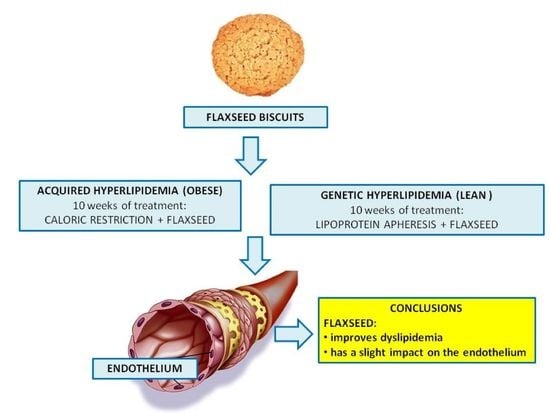
Effect of Flaxseed (Linum usitatissimum L.) Supplementation on Vascular Endothelial Cell Morphology and Function in Patients with Dyslipidaemia—A Preliminary Observation
Abstract
:1. Introduction
2. Methods
2.1. Study Design
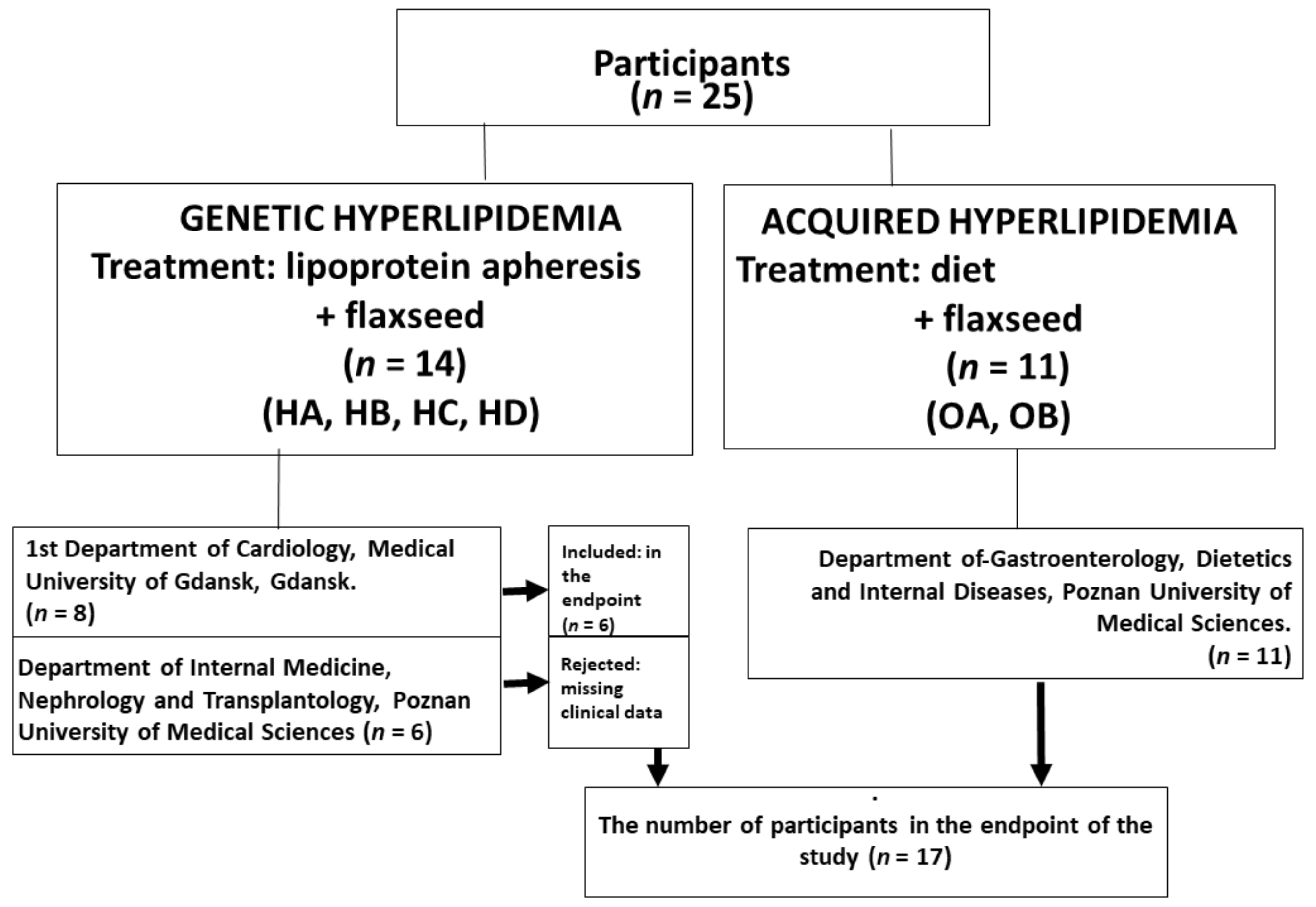
2.2. Study Population
2.3. Flaxseed Supplementation and the Study Protocol
2.4. Study Limitation
2.5. Biochemical Analyses
2.6. Cell Culture
2.7. Experimental Design
2.8. Endothelial Cell Proliferation
2.9. Light Microscopy
2.10. Electron Microscopy
2.11. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Effect of Flaxseed on Endothelial Cells’ Biomarkers
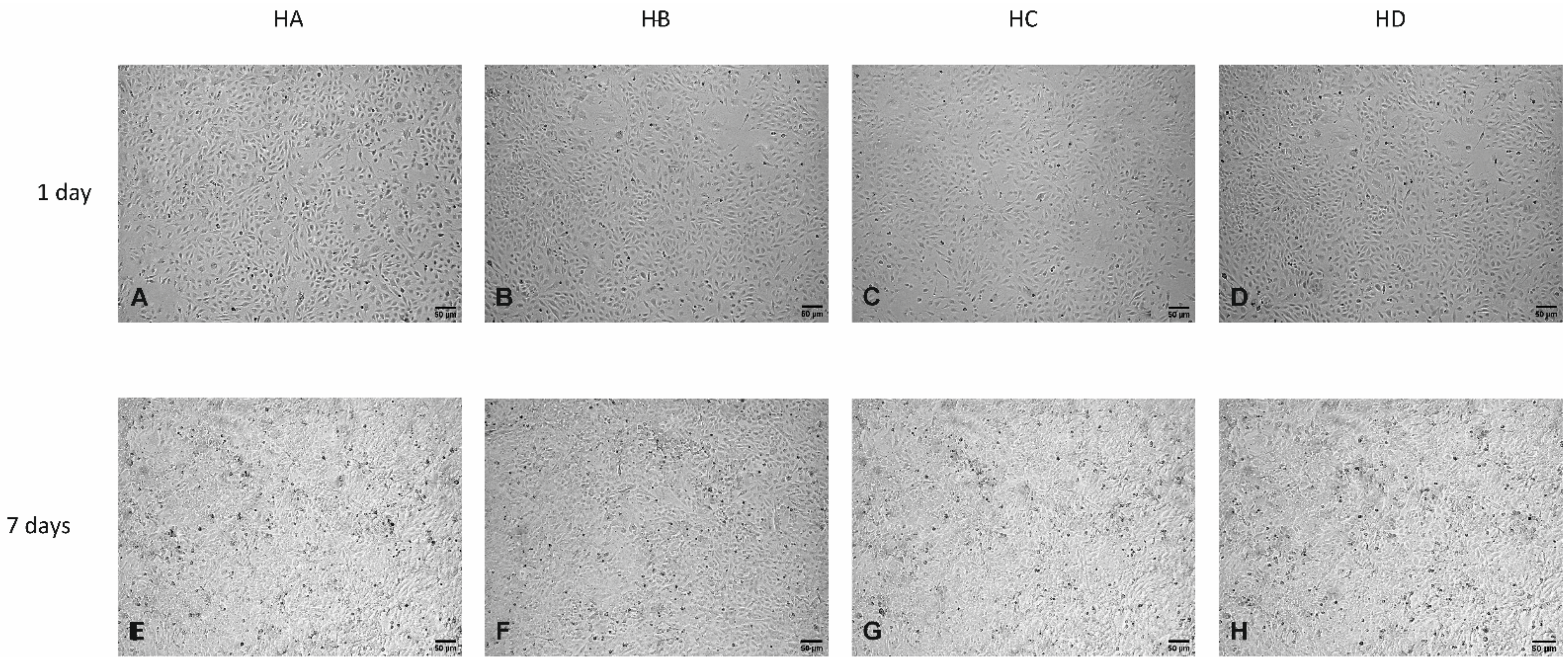
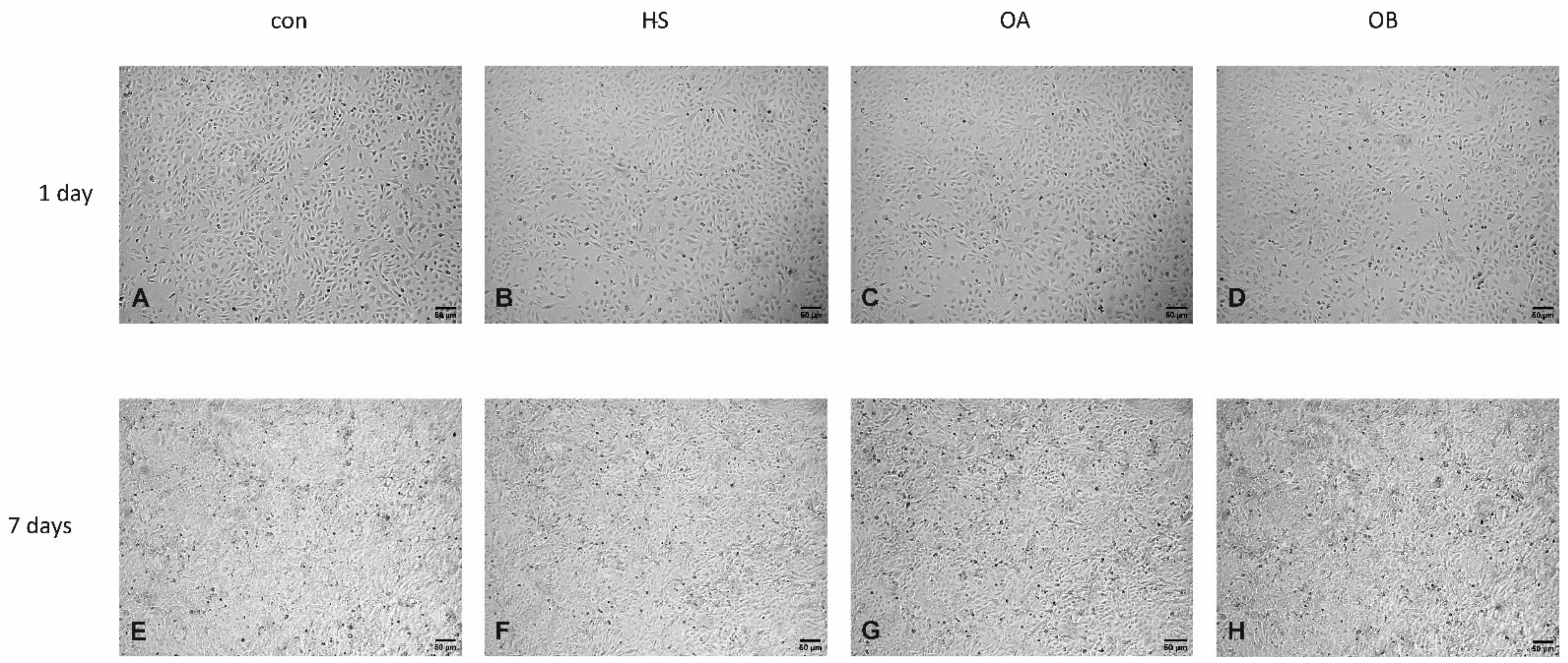
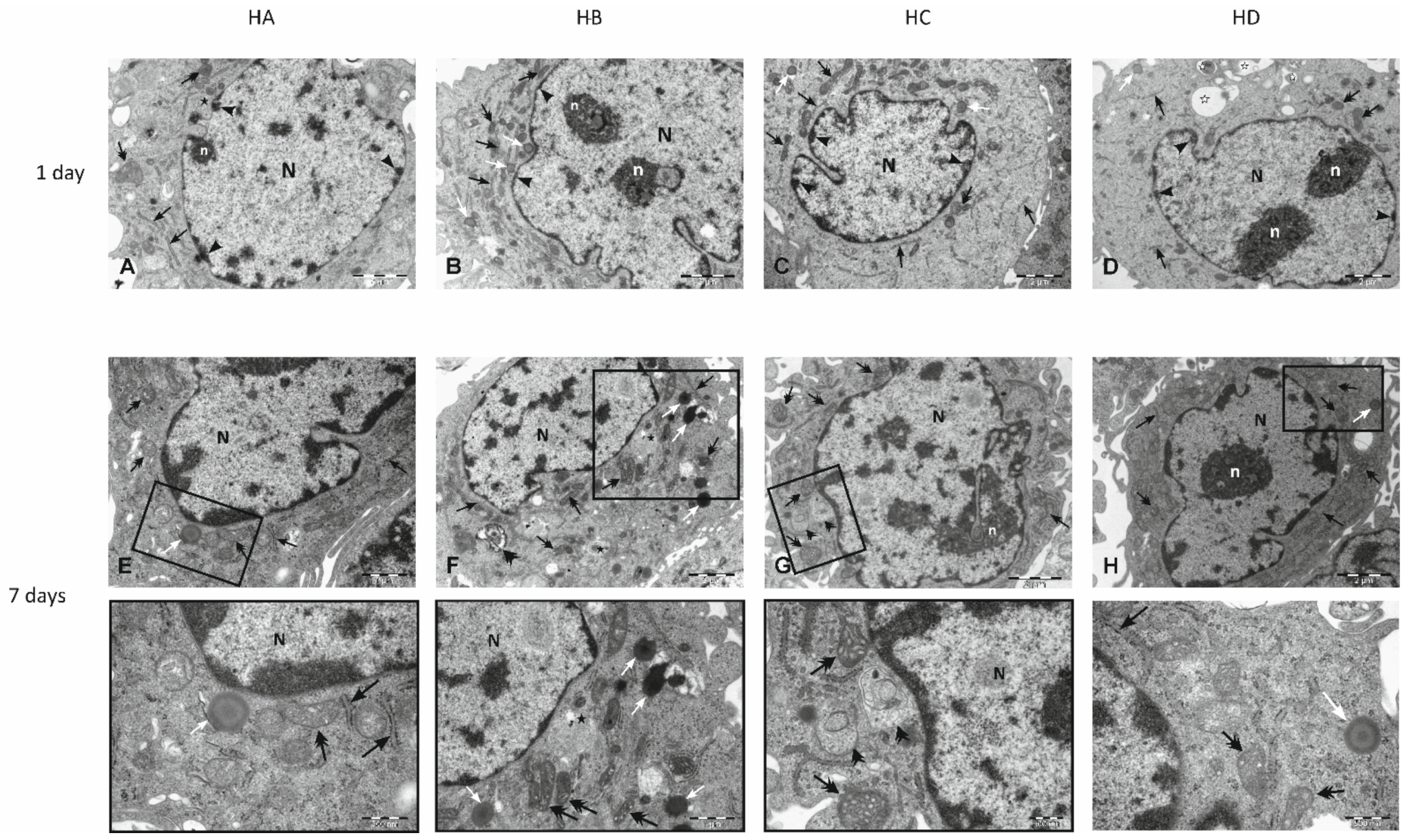
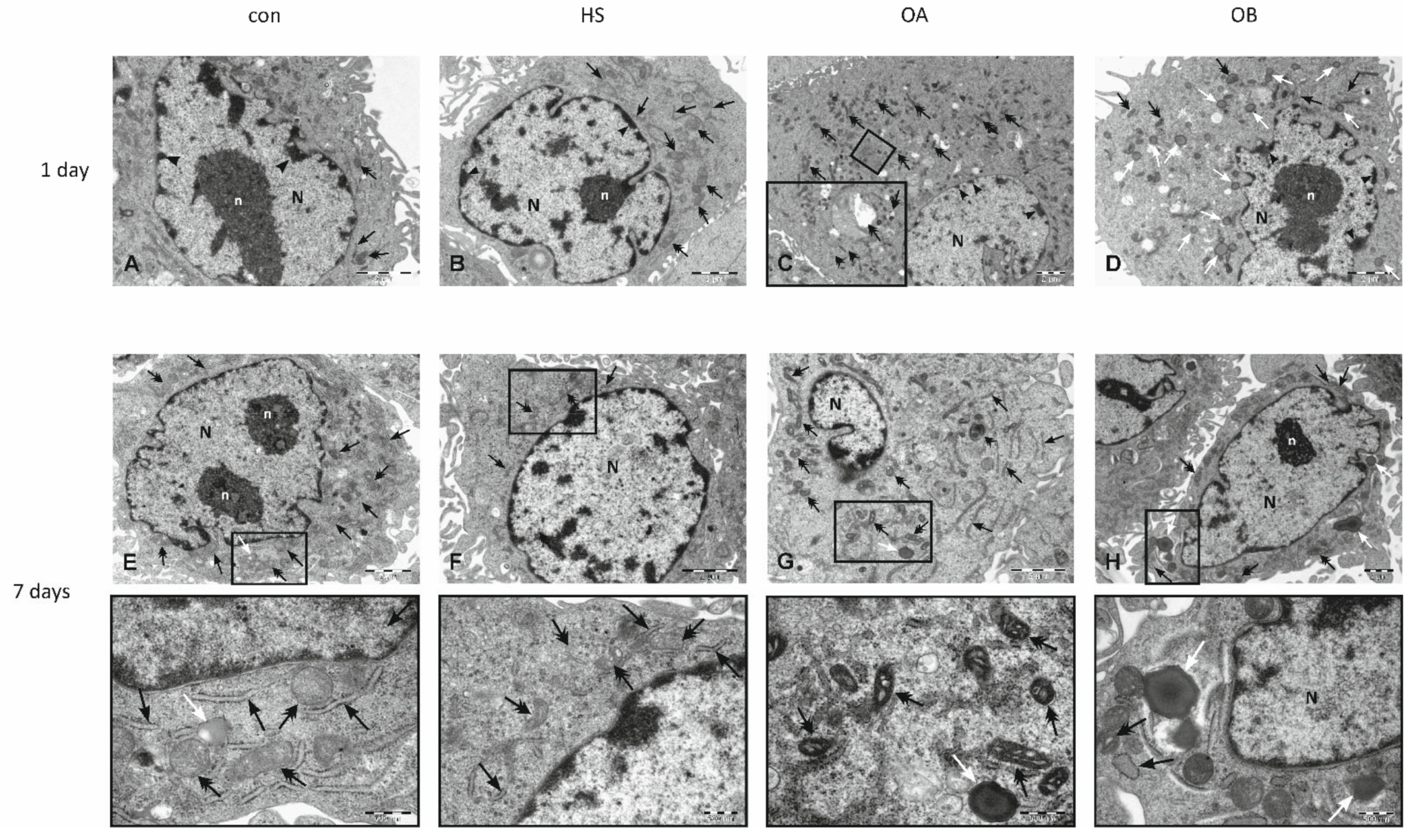
3.3. Effect of Flaxseed on Endothelial Cell Morphology
Light Microscopy
3.4. Electron Microscopy
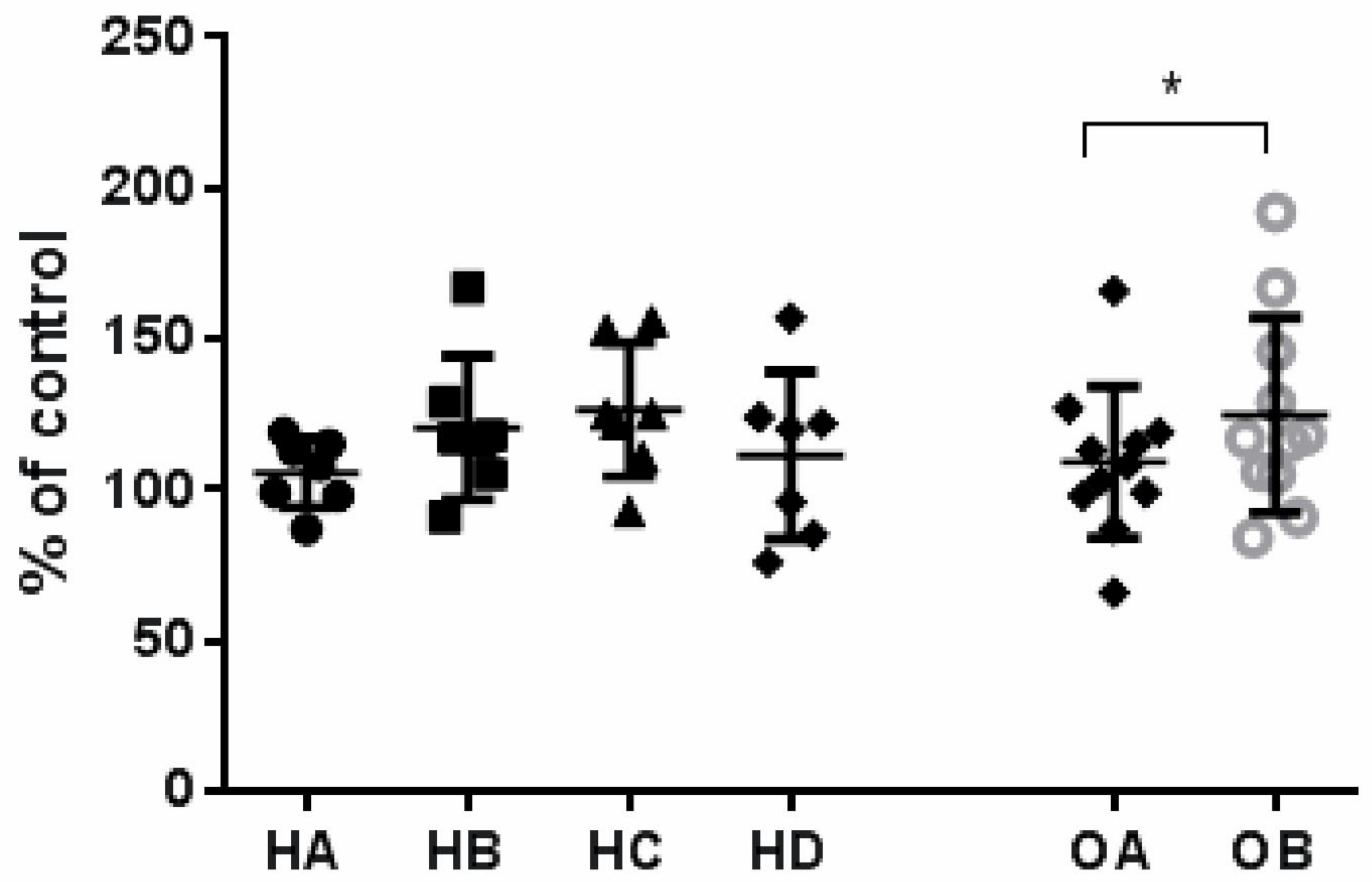
3.5. Effect of Flaxseed on Endothelial Cell Proliferation
3.6. Effect of Flaxseed on Parameters of Inflammation
3.7. Effect of Flaxseed on TC, LDL, Oxy-LDL, HDL and TG Levels
3.8. Side Effects and Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation. The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers-An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leyva, D.; Dupasquier, C.M.