Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature
Abstract
:1. Introduction
2. Cancer Cachexia
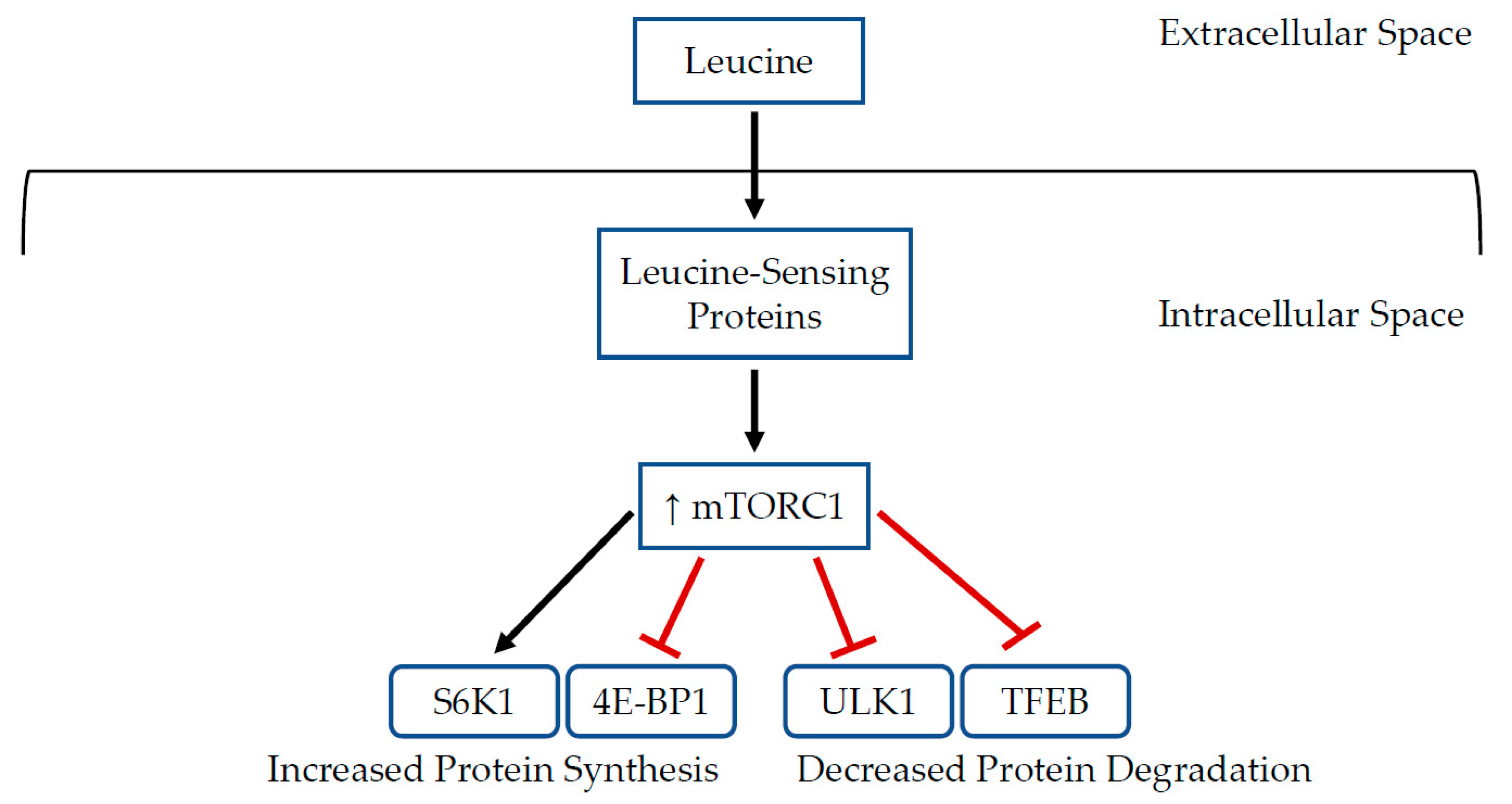
3. Role of Leucine in Muscle Metabolism
4. Role of Leucine in Immune Function
5. Methods
6. Results
6.1. Skeletal and Cardiac Muscle Effects
6.2. Inflammatory Effects
6.3. Tumor Growth Effects
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E.R. Do Patients with Weight Loss Have a Worse Outcome When Undergoing Chemotherapy for Lung Cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Wallengren, O.; Lundholm, K.; Bosaeus, I. Diagnostic Criteria of Cancer Cachexia: Relation to Quality of Life, Exercise Capacity and Survival in Unselected Palliative Care Patients. Support. Care Cancer 2013, 21, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.C.; Weintraub, M.; Blackburn, G.L.; Bistrian, B.R. Branched Chain Amino Acids as the Protein Component of Parenteral Nutrition in Cancer Cachexia. Br. J. Surg. 1989, 76, 149–153. [Google Scholar] [CrossRef]
- Choudry, H.A.; Pan, M.; Karinch, A.M.; Souba, W.W. Branched-Chain Amino Acid-Enriched Nutritional Support in Surgical and Cancer Patients. J. Nutr. 2006, 136, 314S–318S. [Google Scholar] [CrossRef]
- Asp, M.L.; Tian, M.; Kliewer, K.L.; Belury, M.A. Rosiglitazone Delayed Weight Loss and Anorexia While Attenuating Adipose Depletion in Mice with Cancer Cachexia. Cancer Biol. Ther. 2011, 12, 957–965. [Google Scholar] [CrossRef] [Green Version]
- DeWys, W.D. Pathophysiology of Cancer Cachexia: Current Understanding and Areas for Future Research. Cancer Res. 1982, 42, 721s–725s. [Google Scholar]
- Dhanapal, R.; Saraswathi, T.; Govind, R.N. Cancer Cachexia. J. Oral Maxillofac. Pathol. 2011, 15, 257–260. [Google Scholar] [CrossRef]
- WHO. Cancer—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 October 2021).
