Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Analytical Methods
2.3. Definitions
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
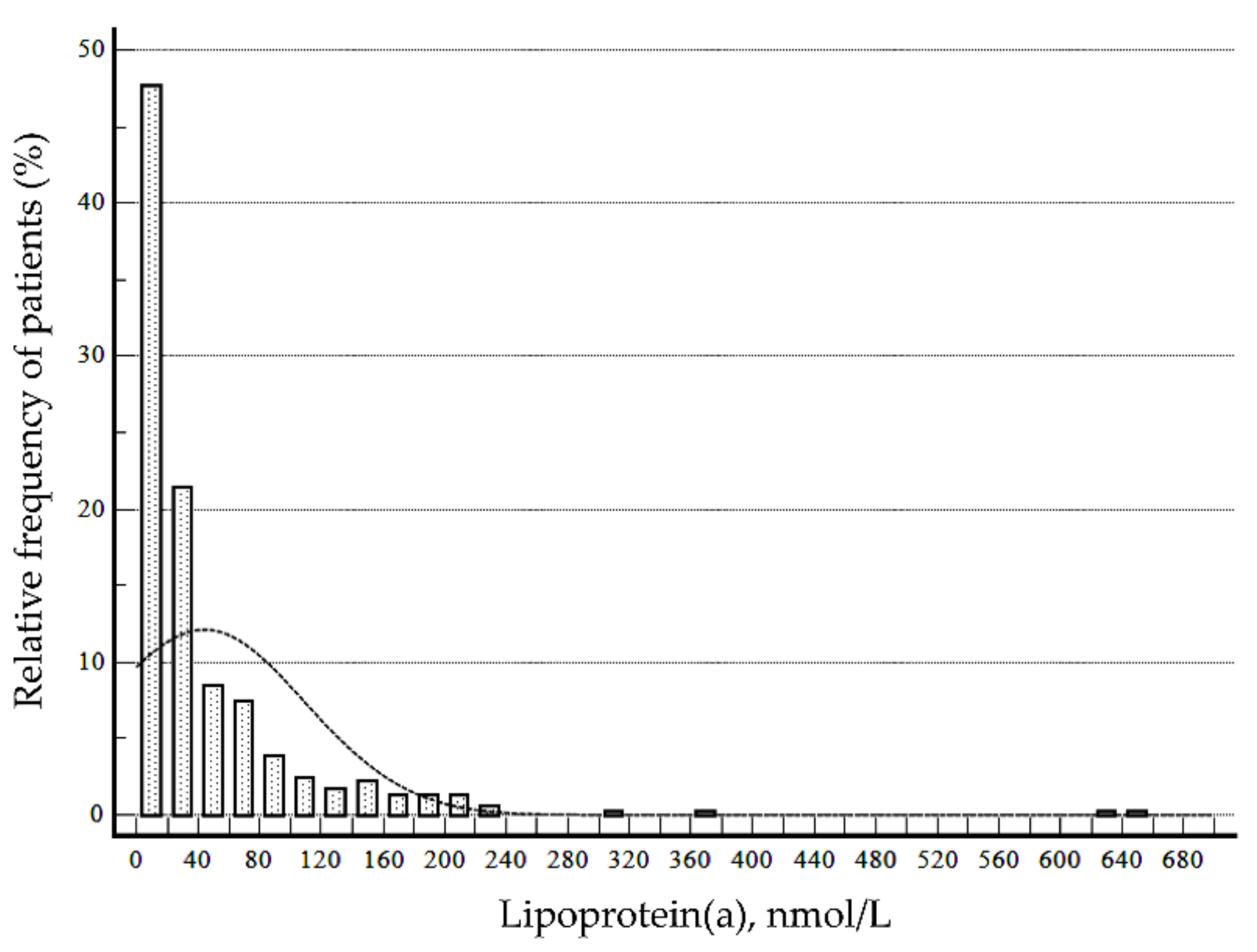
3.1. Characteristics of Study Subjects and Baseline Serum Lipoprotein(a)
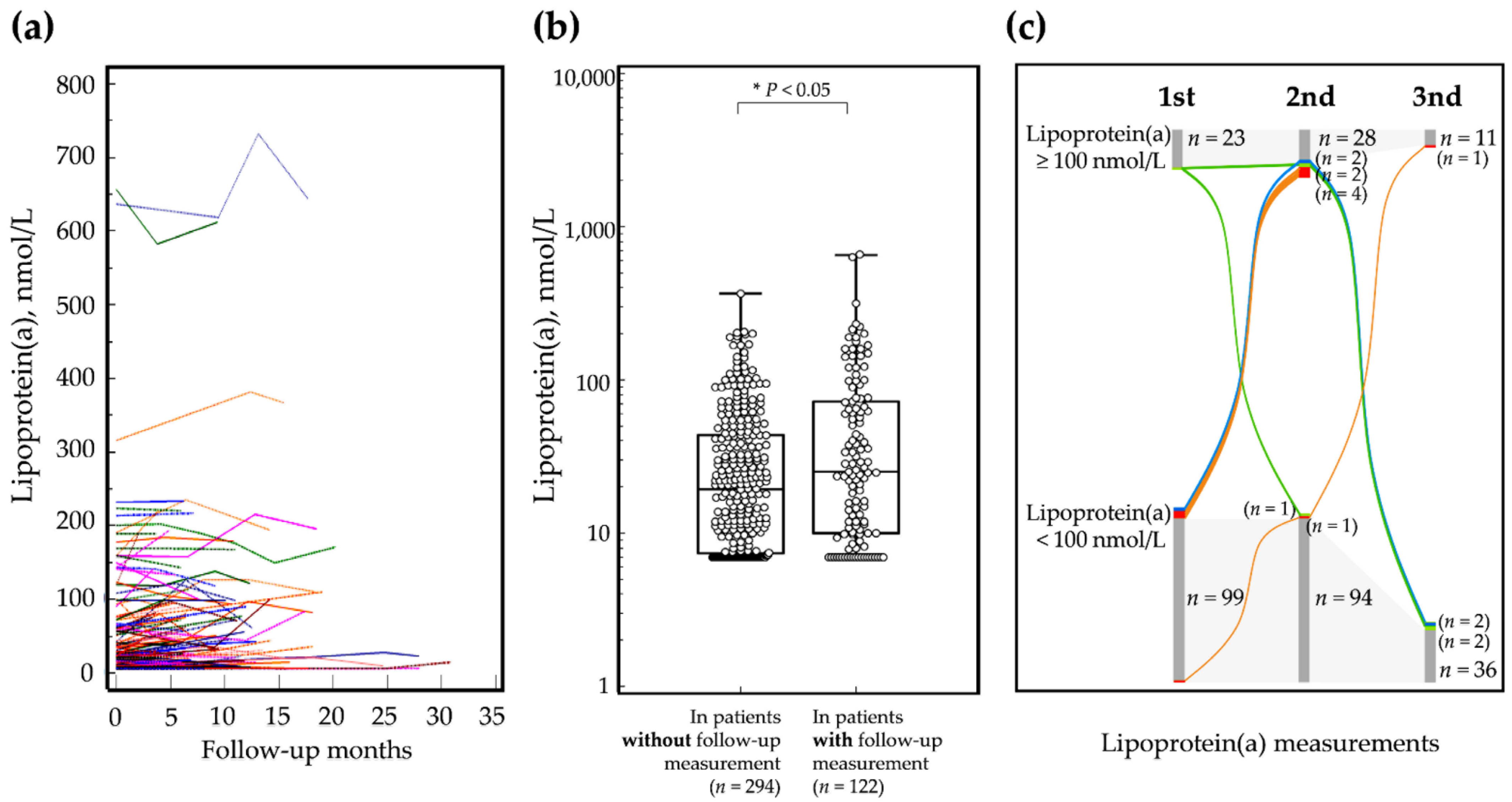
3.2. Serum Lipoprotein(a) Level during Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.L.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Ruotsalainen, S.; Handsaker, R.E.; Alver, M.; Bloom, J.; Poterba, T.; Seed, C.; Ernst, J.; Chaffin, M.; Engreitz, J.; et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat. Commun. 2018, 9, 2606. [Google Scholar] [CrossRef] [Green Version]
- Fogacci, F.; Cicero, A.F.G.; D’Addato, S.; D’Agostini, L.; Rosticci, M.; Giovannini, M.; Bertagnin, E.; Borghi, C. Serum lipoprotein(a) level as long-term predictor of cardiovascular mortality in a large sample of subjects in primary cardiovascular prevention: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2016, 37, 49–55. [Google Scholar] [CrossRef]
- Koschinsky, M.L.; Kronenberg, F. The long journey of lipoprotein(a) from cardiovascular curiosity to therapeutic target. Atherosclerosis 2022, 349, 1–6. [Google Scholar] [CrossRef]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Kohn, B.; Ashraf, A.P.; Wilson, D.P. Should Lipoprotein(a) be Measured in Youth? J. Pediatr. 2021, 228, 285–289. [Google Scholar] [CrossRef]
- Mehta, A.; Jain, V.; Saeed, A.; Saseen, J.J.; Gulati, M.; Ballantyne, C.M.; Virani, S.S. Lipoprotein(a) and ethnicities. Atherosclerosis 2022, 349, 42–52. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Berglund, L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis 2022, 349, 53–62. [Google Scholar] [CrossRef]
- Wilson, D.P.; Koschinsky, M.L.; Moriarty, P.M. Expert position statements: Comparison of recommendations for the care of adults and youth with elevated lipoprotein(a). Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 159–173. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J. Lipoprotein (a) measurements for clinical application. J. Lipid Res. 2016, 57, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Chemello, K.; Chan, D.C.; Lambert, G.; Watts, G.F. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis 2022, 349, 82–91. [Google Scholar] [CrossRef]
- ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [CrossRef] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Derosa, G.; D’Angelo, A.; Ventura, F.; Rizzoli, E.; D’Addato, S.; Borghi, C.; on behalf of the Brisighella Heart Study Group. Lipoprotein(a) Serum Levels Predict Pulse Wave Velocity in Subjects in Primary Prevention for Cardiovascular Disease with Large Apo(a) Isoforms: Data from the Brisighella Heart Study. Biomedicines 2022, 10, 656. [Google Scholar] [CrossRef]
- Lee, H.; Park, K.S.; Jeon, Y.-J.; Park, E.J.; Park, S.; Ann, S.H.; Kim, Y.-G.; Lee, Y.; Choi, S.H.; Park, G.-M. Lipoprotein(a) and subclinical coronary atherosclerosis in asymptomatic individuals. Atherosclerosis 2022, 349, 190–195. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Ballantyne, C.M. Existing and emerging strategies to lower Lipoprotein(a). Atherosclerosis 2022, 349, 110–122. [Google Scholar] [CrossRef]
- Rhee, E.-J.; Kim, H.C.; Kim, J.H.; Lee, E.Y.; Kim, B.J.; Kim, E.M.; Song, Y.; Lim, J.H.; Kim, H.J.; Choi, S.; et al. 2018 Guidelines for the management of dyslipidemia. Korean J. Intern. Med. 2019, 34, 723–771. [Google Scholar] [CrossRef] [Green Version]
- De Boer, L.M.; Hof, M.H.; Wiegman, A.; Stroobants, A.K.; Kastelein, J.J.; Hutten, B.A. Lipoprotein(a) levels from childhood to adulthood: Data in nearly 3,000 children who visited a pediatric lipid clinic. Atherosclerosis 2022, 349, 227–232. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, E.Y.; Kim, J.H.; Yoo, J.-H.; Yi, K.H.; Chae, H.W.; Choi, J.-H.; Kim, J.Y.; Hwang, I.T.; the Committee of Dyslipidemia of Korean Children and Adolescents on behalf of Korean Society of Pediatric Endocrinology (KSPE). 2017 Clinical practice guidelines for dyslipidemia of Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 199–207. [Google Scholar] [CrossRef]
- Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [CrossRef] [Green Version]
- Ruhaak, L.; Cobbaert, C. Quantifying apolipoprotein(a) in the era of proteoforms and precision medicine. Clin. Chim. Acta 2020, 511, 260–268. [Google Scholar] [CrossRef]