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Bloedon, L.T.; Balikai, S.; Chittams, J.; Cunnane, S.C.; Berlin, J.A.; Rader, D.J.; Szapary, P.O. Flaxseed and cardiovascular risk factors: Results from a double blind, randomised, controlled clinical trial. J. Am. Coll. Nutr. 2008, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Askarpour, M.; Salamat, S.; Ghaedi, E.; Symonds, M.E.; Miraghajani, M. Effect of flaxseed supplementation on lipid profile: An updated systematic review and dose-response meta-analysis of sixty-two randomised controlled trials. Pharmacol. Res. 2020, 152, 104622. [Google Scholar] [CrossRef] [PubMed]
- Kanikowska, D.; Korybalska, K.; Mickiewicz, A.; Rutkowski, R.; Kuchta, A.; Sato, M.; Kreft, E.; Fijalkowski, M.; Gruchala, M.; Jankowski, M.; et al. Flaxseed (Linum usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia-A Pilot Study. Nutrients 2020, 12, 1137. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, E.; Flammer, A.J.; Lerman, L.O.; Elizaga, J.; Lerman, A.; Fernandez-Aviles, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Charakida, M.; Tousoulis, D.; Skoumas, I.; Pitsavos, C.; Vasiliadou, C.; Stefanadi, E.; Antoniades, C.; Latsios, G.; Siasos, G.; Stefanadis, C. Inflammatory and thrombotic processes are associated with vascular dysfunction in children with familial hypercholesterolemia. Atherosclerosis 2009, 204, 532–537. [Google Scholar] [CrossRef]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Karimi, M.; Hadi, A.; Ghaedi, E.; Symonds, M.E.; Miraghajani, M.; Javadian, P. Effect of flaxseed supplementation on markers of inflammation and endothelial function. A systematic review and meta-analysis. Cytokine 2020, 126, 154922. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Bluher, M.; Dawczynski, C. Benefits of foods supplemented with vegetable oils rich in alpha-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomised, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef]
- Pan, A.; Yu, D.; Mark-Wahnefried, W.; Franco, O.H.; Lin, X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am. J. Clin. Nutr. 2009, 90, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.; Chahal, N.; Manlhiot, C.; Niedra, E.; McCrindle, B.W. Flaxseed in pediatric hyperlipidemia: A placebo-controlled, blinded, randomised clinical trial of dietary flaxseed supplementation for children and adolescents with hypercholesterolemia. JAMA Pediatr. 2013, 167, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridisation. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [Green Version]
- Formanowicz, D.; Malinska, A.; Nowicki, M.; Kowalska, K.; Gruca-Stryjak, K.; Breborowicz, G.; Korybalska, K. Preeclampsia with Intrauterine Growth Restriction Generates Morphological Changes in Endothelial Cells Associated with Mitochondrial Swelling-An In Vitro Study. J. Clin. Med. 2019, 8, 1994. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Witowski, J.; Korybalska, K.; Wisniewska, J.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jorres, A. Effect of glucose degradation products on human peritoneal mesothelial cell function. J. Am. Soc. Nephrol. 2000, 11, 729–739. [Google Scholar] [CrossRef]