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-Tissue Communication in Cancer Cachexia. Nat. Rev. Endocrinol. 2019, 15, 9–20. [Google Scholar] [CrossRef]
- Tsoli, M.; Robertson, G. Cancer Cachexia: Malignant Inflammation, Tumorkines, and Metabolic Mayhem. Trends Endocrinol. Metab. 2013, 24, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Barkhudaryan, A.; Scherbakov, N.; Springer, J.; Doehner, W. Cardiac Muscle Wasting in Individuals with Cancer Cachexia. ESC Heart Fail. 2017, 4, 458–467. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; Backer, F.D.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring Specific Lactobacilli Levels Decreases Inflammation and Muscle Atrophy Markers in an Acute Leukemia Mouse Model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetto, A.; Kays, J.K.; Parker, V.A.; Matthews, R.R.; Barreto, R.; Puppa, M.J.; Kang, K.S.; Carson, J.A.; Guise, T.A.; Mohammad, K.S.; et al. Differential Bone Loss in Mouse Models of Colon Cancer Cachexia. Front. Physiol. 2017, 7, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwarkasing, J.T.; Boekschoten, M.V.; Argilès, J.M.; van Dijk, M.; Busquets, S.; Penna, F.; Toledo, M.; Laviano, A.; Witkamp, R.F.; van Norren, K. Differences in Food Intake of Tumour-Bearing Cachectic Mice Are Associated with Hypothalamic Serotonin Signalling. J. Cachexia Sarcopenia Muscle 2015, 6, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Nishijima, Y.; Asp, M.L.; Stout, M.B.; Reiser, P.J.; Belury, M.A. Cardiac Alterations in Cancer-Induced Cachexia in Mice. Int. J. Oncol. 2010, 37, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Bosaeus, I.; Daneryd, P.; Svanberg, E.; Lundholm, K. Dietary Intake and Resting Energy Expenditure in Relation to Weight Loss in Unselected Cancer Patients. Int. J. Cancer 2001, 93, 380–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, D.T.; Feurer, I.D.; Knox, L.S.; Crosby, L.O.; Buzby, G.P.; Mullen, J.L. Energy Expenditure in Malnourished Gastrointestinal Cancer Patients. Cancer 1984, 53, 1265–1273. [Google Scholar] [CrossRef]
- Knox, L.S.; Crosby, L.O.; Feurer, I.D.; Buzby, G.P.; Miller, C.L.; Mullen, J.L. Energy Expenditure in Malnourished Cancer Patients. Ann. Surg. 1983, 197, 152–162. [Google Scholar] [CrossRef]
- Souza, M.T.P.; Singer, P.; Ozorio, G.A.; Rosa, V.M.; Alves, M.M.F.; Mendoza López, R.V.; Waitzberg, D.L. Resting Energy Expenditure and Body Composition in Patients with Head and Neck Cancer: An Observational Study Leading to a New Predictive Equation. Nutrition 2018, 51–52, 60–65. [Google Scholar] [CrossRef]
- den Brekel, A.J.S.; Schols, A.M.W.J.; ten Velde, G.P.M.; Buurman, W.A.; Wouters, E.F.M. Analysis of the Energy Balance in Lung Cancer Patients. Cancer Res. 1994, 54, 6430–6433. [Google Scholar]
- Chen, W.-J.; Chung, Y.-C. Energy Expenditure in Patients with Hepatocellular Carcinoma. Cancer 1994, 73, 590–595. [Google Scholar] [CrossRef]
- Jebb, S.A.; Osborne, R.J.; Dixon, A.K.; Bleehen, N.M.; Elia, M. Measurements of Resting Energy Expenditure and Body Composition before and after Treatment of Small Cell Lung Cancer. Ann. Oncol. 1994, 5, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.R.; McMillan, D.C.; Watson, W.S.; Milroy, R.; McArdle, C.S. Longitudinal Study of Resting Energy Expenditure, Body Cell Mass and the Inflammatory Response in Male Patients with Non-Small Cell Lung Cancer. Lung Cancer 2001, 32, 307–312. [Google Scholar] [CrossRef]
- Friesen, D.E.; Baracos, V.E.; Tuszynski, J.A. Modeling the Energetic Cost of Cancer as a Result of Altered Energy Metabolism: Implications for Cachexia. Theor. Biol. Med. Model 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, S.A.; Tisdale, M.J. Effect of Cancer Cachexia on Triacylglycerol/Fatty Acid Substrate Cycling in White Adipose Tissue. Lipids 2004, 39, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Baracos, V.E. Computational Modeling of Cancer Cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Kir, S.; Spiegelman, B.M. Cachexia & brown fat: A burning issue in cancer. Trends Cancer 2016, 2, 461–463. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Schiessel, D.L.; Baracos, V.E. Barriers to Cancer Nutrition Therapy: Excess Catabolism of Muscle and Adipose Tissues Induced by Tumour Products and Chemotherapy. Proc. Nutr. Soc. 2018, 77, 394–402. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-Gut Axis and Its Role in the Control of Food Intake. J. Physiol. Pharm. 2004, 55, 137–154. [Google Scholar]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The Central Role of Hypothalamic Inflammation in the Acute Illness Response and Cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Olson, B.; Diba, P.; Korzun, T.; Marks, D.L. Neural Mechanisms of Cancer Cachexia. Cancers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Zhu, X.; Szumowski, M.; Scott, G.D.; Grossberg, A.J.; Levasseur, P.R.; Graham, K.; Khan, S.; Damaraju, S.; Colmers, W.F.; et al. Central Nervous System Inflammation Induces Muscle Atrophy via Activation of the Hypothalamic–Pituitary–Adrenal Axis. J. Exp. Med. 2011, 208, 2449–2463. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Scarlett, J.M.; Marks, D.L. Hypothalamic Mechanisms in Cachexia. Physiol. Behav. 2010, 100, 478–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, N.; Stephens, N.A.; Fearon, K.C.H. Muscle Wasting in Cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2215–2229. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Hansell, D.T.; Preston, T.; Plumb, J.A.; Davies, J.; Shapiro, D.; Shenkin, A.; Calman, K.C.; Burns, H.J.G. Influence of Whole Body Protein Turnover Rate on Resting Energy Expenditure in Patients with Cancer. Cancer Res 1988, 48, 2590–2595. [Google Scholar]
- Jeevanandam, M.; Horowitz, G.D.; Lowry, S.F.; Brennan, M.F. Cancer Cachexia and the Rate of Whole Body Lipolysis in Man. Metabolism 1986, 35, 304–310. [Google Scholar] [CrossRef]
- Leeuwen, S.D.Z.; van den Berg, J.W.O.; Wattimena, J.L.D.; van der Gaast, A.; Swart, G.R.; Wilson, J.H.P.; Dagnelie, P.C. Lipolysis and Lipid Oxidation in Weight-Losing Cancer Patients and Healthy Subjects. Metab. Clin. Exp. 2000, 49, 931–936. [Google Scholar] [CrossRef]
- Penna, F.; Costamagna, D.; Pin, F.; Camperi, A.; Fanzani, A.; Chiarpotto, E.M.; Cavallini, G.; Bonelli, G.; Baccino, F.M.; Costelli, P. Autophagic Degradation Contributes to Muscle Wasting in Cancer Cachexia. Am. J. Pathol. 2013, 182, 1367–1378. [Google Scholar] [CrossRef]
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular Mechanisms of Cancer Cachexia-induced Muscle Atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Wang, X.; Gao, T.; Tian, H.; Zhou, D.; Zhang, L.; Li, G.; Wang, X. The Autophagic-Lysosomal and Ubiquitin Proteasome Systems Are Simultaneously Activated in the Skeletal Muscle of Gastric Cancer Patients with Cachexia. Am. J. Clin. Nutr. 2020, 111, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease States. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of Muscle Wasting—The Role of the Ubiquitin–Proteasome Pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Ravid, T.; Hochstrasser, M. Diversity of Degradation Signals in the Ubiquitin–Proteasome System. Nat. Rev. Mol. Cell Biol. 2008, 9, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Le, W.-D. Autophagy and Ubiquitin-Proteasome System. Adv. Exp. Med. Biol. 2019, 1206, 527–550. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Mammalian Autophagy: Core Molecular Machinery and Signaling Regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of Mammalian Autophagy in Physiology and Pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, M.-S. Autophagy—A Key Player in Cellular and Body Metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-Proteasome System (UPS) and Autophagy Two Main Protein Degradation Machineries in Response to Cell Stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef]
- Del Fabbro, E. Combination Therapy in Cachexia. Ann. Palliat. Med. 2019, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.G.; Guadagni, M.; Fiotti, N.; Situlin, R.; Biolo, G. Contraction and Nutrition Interaction Promotes Anabolism in Cachectic Muscle. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 60–67. [Google Scholar] [CrossRef] [PubMed]
- McKeaveney, C.; Maxwell, P.; Noble, H.; Reid, J. A Critical Review of Multimodal Interventions for Cachexia. Adv. Nutr. 2021, 12, 523–532. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The Regulatory Approval of Anamorelin for Treatment of Cachexia in Patients with Non-Small Cell Lung Cancer, Gastric Cancer, Pancreatic Cancer, and Colorectal Cancer in Japan: Facts and Numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of Malnutrition in Patients at First Medical Oncology Visit: The PreMiO Study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic Effect of Weight Loss Prior to Chemotherapy in Cancer Patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Solheim, T.S.; Blum, D.; Fayers, P.M.; Hjermstad, M.J.; Stene, G.B.; Strasser, F.; Kaasa, S. Weight Loss, Appetite Loss and Food Intake in Cancer Patients with Cancer Cachexia: Three Peas in a Pod?—Analysis from a Multicenter Cross Sectional Study. Acta Oncol. 2014, 53, 539–546. [Google Scholar] [CrossRef]
- Van der Werf, A.; Arthey, K.; Hiesmayr, M.; Sulz, I.; Schindler, K.; Laviano, A.; Langius, J.; de van der Schueren, M. The Determinants of Reduced Dietary Intake in Hospitalised Colorectal Cancer Patients. Support. Care Cancer 2018, 26, 2039–2047. [Google Scholar] [CrossRef] [Green Version]
- Rüfenacht, U.; Rühlin, M.; Wegmann, M.; Imoberdorf, R.; Ballmer, P.E. Nutritional Counseling Improves Quality of Life and Nutrient Intake in Hospitalized Undernourished Patients. Nutrition 2010, 26, 53–60. [Google Scholar] [CrossRef]
