Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Establishment of a Reporter System to Screen for Polyphenols That Activate the Raldh2 Promoter
2.3. Screening of Polyphenols That Activate Raldh2 Promoter via IN Cell Analyzer 2200
2.4. Quantitative Reverse Transcription-PCR (RT-qPCR)
2.5. Flow Cytometry
2.6. Preparation of Human Peripheral Blood Mononuclear Cells (PBMCs)
2.7. Animal Experiments
2.8. Measurement of Fecal IgA Content via Enzyme-Linked Immunosorbent Assay (ELISA)
3. Results
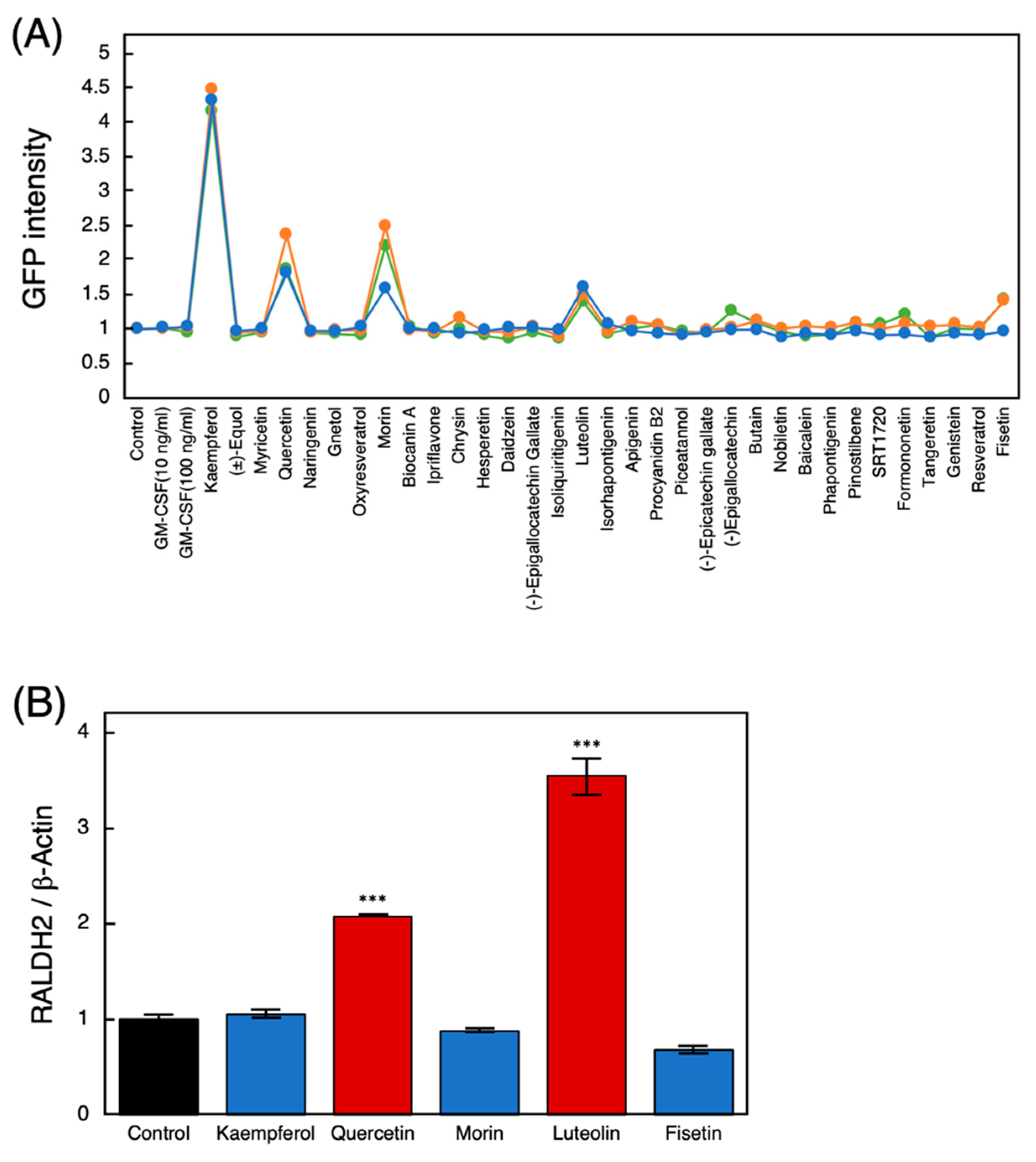
3.1. Screening for Polyphenols That Activate the Raldh2 Promoter
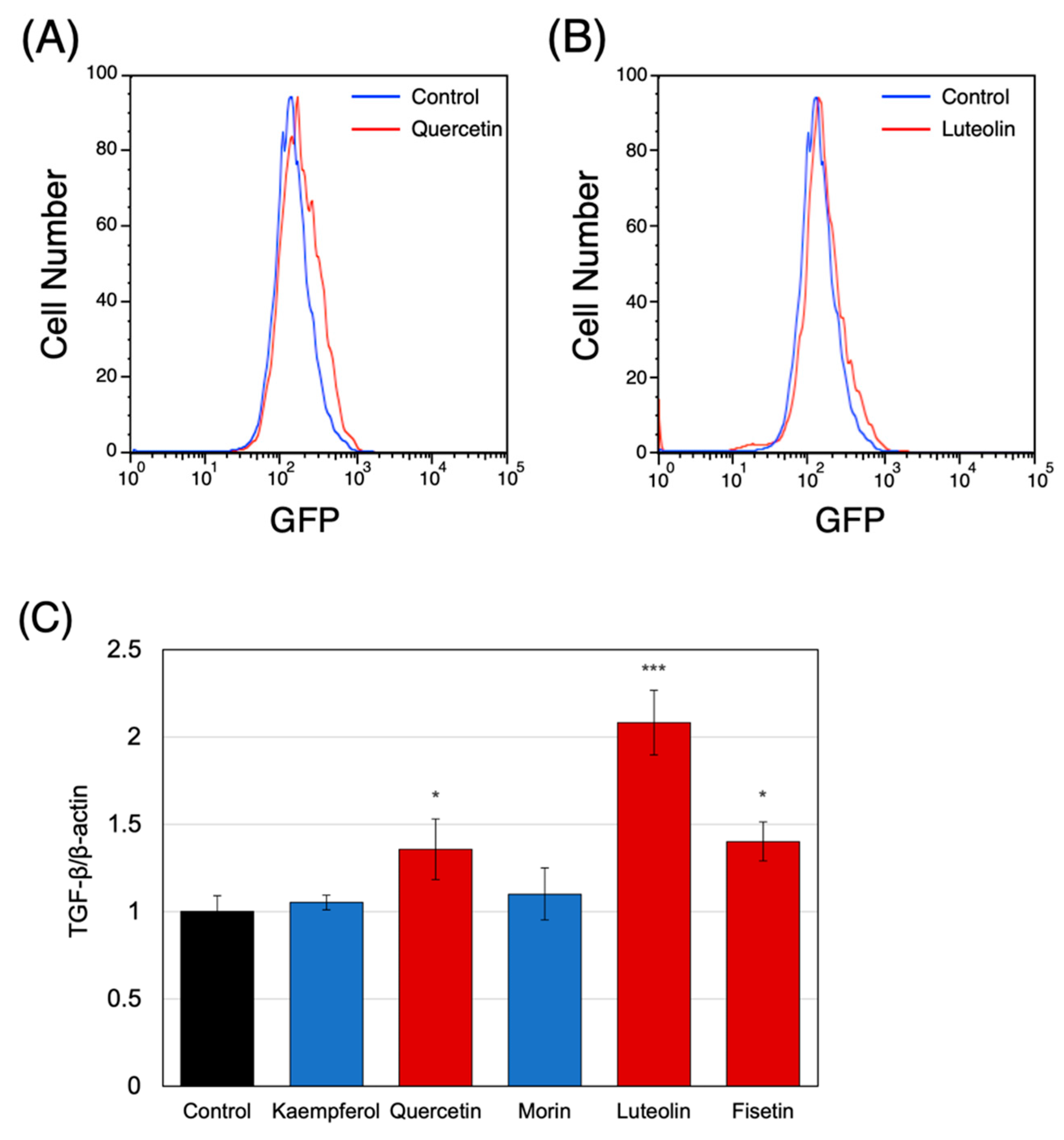
3.2. Effects of the Raldh2-Affecting Polyphenols on THP-1 Cells
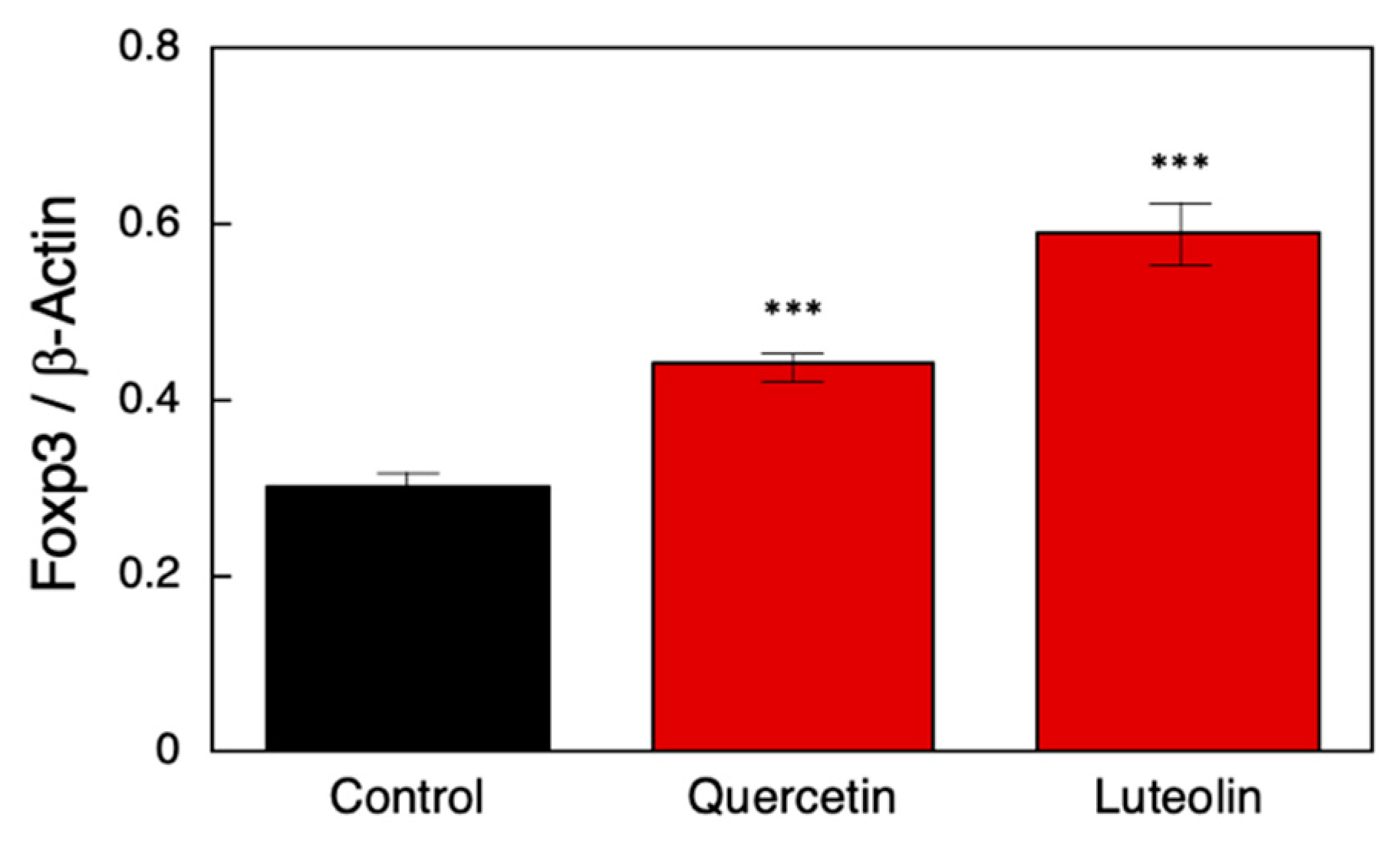