- Avogaro, A.; De Kreutzenberg, S.V. Mechanisms of endothelial dysfunction in obesity. Clin. Chim. Acta 2005, 360, 9–26. [Google Scholar] [CrossRef]
- Buttari, B.; Segoni, L.; Profumo, E.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Businaro, R.; Iuliano, L.; Rigano, R. 7-Oxo-cholesterol potentiates pro-inflammatory signaling in human M1 and M2 macrophages. Biochem. Pharmacol. 2013, 86, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.M.; Thompson, L.U.; Dabrosin, C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res. 2007, 13, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Korybalska, K.; Luczak, J.; Swora-Cwynar, E.; Kanikowska, A.; Czepulis, N.; Kanikowska, D.; Skalisz, H.; Breborowicz, A.; Grzymislawski, M.; Witowski, J. Weight loss-dependent and -independent effects of moderate calorie restriction on endothelial cell markers in obesity. J. Physiol. Pharmacol. 2017, 68, 597–608. [Google Scholar]
- Korybalska, K.; Swora-Cwynar, E.; Luczak, J.; Kanikowska, A.; Czepulis, N.; Rutkowski, R.; Breborowicz, A.; Grzymislawski, M.; Witowski, J. Association of endothelial proliferation with the magnitude of weight loss during calorie restriction. Angiogenesis 2016, 19, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Bai, Y.P.; Gao, H.C.; Wang, X.; Li, L.F.; Zhang, G.G.; Hu, C.P. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis 2014, 235, 310–317. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Gerencser, A.A.; Doczi, J.; Torocsik, B.; Bossy-Wetzel, E.; dam-Vizi, V. Mitochondrial swelling measurement in situ by optimised spatial filtering: Astrocyte-neuron differences. Biophys. J. 2008, 95, 2583–2598. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Zhang, Y.; Jin, K.; Lu, Z.; Zeng, Z.; Xiong, W. Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell Biosci. 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Hanson, P.I.; Cashikar, A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef]
- Haldar, S.; Wong, L.H.; Tay, S.L.; Jacoby, J.J.; He, P.; Osman, F.; Ponnalagu, S.; Jiang, Y.R.; Lian, H.P.R.; Henry, C.J. Two Blends of Refined Rice Bran, Flaxseed, and Sesame Seed Oils Affect the Blood Lipid Profile of Chinese Adults with Borderline Hypercholesterolemia to a Similar Extent as Refined Olive Oil. J. Nutr. 2020, 150, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.N.; Roth, M.T. Evidence for the cardioprotective effects of omega-3 Fatty acids. Ann. Pharm. 2002, 36, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Earnest, C.P.; Tinsley, G.M.; Izidoro, L.F.M.; Macedo, R.C.O. Small dense low-density lipoprotein-cholesterol (sdLDL-C): Analysis, effects on cardiovascular endpoints and dietary strategies. Prog. Cardiovasc. Dis. 2020, 63, 503–509. [Google Scholar] [CrossRef] [PubMed]

| GROUP 1 (Treated with Apheresis + Flaxseed Biscuits) n = 11 | GROUP 2 (Treated with Diet + Flaxseed Biscuits) n = 11 | p Value | |
|---|---|---|---|
| Sex, M/F | 7/4 | 1/10 | |