- Uster, A.; Ruefenacht, U.; Ruehlin, M.; Pless, M.; Siano, M.; Haefner, M.; Imoberdorf, R.; Ballmer, P.E. Influence of a Nutritional Intervention on Dietary Intake and Quality of Life in Cancer Patients: A Randomized Controlled Trial. Nutrition 2013, 29, 1342–1349. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-C.; Shih, Y.-L.; Lu, C.-Y.; Hsieh, J.-S.; Chuang, J.-F.; Chen, F.-M.; Ma, C.-J.; Wang, J.-Y. Randomized, Controlled Study of Branched Chain Amino Acid-Enriched Total Parenteral Nutrition in Malnourished Patients with Gastrointestinal Cancer Undergoing Surgery. Am. Surg. 2008, 74, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Tanada, Y.; Shioi, T.; Kato, T.; Kawamoto, A.; Okuda, J.; Kimura, T. Branched-Chain Amino Acids Ameliorate Heart Failure with Cardiac Cachexia in Rats. Life Sci. 2015, 137, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayek, J.A.; Bistrian, B.R.; Hehir, D.J.; Martin, R.; Moldawer, L.L.; Blackburn, G.L. Improved Protein Kinetics and Albumin Synthesis by Branched Chain Amino Acid-Enriched Total Parenteral Nutrition in Cancer Cachexia: A Prospective Randomized Crossover Trial. Cancer 1986, 58, 147–157. [Google Scholar] [CrossRef]
- Anthony, J.C.; Lang, C.H.; Crozier, S.J.; Anthony, T.G.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Contribution of Insulin to the Translational Control of Protein Synthesis in Skeletal Muscle by Leucine. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1092–E1101. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.C.; Reiter, A.K.; Anthony, T.G.; Crozier, S.J.; Lang, C.H.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Orally Administered Leucine Enhances Protein Synthesis in Skeletal Muscle of Diabetic Rats in the Absence of Increases in 4E-BP1 or S6K1 Phosphorylation. Diabetes 2002, 51, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Blomstrand, E.; Saltin, B. BCAA Intake Affects Protein Metabolism in Muscle after but Not during Exercise in Humans. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E365–E374. [Google Scholar] [CrossRef]
- Louard, R.J.; Barrett, E.J.; Gelfand, R.A. Effect of Infused Branched-Chain Amino Acids on Muscle and Whole-Body Amino Acid Metabolism in Man. Clin. Sci. 1990, 79, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Alvestrand, A.; Hagenfeldt, L.; Merli, M.; Oureshi, A.; Eriksson, L.S. Influence of Leucine Infusion on Intracellular Amino Acids in Humans. Eur. J. Clin. Investig. 1990, 20, 293–298. [Google Scholar] [CrossRef]
- Nair, K.S.; Schwartz, R.G.; Welle, S. Leucine as a Regulator of Whole Body and Skeletal Muscle Protein Metabolism in Humans. Am. J. Physiol. Endocrinol. Metab. 1992, 263, E928–E934. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Leucine and Protein Synthesis: MTor and Beyond. Nutr. Rev. 2007, 65, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimobayashi, M.; Hall, M.N. Making New Contacts: The MTOR Network in Metabolism and Signalling Crosstalk. Nat. Rev. Mol. Cell. Biol. 2014, 15, 155–162. [Google Scholar] [CrossRef]
- Dennis, M.D.; Jefferson, L.S.; Kimball, S.R. Role of P70S6K1-Mediated Phosphorylation of EIF4B and PDCD4 Proteins in the Regulation of Protein Synthesis. J. Biol. Chem. 2012, 287, 42890–42899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino Acid-Dependent Control of MTORC1 Signaling: A Variety of Regulatory Modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A Unifying Model for MTORC1-Mediated Regulation of MRNA Translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-Dependent MTORC1 Association with the ULK1–Atg13–FIP200 Complex Required for Autophagy. Moll. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 Functions as a Transcriptional Regulator of Autophagy by Preventing Nuclear Transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, G.; Esposito, A.; Choi, H.; Matarese, M.; Benedetti, V.; Di Malta, C.; Monfregola, J.; Medina, D.L.; Lippincott-Schwartz, J.; Ballabio, A. MTOR-Dependent Phosphorylation Controls TFEB Nuclear Export. Nat. Commun. 2018, 9, 3312. [Google Scholar] [CrossRef]
- Vega-Rubin-de-Celis, S.; Peña-Llopis, S.; Konda, M.; Brugarolas, J. Multistep Regulation of TFEB by MTORC1. Autophagy 2017, 13, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets MTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfson, R.L.; Sabatini, D.M. The Dawn of the Age of Amino Acid Sensors for the MTORC1 Pathway. Cell. Metab. 2017, 26, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, K. Cell Signalling: How MTORC1 Senses Leucine. Nat. Rev. Mol. Cell. Biol. 2015, 16, 699. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Park, S.J.; Stamatakou, E.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Leucine Regulates Autophagy via Acetylation of the MTORC1 Component Raptor. Nat. Commun. 2020, 11, 3148. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 Is a Leucine Sensor for the MTORC1 Pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-TRNA Synthetase Is an Intracellular Leucine Sensor for the MTORC1-Signaling Pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, C.; Lee, M.; Wang, H.; Kim, K.; Park, S.J.; Yoon, I.; Jang, J.; Zhao, H.; Kim, H.K.; et al. Control of Leucine-Dependent MTORC1 Pathway through Chemical Intervention of Leucyl-TRNA Synthetase and RagD Interaction. Nat. Commun. 2017, 8, 732. [Google Scholar] [CrossRef]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural Basis for Leucine Sensing by the Sestrin2-MTORC1 Pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Son, S.M.; Park, S.J.; Lee, H.; Siddiqi, F.; Lee, J.E.; Menzies, F.M.; Rubinsztein, D.C. Leucine Signals to MTORC1 via Its Metabolite Acetyl-Coenzyme, A. Cell Metab. 2019, 29, 192–201.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine Supplementation of a Low-Protein Mixed Macronutrient Beverage Enhances Myofibrillar Protein Synthesis in Young Men: A Double-Blind, Randomized Trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A High Proportion of Leucine Is Required for Optimal Stimulation of the Rate of Muscle Protein Synthesis by Essential Amino Acids in the Elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Koopman, R.; Verdijk, L.; Manders, R.J.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.; van Loon, L.J. Co-Ingestion of Protein and Leucine Stimulates Muscle Protein Synthesis Rates to the Same Extent in Young and Elderly Lean Men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopman, R.; Wagenmakers, A.J.M.; Manders, R.J.F.; Zorenc, A.H.G.; Senden, J.M.G.; Gorselink, M.; Keizer, H.A.; van Loon, L.J.C. Combined Ingestion of Protein and Free Leucine with Carbohydrate Increases Postexercise Muscle Protein Synthesis in Vivo in Male Subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E645–E653. [Google Scholar] [CrossRef]
- Murphy, C.H.; Saddler, N.I.; Devries, M.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Leucine Supplementation Enhances Integrative Myofibrillar Protein Synthesis in Free-Living Older Men Consuming Lower- and Higher-Protein Diets: A Parallel-Group Crossover Study. Am. J. Clin. Nutr. 2016, 104, 1594–1606. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine Supplementation Improves Muscle Protein Synthesis in Elderly Men Independently of Hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef]
- Araki, K.; Ellebedy, A.H.; Ahmed, R. TOR in the Immune System. Curr. Opin. Cell Biol. 2011, 23, 707–715. [Google Scholar] [CrossRef]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by MTOR. Ann. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [Green Version]
- Delgoffe, G.M.; Powell, J.D. MTOR: Taking Cues from the Immune Microenvironment. Immunology 2009, 127, 459–465. [Google Scholar] [CrossRef]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. MTOR Regulates Memory CD8 T-Cell Differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, K.; Youngblood, B.; Ahmed, R. The Role of MTOR in Memory CD8+ T-Cell Differentiation. Immunol. Rev. 2010, 235, 234–243. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The MTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Salmond, R.J.; Zamoyska, R. The Influence of MTOR on T Helper Cell Differentiation and Dendritic Cell Function. Eur. J. Immunol. 2011, 41, 2137–2141. [Google Scholar] [CrossRef]
- van de Laar, L.; Buitenhuis, M.; Wensveen, F.M.; Janssen, H.L.A.; Coffer, P.J.; Woltman, A.M. Human CD34-Derived Myeloid Dendritic Cell Development Requires Intact Phosphatidylinositol 3-Kinase–Protein Kinase B–Mammalian Target of Rapamycin Signaling. J. Immunol. 2010, 184, 6600–6611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathaliyawala, T.; O’Gorman, W.E.; Greter, M.; Bogunovic, M.; Konjufca, V.; Hou, Z.E.; Nolan, G.P.; Miller, M.J.; Merad, M.; Reizis, B. Mammalian Target of Rapamycin Controls Dendritic Cell Development Downstream of Flt3 Ligand Signaling. Immunity 2010, 33, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichhart, T.; Hengstschläger, M.; Linke, M. Regulation of Innate Immune Cell Function by MTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory Functions of MTOR Inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.M.; Kolbe, T.; Stulnig, T.M.; Hörl, W.H.; Hengstschläger, M.; et al. The TSC-MTOR Signaling Pathway Regulates the Innate Inflammatory Response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.K.; Wang, R.; Mackman, N.; Luyendyk, J.P. Rapamycin Enhances LPS Induction of Tissue Factor and Tumor Necrosis Factor-α Expression in Macrophages by Reducing IL-10 Expression. Mol. Immunol. 2009, 46, 2249–2255. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, M.; Nagai, S.; Kondo, S.; Mizuno, S.; Nakamura, K.; Tanabe, M.; Takeuchi, T.; Matsuda, S.; Koyasu, S. Mammalian Target of Rapamycin and Glycogen Synthase Kinase 3 Differentially Regulate Lipopolysaccharide-Induced Interleukin-12 Production in Dendritic Cells. Blood 2008, 112, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, F.; Heit, A.; Dreher, S.; Eisenächer, K.; Mages, J.; Haas, T.; Krug, A.; Janssen, K.-P.; Kirschning, C.J.; Wagner, H. Mammalian Target of Rapamycin (MTOR) Orchestrates the Defense Program of Innate Immune Cells. Eur. J. Immunol. 2008, 38, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Song, C.-H.; Lee, J.-S.; Jung, S.-B.; Oh, J.-H.; Park, J.; Kim, H.-J.; Park, J.-K.; Paik, T.-H.; Jo, E.-K. Intracellular Network of Phosphatidylinositol 3-Kinase, Mammalian Target of the Rapamycin/70 KDa Ribosomal S6 Kinase 1, and Mitogen-Activated Protein Kinases Pathways for Regulating Mycobacteria-Induced IL-23 Expression in Human Macrophages. Cell. Microbiol. 2006, 8, 1158–1171. [Google Scholar] [CrossRef]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.-Y.; Baldwin, A.S. Akt-Dependent Regulation of NF-ΚB Is Controlled by MTOR and Raptor in Association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanian, S.M.; Dinarvand, P.; Smith, S.A.; Rezaie, A.R. Inorganic Polyphosphate Elicits Pro-Inflammatory Responses through Activation of the Mammalian Target of Rapamycin Complexes 1 and 2 in Vascular Endothelial Cells. J. Thromb. Haemost. 2015, 13, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Ozawa, Y.; Kamoshita, M.; Osada, H.; Toda, E.; Kurihara, T.; Nagai, N.; Umezawa, K.; Tsubota, K. The Neuroprotective Effect of Rapamycin as a Modulator of the MTOR-NF-ΚB Axis during Retinal Inflammation. PLoS ONE 2016, 11, e0146517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, A.; Bahreyni, A.; Boroumand, N.; Roshan, M.; Khazaei, M.; Ryzhikov, M.; Soleimanpour, S.; Avan, A.; Hassanian, S.M. Therapeutic Potency of MTOR Signaling Pharmacological Inhibitors in the Treatment of Proinflammatory Diseases, Current Status, and Perspectives. J. Cell. Physiol. 2018, 233, 4783–4790. [Google Scholar] [CrossRef]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef]
- Matsumoto, K.; Koba, T.; Hamada, K.; Sakurai, M.; Higuchi, T.; Miyata, H. Branched-Chain Amino Acid Supplementation Attenuates Muscle Soreness, Muscle Damage and Inflammation during an Intensive Training Program. J. Sports Med. Phys. Fit. 2009, 49, 424–431. [Google Scholar]
- Aoyagi, T.; Kusakari, Y.; Xiao, C.-Y.; Inouye, B.T.; Takahashi, M.; Scherrer-Crosbie, M.; Rosenzweig, A.; Hara, K.; Matsui, T. Cardiac MTOR Protects the Heart against Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H75–H85. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Miura, K.; Nakano, S.; Suzuki, K.; Bannai, M.; Inoue, Y. Leucine-Enriched Essential Amino Acids Attenuate Inflammation in Rat Muscle and Enhance Muscle Repair after Eccentric Contraction. Amino Acids 2016, 48, 2145–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Kusakari, Y.; Xiao, C.-Y.; Kinsella, S.D.; Rosenberg, M.A.; Scherrer-Crosbie, M.; Hara, K.; Rosenzweig, A.; Matsui, T. MTOR Attenuates the Inflammatory Response in Cardiomyocytes and Prevents Cardiac Dysfunction in Pathological Hypertrophy. Am. J. Physiol. Cell Physiol. 2010, 299, C1256–C1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-Leucine Dietary Supplementation Modulates Muscle Protein Degradation and Increases pro-Inflammatory Cytokines in Tumour-Bearing Rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Viana, L.R.; Lopes-Aguiar, L.; Canevarolo, R.; Colombera, M.C.; Valentim, R.R.; Garcia-Fóssa, F.; de Sousa, L.M.; Castelucci, B.G.; et al. Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers 2020, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Marcondes, M.C.C.; Ventrucci, G.; Toledo, M.T.; Cury, L.; Cooper, J.C. A Leucine-Supplemented Diet Improved Protein Content of Skeletal Muscle in Young Tumor-Bearing Rats. Braz. J. Med. Biol. Res. 2003, 36, 1589–1594. [Google Scholar] [CrossRef] [Green Version]
- Van Norren, K.; Kegler, D.; Argilés, J.M.; Luiking, Y.; Gorselink, M.; Laviano, A.; Arts, K.; Faber, J.; Jansen, H.; van der Beek, E.M.; et al. Dietary Supplementation with a Specific Combination of High Protein, Leucine, and Fish Oil Improves Muscle Function and Daily Activity in Tumour-Bearing Cachectic Mice. Br. J. Cancer 2009, 100, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.J.; van Helvoort, A.; Kegler, D.; Argilès, J.M.; Luiking, Y.C.; Laviano, A.; van Bergenhenegouwen, J.; Deutz, N.E.P.; Haagsman, H.P.; Gorselink, M.; et al. Dose-Dependent Effects of Leucine Supplementation on Preservation of Muscle Mass in Cancer Cachectic Mice. Oncol. Rep. 2011, 26, 247–254. [Google Scholar] [CrossRef]