3.3. Effect of RALDH2 Promoter-Activating Polyphenols on Treg Induction
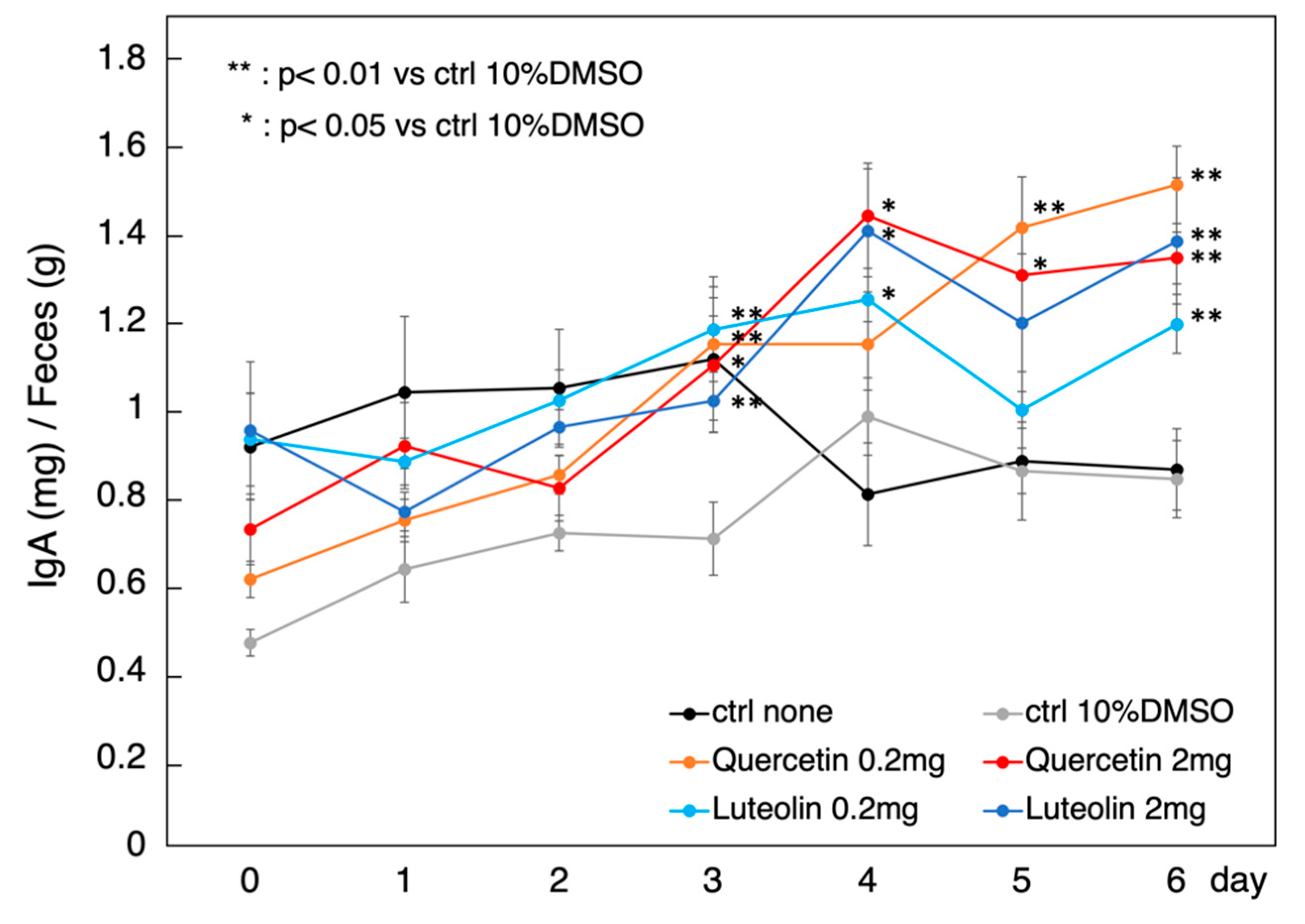
3.4. Effect of Orally Administered Polyphenols on Treg Function In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, A.; He, D.; Xu, H.B.; Geng, C.A.; Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 2015, 5, 14046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal Immun. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 2009, 21, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Wang, Y.C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann–Pick c1-like 1 in caco-2 cells and rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- De la Peña, J.B.; Kim, C.A.; Lee, H.L.; Yoon, S.Y.; Kim, H.J.; Hong, E.Y.; Kim, G.H.; Ryu, J.H.; Lee, Y.S.; Kim, K.M.; et al. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Arch. Pharm. Res. 2014, 37, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yamamoto, K.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of luteolin: Role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors 2013, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Eksteen, B.; Song, S.Y.; Junt, T.; Senman, B.; Otipoby, K.L.; Yokota, A.; Takeuchi, H.; Ricciardi-Castagnoli, P.; et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006, 314, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orihara, K.; Narita, M.; Tobe, T.; Akasawa, A.; Ohya, Y.; Matsumoto, K.; Saito, H. Circulating Foxp3+CD4+ cell numbers in atopic patients and healthy control subjects. J. Allergy Clin. Immunol. 2007, 120, 960–962. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiki, T.; Shinozaki, R.; Udono, M.; Katakura, Y. Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells. Nutrients 2022, 14, 2862. https://doi.org/10.3390/nu14142862
Fujiki T, Shinozaki R, Udono M, Katakura Y. Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells. Nutrients. 2022; 14(14):2862. https://doi.org/10.3390/nu14142862
Chicago/Turabian StyleFujiki, Tsukasa, Ryosuke Shinozaki, Miyako Udono, and Yoshinori Katakura. 2022. "Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells" Nutrients 14, no. 14: 2862. https://doi.org/10.3390/nu14142862
APA StyleFujiki, T., Shinozaki, R., Udono, M., & Katakura, Y. (2022). Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells. Nutrients, 14(14), 2862. https://doi.org/10.3390/nu14142862