- Scharnagl, H.; Stojakovic, T.; Dieplinger, B.; Dieplinger, H.; Erhart, G.; Kostner, G.M.; Herrmann, M.; März, W.; Grammer, T.B. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis 2019, 289, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Choe, Y.H.; Choi, Y.; Kim, J.Q. Lipoprotein(a) in Korean Children and a History of Coronary or Cerebral Vascular Events in Their Older Family Members. Ann. Clin. Biochem. Int. J. Lab. Med. 1997, 34, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kwon, A.; Oh, Y.; Lee, S.G.; Lee, E.H. Time Points for Gonadotropin-Releasing Hormone Stimulation Test Results in Korean Children. J. Clin. Med. 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Schienkiewitz, A.; Truthmann, J.; Ernert, A.; Wiegand, S.; Schwab, K.O.; Scheidt-Nave, C. Age, maturation and serum lipid parameters: Findings from the German Health Survey for Children and Adolescents. BMC Public Health 2019, 19, 1627. [Google Scholar] [CrossRef]
- Martin, S.S.; Blaha, M.J.; Toth, P.P.; Joshi, P.H.; McEvoy, J.W.; Ahmed, H.M.; Elshazly, M.B.; Swiger, K.J.; Michos, E.D.; Kwiterovich, P.O.; et al. Very Large Database of Lipids: Rationale and Design. Clin. Cardiol. 2013, 36, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Benn, M.; Watts, G.F.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur. Heart J. 2016, 37, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef]
- Toft-Nielsen, F.; Emanuelsson, F.; Benn, M. Familial Hypercholesterolemia Prevalence Among Ethnicities—Systematic Review and Meta-Analysis. Front. Genet. 2022, 13, 840797. [Google Scholar] [CrossRef]
- Jung, K.J.; Koh, H.; Choi, Y.; Lee, S.J.; Ji, E.; Jee, S.H. Familial hypercholesterolemia and atherosclerotic cardiovascular mortality among Korean adults with low levels of serum cholesterol. Atherosclerosis 2018, 278, 103–109. [Google Scholar] [CrossRef]
- Dzobo, K.E.; Kraaijenhof, J.M.; Stroes, E.S.; Nurmohamed, N.S.; Kroon, J. Lipoprotein(a): An underestimated inflammatory mastermind. Atherosclerosis 2022, 349, 101–109. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 416) | Boy (n = 124) | Girl (n = 292) | |
|---|---|---|---|---|
| Age, years (median, IQR) | 11.1 (9.8 to 13.9) | 13.5 (10.8 to 16.2) | 10.7 (9.5 to 12.1) | |