- Salomão, E.M.; Gomes-Marcondes, M.C.C. Light Aerobic Physical Exercise in Combination with Leucine and/or Glutamine-Rich Diet Can Improve the Body Composition and Muscle Protein Metabolism in Young Tumor-Bearing Rats. J. Physiol. Biochem. 2012, 68, 493–501. [Google Scholar] [CrossRef]
- Salomão, E.M.; Toneto, A.T.; Silva, G.O.; Gomes-Marcondes, M.C.C. Physical Exercise and a Leucine-Rich Diet Modulate the Muscle Protein Metabolism in Walker Tumor-Bearing Rats. Nutr. Cancer 2010, 62, 1095–1104. [Google Scholar] [CrossRef]
- Toneto, A.T.; Ferreira Ramos, L.A.; Salomão, E.M.; Tomasin, R.; Aereas, M.A.; Gomes-Marcondes, M.C.C. Nutritional Leucine Supplementation Attenuates Cardiac Failure in Tumour-Bearing Cachectic Animals. J. Cachexia Sarcopenia Muscle 2016, 7, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Ventrucci, G.; Mello, M.a.R.; Gomes-Marcondes, M.C.C. Proteasome Activity Is Altered in Skeletal Muscle Tissue of Tumour-Bearing Rats a Leucine-Rich Diet. Endocr. -Relat. Cancer 2004, 11, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.R.; Canevarolo, R.; Luiz, A.C.P.; Soares, R.F.; Lubaczeuski, C.; de Mattos Zeri, A.C.; Gomes-Marcondes, M.C.C. Leucine-Rich Diet Alters the 1H-NMR Based Metabolomic Profile without Changing the Walker-256 Tumour Mass in Rats. BMC Cancer 2016, 16, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viana, L.R.; Chiocchetti, G.d.M.e.; Oroy, L.; Vieira, W.F.; Busanello, E.N.B.; Marques, A.C.; Salgado, C.d.M.; de Oliveira, A.L.R.; Vieira, A.S.; Suarez, P.S.; et al. Leucine-Rich Diet Improved Muscle Function in Cachectic Walker 256 Tumour-Bearing Wistar Rats. Cells 2021, 10, 3272. [Google Scholar] [CrossRef] [PubMed]
- Khal, J.; Wyke, S.M.; Russell, S.T.; Hine, A.V.; Tisdale, M.J. Expression of the Ubiquitin-Proteasome Pathway and Muscle Loss in Experimental Cancer Cachexia. Br. J. Cancer 2005, 93, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Penna, F.; Ballarò, R.; Martinez-Cristobal, P.; Sala, D.; Sebastian, D.; Busquets, S.; Muscaritoli, M.; Argilés, J.M.; Costelli, P.; Zorzano, A. Autophagy Exacerbates Muscle Wasting in Cancer Cachexia and Impairs Mitochondrial Function. J. Mol. Biol. 2019, 431, 2674–2686. [Google Scholar] [CrossRef]
- Sandri, M. Protein Breakdown in Cancer Cachexia. Semin. Cell Dev. Biol. 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Plas, R.L.C.; Poland, M.; Faber, J.; Argilès, J.; van Dijk, M.; Laviano, A.; Meijerink, J.; Witkamp, R.F.; van Helvoort, A.; van Norren, K. A Diet Rich in Fish Oil and Leucine Ameliorates Hypercalcemia in Tumour-Induced Cachectic Mice. Int. J. Mol. Sci. 2019, 20, 4978. [Google Scholar] [CrossRef] [Green Version]
- Goldner, W. Cancer-Related Hypercalcemia. JOP 2016, 12, 426–432. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. N. Am. J. Med. Sci. 2015, 7, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Faber, J.; Vos, P.; Kegler, D.; van Norren, K.; Argilés, J.M.; Laviano, A.; Garssen, J.; van Helvoort, A. Beneficial Immune Modulatory Effects of a Specific Nutritional Combination in a Murine Model for Cancer Cachexia. Br. J. Cancer 2008, 99, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- Nishio, Y.; Kakizoe, T.; Ohtani, M.; Sato, S.; Sugimura, T.; Fukushima, S. L-Isoleucine and L-Leucine: Tumor Promoters of Bladder Cancer in Rats. Science 1986, 231, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-L.; Wei, M.; Yunoki, T.; Kakehashi, A.; Yamano, S.; Kato, M.; Wanibuchi, H. Long-Term Treatment with l-Isoleucine or l-Leucine in AIN-93G Diet Has Promoting Effects on Rat Bladder Carcinogenesis. Food Chem. Toxicol. 2012, 50, 3934–3940. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine Supplementation Differentially Enhances Pancreatic Cancer Growth in Lean and Overweight Mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballarò, R.; Costelli, P.; Penna, F. Animal Models for Cancer Cachexia. Curr. Opin. Supportive Palliat. Care 2016, 10, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, N.; Walsh, D. Animal Models of the Cancer Anorexia–Cachexia Syndrome. Support. Care Cancer 2011, 19, 1451–1463. [Google Scholar] [CrossRef]
- Penna, F.; Busquets, S.; Argilés, J.M. Experimental Cancer Cachexia: Evolving Strategies for Getting Closer to the Human Scenario. Semin. Cell. Dev. Biol. 2016, 54, 20–27. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Demetrius, L. Of Mice and Men. EMBO Rep. 2005, 6, S39–S44. [Google Scholar] [CrossRef]
- Schöch, G.; Topp, H.; Held, A.; Heller-Schöch, G.; Ballauff, A.; Manz, F.; Sander, G. Interrelation between Whole-Body Turnover Rates of RNA and Protein. Eur. J. Clin. Nutr. 1990, 44, 647–658. [Google Scholar]
| Author(s) | Animals | Dietary Details | Experimental Protocol | Major Effect(s) |
|---|---|---|---|---|
| Cruz et al., 2017 [124] | Walker-256 tumor model, female Wistar rats (n = 72, 90 days old, weighing 180–200 g) | C = 18% protein E = 18% protein + 3% leucine | 12 groups according to Walker-256 status, tumor growth period, and diet | Attenuated skeletal muscle and protein content loss |