| Age, years | 51.0 (29.0–79.0) | 60.0 (35.0–73.0) | 0.5612 |
| BMI, kg/m2 | 25.6 (18.2–32.0) | 35.5 (31.6–47.6) | 0.0001 |
| Underlying condition: | |||
| 2 | NA | |
| 2 | NA | |
| 2 | NA | |
| 2 | NA | |
| 5 | NA | |
| Dyslipidaemia, Y/N | 11/0 | 11/0 | |
| Coronary artery disease, Y/N | 7/4 | 0/11 | |
| Stroke, Y/N | 2/9 | 0/11 | |
| Diabetes, Y/N | 1/10 | 3/8 | |
| Hypertension, Y/N | 5/6 | 8/3 | |
| Time on Lipoprotein apheresis, months | 26.5 (19.0–38.0) | NA | |
| Lipid profile and proinflammatory markers before commencement of lipoprotein apheresis/diets: | |||
| Total cholesterol, mg/dL | 239.5 (191.0–367.0) | 239.0 (178.0–271.0) | 0.0591 |
| LDL, mg/dL | 152.8 (118.0–152.5) | 150.7 (102.4–182.4) | 0.0157 |
| HDL, mg/dL | 49.0 (39.0–77.0) | 52.0 (41.0–65.0) | 0.0940 |
| Triglycerides, mg/dL | 294.5 (187.0–735.0) | 167.0 (76.0–267.0) | 0.4421 |
| Leptin, ng/mL | 15.0 (2.3–89.6) | 71.4 (18.2–156.4) | 0.0031 |
| Adiponectin, ng/mL | 438.5 (161.8–648.1) | 598.1 (258.6–804.1) | 0.1330 |
| hsCRP, µg/mL | 482.5 (301.0–2930.0) | 400.4 (141.5–798.3) | 0.8404 |
| hsIL-1β, pg/mL | 0.444 (0.240–0.992) | 0.382 (0.339–0.669) | 0.4479 |
| hsIL-6, pg/mL | 10.0 (7.7–37.0) | 9.3 (7.6–25.4) | 0.4299 |
| hsTNFα, pg/mL | 0.674 (0.392–2.68) | 0.958 (0.517–1.53) | 0.0754 |
| Additional medication: | |||
| 6/5 | 0/11 | |
| 6/5 | 0/11 | |
| 1/10 | 0/11 | |
| Parameters | Group 1 (n = 11) | Group 2 (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| HA (Run in) | HB (Flaxseed) | HC (Wash out) | HD (Placebo) | p Value Group 1 | OA (Run in) | OB (Flaxseed) | p Value Group 2 | |
