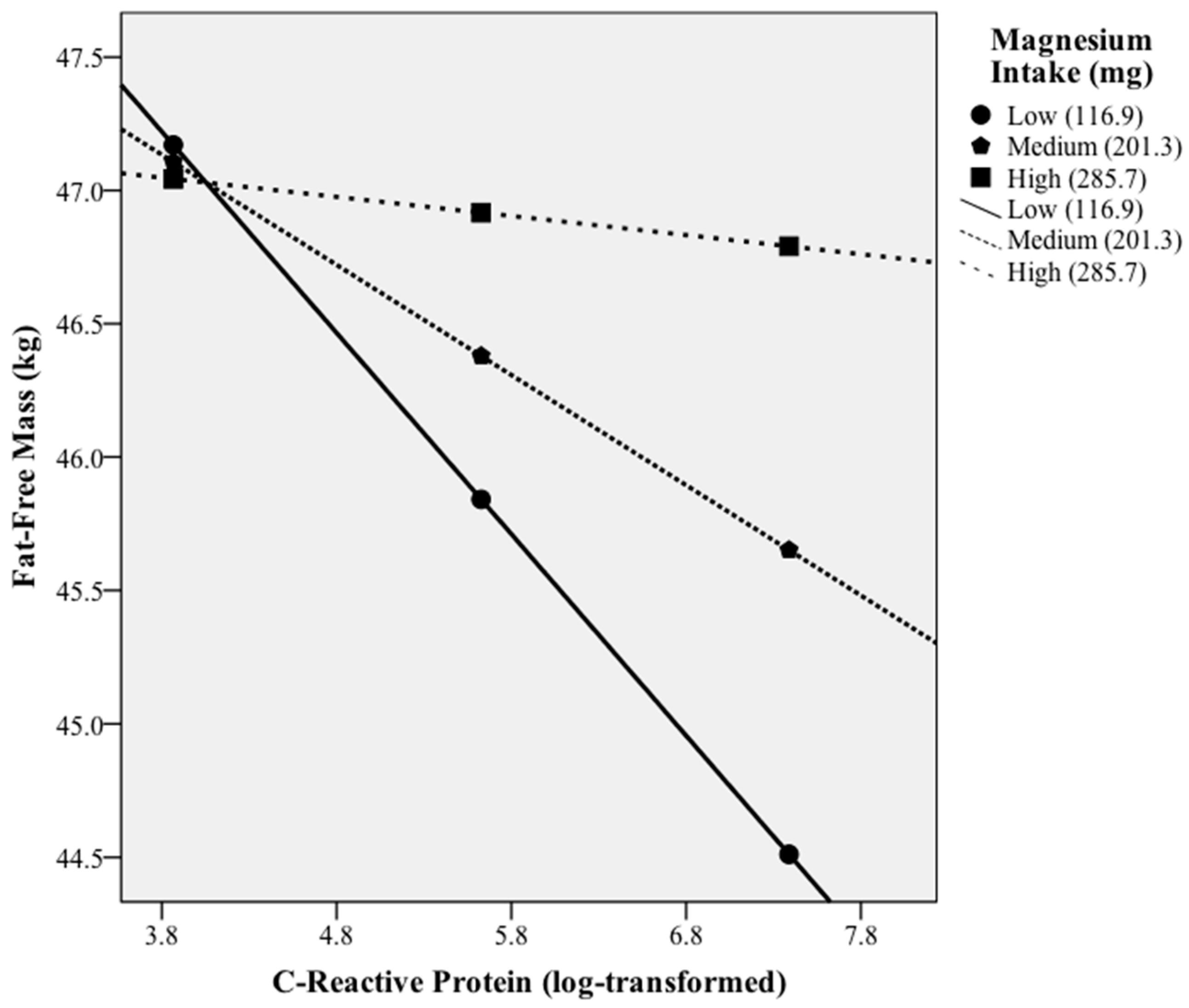
3.4. Moderation of Magnesium Intake on C-Reactive Protein and Fat-Free Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chutia, H.; Lynrah, K.G. Association of Serum Magnesium Deficiency with Insulin Resistance in Type 2 Diabetes Mellitus. J. Lab. Physicians 2015, 7, 75–78. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S.C., Jr. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Bahramimoghadam, S.; Sharifzadeh, F.; Asemi, Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2018, 43, 565–570. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Goldman, J.; Ahuja, J.; Rhodes, D.; Lacomb, R. What We Eat in America, NHANES 2005-2006, Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium; US Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2009.