| Cruz et al., 2020 [125] | Walker-256 tumor model, male Wistar rats (n = 72, 90 days old, weighing 350–380 g) | C = 18% protein E = 18% protein + 3% leucine | 4 groups according to Walker-256 status and diet | Attenuated energy production |
| Faber et al., 2008 [141] | C26 tumor model, male CD2F1 mice (6 to 7 weeks old) | C = 12.6% protein E = 15.1% protein ± 1.6% leucine and/or fish oil, or 21% protein ± 2.1% leucine and/or fish oil | 6 groups according to C26 status and diet (control, single nutrient additive, or combination) | Combined diet led to reduced inflammation and improved immune competence |
| Gomes-Marcondes et al., 2003 [126] | Walker-256 tumor model, male Wistar rats (n = 36, 25 days old) | C = 18% protein E = 15% protein + 3% leucine | 4 groups according to Walker-256 status and diet | Attenuation of lean carcass mass and muscle myosin loss |
| Liu et al., 2014 [144] | Panco02 tumor model, male C57BL/6 mice (n = 88, 6 to 8 weeks old—diet initiation, 23 weeks of age—tumor injection) | C = ~16% protein E = ~16% protein + 5% leucine | At 6 to 8 weeks, 4 groups according to diet and calorie restriction. After 23 weeks, some mice were euthanized while the remainder were redistributed into 4 groups according to Panco02 status | Enhanced tumor growth |
| Peters et al., 2011 [128] | C26 tumor model, male CD2F1 mice (n = 38, 6–7 weeks old) | C = 8.7% of protein as leucine E = 9.6% or 14.8% of protein as leucine | 4 groups according to C26 status and diet (low and high leucine feeding) | Reduced skeletal muscle wasting |
| Plas et al., 2019 [138] | C26 tumor model, male CD2F1 mice (6–7 weeks old) | C = 12.6% protein E = 15.1% protein ± 1.6% leucine and/or fish oil | 53 groups according to C26 status and diet (control, single nutrient additive, or combination) | Combined diet reduced elevated plasma PGE-2 and PTHrP levels |
| Salomão et al., 2010 [130] | Walker-256 tumor model, male Wistar rats (n = 93, 21 days old) | C = 18% protein E = 18% protein + 3% leucine | At 21 days, 4 groups according to exercise and diet. After 60 days, rats were redistributed into 8 groups according to Walker-256 status | Exercise and leucine supplementation in conjunction led to decreased negative alterations in protein turnover |
| Salomão et al., 2012 [129] | Walker-256 tumor model, male Wistar rats (n = 80, 35 ± 2 days old) | C = 18% protein E = 18% protein + 3% leucine or 4% glutamine, or both | 8 groups according to Walker-256 status, exercise, and diet | Exercise and leucine supplementation in conjunction led to decreased negative alterations in protein turnover and carcass nitrogen content |
| Toneto et al., 2016 [131] | Walker-256 tumor model, male Wistar rats (n = 20, 90 days old) | C = 18% protein E = 18% protein + 3% leucine | 4 groups according to Walker-256 status and diet | Attenuated cardiac failure |
| van Norren et al., 2009 [127] | C26 tumor model, male CD2F1 mice (6–7 weeks old) | C = 12.6% protein E = 15.1% protein ± 1.6% leucine and/or fish oil | 6 groups according to C26 status and diet (control, single-nutrient additive, or combination) | Reduced loss of carcass, skeletal muscle, and fat mass loss with leucine-rich diet alone, combined diet resulted in a greater reduction in cachectic symptoms and improved functional performance |
| Ventrucci et al., 2004 [132] | Walker-256 tumor model, pregnant female Wistar rats (n = 60, 45 days old) | C = 18% protein E = 15% protein + 3% leucine | 6 groups according to Walker-256 status, diet, and pair feeding | Reduced 20S, 19S, and 11S proteasome content and increased protein synthesis |
| Viana et al., 2016 [133] | Walker-256 tumor model, female Wistar rats (n = 35, 90 ± 10 days old, weighing 265 ± 10 g) | C = 18% protein E = 18% protein + 3% leucine | 4 groups according to Walker-256 status and diet | Alterations in 23 serum metabolites with no increase in tumor size |
| Viana et al., 2021 [134] | Walker-256 tumor model, male Wistar rats (n = 24, 12 weeks old) | C = 18% protein E = 18% protein + 3% leucine | 4 groups according to Walker-256 status and diet | Improved muscle strength and behavioral performance, no impact on walking test, inflammation status, or muscle oxidative capacity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. https://doi.org/10.3390/nu14142824
Beaudry AG, Law ML. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients. 2022; 14(14):2824. https://doi.org/10.3390/nu14142824
Chicago/Turabian StyleBeaudry, Anna G., and Michelle L. Law. 2022. "Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature" Nutrients 14, no. 14: 2824. https://doi.org/10.3390/nu14142824
APA StyleBeaudry, A. G., & Law, M. L. (2022). Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients, 14(14), 2824. https://doi.org/10.3390/nu14142824