| Endothelial cell biomarkers | ||||||||
| VEGF, pg/mL | 57.0 (42.0–3172.0) | 41.5 ** (17.0–3063.0) | 45.5 (27.0–2765.0) | 50.0 (35.0–2791.0) | 0.024 | 50.0 (16.0–103.0) | 67.0 (15.0–158.0) | 0.168 |
| sICAM-1, ng/mL | 141.5 (77.0–262.0) | 131.5 (81.0–234.0) | 171.0 (40.0–279.0) | 231.5 (129.0–276.0) | 0.154 | 148.0 (95.0–295.0) | 118.0 (33.0–288.0) | 0.118 |
| sVCAM-1, ng/mL | 88.5 (54.0–148.0) | 91.0 (52.0–201.0) | 134.5 (64.0–151.0) | 88.5 (35.0–147.0) | 0.522 | 84.0 (35.0–142.0) | 74.0 (22.0–121.0) | 0.577 |
| oxy-LDL, ng/mL | 103.0 (94.0–623.0) | 103 (84.0–575.0) | 100.5 (86.0–271.0) | 104.0 (70.0–212.0 | 0.795 | 95.0 (80.0–267.0) | 97.0 (79.0–234.0) | 0.646 |
| Parameters | Group 2 (n = 11) | ||
|---|---|---|---|
| OA (Run in) | OB (Flaxseed) | p Value | |
| hsCRP, µg/mL | 400.4 (141.5–798.3) | 494.3 (252.3–967.3) | 0.840 |
| hsTNF alpha, pg/mL | 0.958 (0.517–1.538) | 0.858 (0.475–1.299) | 0.075 |
| hsIL-1β, pg/mL | 0.382 (0.339–0.669) | 0.405 (0.327–0.722) | 0.174 |
| hsIL-6, pg/mL | 9.3 (7.6–25.4) | 9.3 (7.5–16.0) | 0.562 |
| LDL, mg/dL | 150.7 (102.4–182.4) | 143.2 (99.3–173.6) | 0.067 |
| TC, mg/dL | 239.0 (178.0–271.0) | 217.0 (120.6–250.0) | 0.022 |
| HDL-C, mg/dL | 52.0 (41.0–65.0) | 55.0 (44.0–63.0) | 0.210 |