- Ford, E.S.; Mokdad, A.H. Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [Green Version]
- Costello, R.; Wallace, T.C.; Rosanoff, A. Magnesium. Adv. Nutr. Int. Rev. J. 2016, 7, 199–201. [Google Scholar] [CrossRef]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated with Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association Between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Miner. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Dibaba, D.T.; Xun, P.; He, K. Dietary Magnesium Intake Is Inversely Associated with Serum C-reactive Protein Levels: Meta-analysis and Systematic Review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artero, E.G.; España-Romero, V.; Jiménez-Pavón, D.; Martinez-Gómez, D.; Warnberg, J.; Gómez-Martínez, S.; González-Gross, M.; Vanhelst, J.; Kafatos, A.; Molnar, D.; et al. Muscular fitness, fatness and inflammatory biomarkers in adolescents. Pediatric Obes. 2014, 9, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance: A Possible Interface of Inflammation and Metabolism in Obesity-Related Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, A.S.; Cardoso, R.O.; Carvalho, D.F.; Collet, N.; Medeiros, C.C.M. C-reactive protein and cardiometabolic risk factors in overweight or obese children and adolescents. Rev. De Nutr. 2014, 27, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Chiu, F.-H.; Chuang, C.H.; Li, W.-C.; Weng, Y.-M.; Fann, W.-C.; Lo, H.-Y.; Sun, C.; Wang, S.-H. The association of leptin and C-reactive protein with the cardiovascular risk factors and metabolic syndrome score in Taiwanese adults. Cardiovasc. Diabetol. 2012, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fugoni, V., 3rd. Dietary magnesium usual intake is associated with favorable diabetes-related physiological outcomes and reduced risk of metabolic syndrome: An NHANES 2001–2010 analysis. J. Hum. Nutr. Food Sci. 2014, 2, 1038. [Google Scholar]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Yokota, K.; He, K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet. Med. A J. Br. Diabet. Assoc. 2014, 31, 1301–1309. [Google Scholar] [CrossRef]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatric Diabetes 2017, 18, 895–902. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Whincup, P.H. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am. J. Clin. Nutr. 2007, 86, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13, 6–13. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Malpuech-Brugère, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.-P.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta. (BBA)-Mol. Basis Dis. 2000, 1501, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Menaker, W.; Kleiner, I.S. Effect of Deficiency of Magnesium and Other Minerals on Protein Synthesis. Proc. Soc. Exp. Biol. Med. 1952, 81, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Yin, Z.; Humphries, M.C.; Barbeau, P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am. J. Clin. Nutr. 2005, 81, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zierle-Ghosh, A.; Jan, A. Physiology, Body Mass Index; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Stallmann-Jorgensen, I.S.; Gutin, B.; Hatfield-Laube, J.L.; Humphries, M.C.; Johnson, M.H.; Barbeau, P. General and visceral adiposity in black and white adolescents and their relation with reported physical activity and diet. Int. J. Obes. 2007, 31, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Gutin, B.; Johnson, M.H.; Humphries, M.C.; Hatfield-Laube, J.L.; Kapuku, G.K.; Allison, J.D.; Gower, B.A.; Daniels, S.R.; Barbeau, P. Relationship of Visceral Adiposity to Cardiovascular Disease Risk Factors in Black and White Teens. Obesity 2007, 15, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process. Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Dietary Guidelines for Americans, 2015–2020; Office of Disease Prevent: Atlanta, GA, USA, 2015.
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Institute of Medicine, Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride; National Academy Press: Washingtion, DC, USA, 1997.