| Follow-up measurement, number (median, IQR) | 1.0 (1.0 to 2.0) | 1.0 (1.0 to 1.0) | 1.0 (1.0 to 2.0) | |
| Follow-up duration, months (median, IQR) * | 6.7 (5.5 to 11.8) | 6.0 (3.2 to 10.8) | 6.7 (5.6 to 12.1) | |
| Age distribution (n, %) | 2 to 4 years | 7 (1.7%) | 5 (4.0%) | 2 (0.7%) |
| 5 to 8 years | 53 (12.7%) | 5 (4.0%) | 48 (16.4%) | |
| 9 to 11 years | 197 (47.4%) | 30 (24.2%) | 167 (57.2%) | |
| 12 to 17 years | 159 (38.2%) | 84 (67.7%) | 75 (25.7%) | |
| Lipoprotein(a) level at initial measurement, nmol/L (median, IQR) | Total | 21.5 (8.2 to 51.7) | 17.7 (<7.0 to 36.6) | 22.7 (9.9 to 59.7) |
| 2 to 4 years | 14.1 (8.8 to 17.5) | 14.1 (12.3 to 24.6) | 11.0 (<7.0 to 15.0) | |
| 5 to 8 years | 22.0 (8.1 to 56.7) | <7.0 (<7.0 to 30.7) | 22.4 (9.2 to 60.6) | |
| 9 to 11 years | 24.1 (9.8 to 57.4) | 34.1 (13.1 to 83.2) | 20.8 (7.9 to 51.7) | |
| 12 to 17 years | 20.5 (7.6 to 49.8) | 12.8 (<7.0 to 30.6) | 23.7 (11.7 to 71.7) | |
| Distribution | n | Mean | SD | Min | 2.5th | 5th | 10th | 25th | Med | 75th | 90th | 95th | 97.5th | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 416 | 43.9 | 65.8 | <7.0 | <7.0 | <7.0 | <7.0 | 8.2 | 21.5 | 51.7 | 107.8 | 165.2 | 200.6 | 656.2 |
| Boy | 124 | 38.7 | 70.2 | <7.0 | <7.0 | <7.0 | <7.0 | <7.0 | 17.7 | 36.6 | 93.2 | 148.5 | 193.0 | 656.2 |
| Girl | 292 | 46.2 | 63.9 | <7.0 | <7.0 | <7.0 | <7.0 | 9.9 | 22.7 | 59.7 | 113.8 | 167 | 204.9 | 636.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.; Lee, S.G.; Lee, E.H. Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals. Nutrients 2022, 14, 2820. https://doi.org/10.3390/nu14142820
Choi R, Lee SG, Lee EH. Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals. Nutrients. 2022; 14(14):2820. https://doi.org/10.3390/nu14142820
Chicago/Turabian StyleChoi, Rihwa, Sang Gon Lee, and Eun Hee Lee. 2022. "Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals" Nutrients 14, no. 14: 2820. https://doi.org/10.3390/nu14142820
APA StyleChoi, R., Lee, S. G., & Lee, E. H. (2022). Lipoprotein(a) in the Korean Pediatric Population Visiting Local Clinics and Hospitals. Nutrients, 14(14), 2820. https://doi.org/10.3390/nu14142820