| TG, mg/dL | 167.0 (76.0–267.0) | 125.0 (77.0–200.0) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanikowska, D.; Malińska, A.; Mickiewicz, A.; Zawada, A.; Rutkowski, R.; Pawlaczyk, K.; Sato, M.; Bręborowicz, A.; Witowski, J.; Korybalska, K. Effect of Flaxseed (Linum usitatissimum L.) Supplementation on Vascular Endothelial Cell Morphology and Function in Patients with Dyslipidaemia—A Preliminary Observation. Nutrients 2022, 14, 2879. https://doi.org/10.3390/nu14142879
Kanikowska D, Malińska A, Mickiewicz A, Zawada A, Rutkowski R, Pawlaczyk K, Sato M, Bręborowicz A, Witowski J, Korybalska K. Effect of Flaxseed (Linum usitatissimum L.) Supplementation on Vascular Endothelial Cell Morphology and Function in Patients with Dyslipidaemia—A Preliminary Observation. Nutrients. 2022; 14(14):2879. https://doi.org/10.3390/nu14142879
Chicago/Turabian StyleKanikowska, Dominika, Agnieszka Malińska, Agnieszka Mickiewicz, Agnieszka Zawada, Rafał Rutkowski, Krzysztof Pawlaczyk, Maki Sato, Andrzej Bręborowicz, Janusz Witowski, and Katarzyna Korybalska. 2022. "Effect of Flaxseed (Linum usitatissimum L.) Supplementation on Vascular Endothelial Cell Morphology and Function in Patients with Dyslipidaemia—A Preliminary Observation" Nutrients 14, no. 14: 2879. https://doi.org/10.3390/nu14142879
APA StyleKanikowska, D., Malińska, A., Mickiewicz, A., Zawada, A., Rutkowski, R., Pawlaczyk, K., Sato, M., Bręborowicz, A., Witowski, J., & Korybalska, K. (2022). Effect of Flaxseed (Linum usitatissimum L.) Supplementation on Vascular Endothelial Cell Morphology and Function in Patients with Dyslipidaemia—A Preliminary Observation. Nutrients, 14(14), 2879. https://doi.org/10.3390/nu14142879