- Tarleton, E.K. Factors influencing magnesium consumption among adults in the United States. Nutr. Rev. 2018, 76, 526–538. [Google Scholar] [CrossRef]
- Kim, D.J.; Xun, P.; Liu, K.; Loria, C.; Yokota, K.; Jacobs, D.R.; He, K. Magnesium Intake in Relation to Systemic Inflammation, Insulin Resistance, and the Incidence of Diabetes. Diabetes Care 2010, 33, 2604–2610. [Google Scholar] [CrossRef] [Green Version]
- Simental-Mendia, L.E.; Sahebkar, A.; Rodriguez-Moran, M.; Zambrano-Galvan, G.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Plasma C-reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2017, 23, 4678–4686. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Rashvand, S.; Mobasseri, M.; Tarighat-Esfanjani, A. The Effects of Choline and Magnesium Co-Supplementation on Metabolic Parameters, Inflammation, and Endothelial Dysfunction in Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2019, 38, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Bussière, F.o.I.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Protective effect of calcium deficiency on the inflammatory response in magnesium-deficient rats. Eur. J. Nutr. 2002, 41, 197–202. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanthan, P.; Horwich, T.B.; Tseng, C.H. Relation of Muscle Mass and Fat Mass to Cardiovascular Disease Mortality. Am. J. Cardiol. 2016, 117, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Cross-sectional associations of dietary and circulating magnesium with skeletal muscle mass in the EPIC-Norfolk cohort. Clin. Nutr. 2019, 38, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary Magnesium May Be Protective for Aging of Bone and Skeletal Muscle in Middle and Younger Older Age Men and Women: Cross-Sectional Findings from the UK Biobank Cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Chen, C.; Liu, W.; Zhou, T.; Xun, P.; He, K.; Chen, P. The effect of magnesium supplementation on muscle fitness: A meta-analysis and systematic review. Magnes. Res. 2017, 30, 120–132. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.A.; Monteiro, C.P.; Vasco, A.M.; Baptista, F.; Sardinha, L.B.; Laires, M.J.; Silva, A.M. Magnesium intake mediates the association between bone mineral density and lean soft tissue in elite swimmers. Magnes. Res. 2012, 25, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Abrams, S.A. The Relationship between Magnesium and Calcium Kinetics in 9- to 14-Year-Old Children. J. Bone Miner. Res. 1998, 13, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholst, I.N.; Steinberg, S.F.; Tropper, P.J.; Fox, H.E.; Segre, G.V.; Bilezikian, J.P. The Influence of Hypermagnesemia on Serum Calcium and Parathyroid Hormone Levels in Human Subjects. N. Engl. J. Med. 1984, 310, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J. Effects of Parathyroid Hormone on Skeletal Muscle Protein and Amino Acid Metabolism in the Rat. J. Clin. Investig. 1983, 71, 1806–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Sussman, K.E.; Draznin, B. Calcium-induced inhibition of phosphoserine phosphatase in insulin target cells is mediated by the phosphorylation and activation of inhibitor 1. J. Biol. Chem. 1992, 267, 5959–5963. [Google Scholar] [CrossRef]
- Sinha, I.; Sakthivel, D.; Varon, D.E. Systemic Regulators of Skeletal Muscle Regeneration in Obesity. Front. Endocrinol. 2017, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewski, M.; Charchar, F.J.; Przybycin, M.; Crawford, L.; Wallace, A.M.; Gosek, K.; Lowe, G.D.; Zukowska-Szczechowska, E.; Grzeszczak, W.; Sattar, N.; et al. Strikingly Low Circulating CRP Concentrations in Ultramarathon Runners Independent of Markers of Adiposity. How Low Can You Go 2003, 23, 1640–1644. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Sugiyama, A.; Aye, N.N.; Katahira, S.; Hagihara, A.; Hashimoto, K. Effects of magnesium sulfate on the canine cardiovascular system complicating astemizole overdose. J. Cardiovasc. Pharmacol. 1997, 29, 795–800. [Google Scholar] [CrossRef]
- Ma, Y.; Olendzki, B.C.; Pagoto, S.L.; Hurley, T.G.; Magner, R.P.; Ockene, I.S.; Schneider, K.L.; Merriam, P.A.; Hébert, J.R. Number of 24-Hour Diet Recalls Needed to Estimate Energy Intake. Ann. Epidemiol. 2009, 19, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Raina, S.K. Limitations of 24-hour Recall Method: Micronutrient Intake and the Presence of the Metabolic Syndrome. N. Am. J. Med. Sci. 2013, 5, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, B.; Dick, K.; Nelson, M. A comparison of four dietary assessment methods in materially deprived households in England. Public Health Nutr. 2008, 11, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Demographics | p-Value | ||||
|---|---|---|---|---|---|---|
| Male | Female | Black | White | Sex | Race | |
| General Demographics | ||||||
| Number of subjects | 381 | 385 | 389 | 377 | ||
| Age (years) | 16.12 ± 1.25 | 16.13 ± 1.21 | 16.18 ± 1.23 | 16.07 ± 1.20 | 0.87 | 0.30 |
| Male/Female Ratio | N/A | N/A | 0.98 | 0.99 | N/A | 0.95 |
| Black/White Ratio | 0.96 | 0.97 | N/A | N/A | 0.95 | N/A |
| BMI (kg/m2) | 22.88 ± 4.74 | 23.21 ± 3.51 | 24.32 ± 5.86 | 22.03 ± 4.09 | 0.14 | <0.001 |
| Physical Activity (minutes) | 53.98 ± 31.98 | 34.06 ± 21.70 | 44.04 ± 30.38 | 43.83 ± 27.64 | <0.001 | 0.61 |
| Tanner Stage | 4.30 ± 0.77 (n = 371) | 4.38 ± 12.32 (n = 375) | 4.41 ± 0.73 (n = 368) | 4.28 ± 0.73 (n = 378) | 0.19 | 0.02 |
| DEXA Measurements (n) | 376 | 380 | 372 | 384 | ||
| Fat-Free Mass (kg) | 52.75 ± 8.78 | 40.25 ± 6.03 | 48.42 ± 10.01 | 44.59 ± 9.29 | <0.001 | <0.001 |
| Fat Mass (kg) | 13.42 ± 10.06 | 19.23 ± 10.35 | 17.35 ± 11.91 | 15.36 ± 8.46 | <0.001 | <0.001 |
| Inflammation Measurements | ||||||
| hs-CRP (mg/L) | 0.93 ± 1.79 (n = 307) | 1.21 ± 2.48 (n = 329) | 1.20 ± 2.4 (n = 295) | 0.96 ± 1.94 (n = 341) | 0.10 | 0.17 |
| Leptin (ng/mL) | 6.14 ± 8.78 (n = 310) | 17.41 ± 13.71 (n = 338) | 14.16 ± 14.28 (n = 300) | 10.17 ± 11.29 (n = 348) | <0.001 | <0.001 |
| Resistin (ng/mL) | 10.98 ± 6.09 (n = 324) | 12.35 ± 6.34 (n = 339) | 11.77 ± 6.26 (n = 314) | 11.60 ± 5.12 (n = 349) | <0.001 | 0.72 |
| Adiponectin (μg/mL) | 7.53 ± 4.50 (n = 304) | 9.50 ± 5.36 (n = 294) | 8.50 ± 5.04 (n = 273) | 9.28 ± 5.11 (n = 325) | <0.001 | <0.001 |
| Dietary Intake (per day) | ||||||
| Energy Intake (kcal) | 2239.66 ± 627.84 | 1725.97 ± 570.73 | 1915.16 ± 651.50 | 2045.75 ± 647.24 | <0.001 | <0.001 |
| Magnesium (mg) | 200.66 ± 7.09 | 205.03 ± 7.05 | 187.75 ± 6.92 | 217.95 ± 6.81 | <0.001 | <0.001 |
| Predictors | Magnesium Intake | |
|---|---|---|
| Base Model | Base Model + Physical Activity | |
| hs-CRP | (n = 626) <−0.01 (−0.01, −0.00) * | (n = 542) <−0.01 (−0.01, −0.00) * |
| Leptin | (n = 638) <−0.01 (−0.01, −0.00) ** | (n = 553) <−0.01 (−0.01, −0.00) * |
| Resistin | (n = 653) <−0.01 (−0.00, 0.00) | (n = 565) <−0.01 (−0.00, 0.00) |
| Adiponectin | (n = 594) <0.01 (0.00, 0.00) | (n = 513) <0.01 (−0.01, 0.01) |
| Fat-Free Mass | (n = 745) 0.01 (0.01, 0.02) * | (n = 649) 0.01 (0.01, 0.02) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Chen, L.; Gutin, B.; Huang, Y.; Dong, Y.; Zhu, H. Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents. Nutrients 2022, 14, 2882. https://doi.org/10.3390/nu14142882
Dong Y, Chen L, Gutin B, Huang Y, Dong Y, Zhu H. Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents. Nutrients. 2022; 14(14):2882. https://doi.org/10.3390/nu14142882
Chicago/Turabian StyleDong, Yutong, Li Chen, Bernard Gutin, Ying Huang, Yanbin Dong, and Haidong Zhu. 2022. "Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents" Nutrients 14, no. 14: 2882. https://doi.org/10.3390/nu14142882
APA StyleDong, Y., Chen, L., Gutin, B., Huang, Y., Dong, Y., & Zhu, H. (2022). Magnesium Intake, C-Reactive Protein, and Muscle Mass in Adolescents. Nutrients, 14(14), 2882. https://doi.org/10.3390/nu14142882






