B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Eye Examination
2.3. AMD Classification
2.4. Event of Interest and Time Axis
2.5. Dietary Assessment
2.6. Serum Measurements
2.7. Other Variables
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Description of Vitamin B Dietary Intake
3.3. Multivariate Associations between Dietary Intake of B Vitamins and Risk of AMD
3.4. Multivariate Associations between Serum B Vitamins and Risk of AMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar] [CrossRef]
- Sobrin, L.; Seddon, J.M. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog. Retin. Eye Res. 2014, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Delcourt, C.; Carriere, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W.; Group, P.S. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA Study. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Cougnard-Grégoire, A.; Korobelnik, J.-F.; Schalch, W.; Etheve, S.; Rougier, M.-B.; Féart, C.; Samieri, C.; Delyfer, M.-N.; Delcourt, C. Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2021, 13, 2047. [Google Scholar] [CrossRef]
- Agron, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Gregoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Feart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Mares, J.; Clemons, T.E.; van Asten, F.; Swaroop, A.; Chew, E.Y. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020, 127, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. B Vitamins and Ageing. Biomed. Sci. 2018, 90, 451–470. [Google Scholar] [CrossRef]
- Seddon, J.M.; Gensler, G.; Klein, M.L.; Milton, R.C. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am. J. Ophthalmol. 2006, 141, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Zampatti, S.; Ricci, F.; Cusumano, A.; Marsella, L.T.; Novelli, G.; Giardina, E. Review of nutrient actions on age-related macular degeneration. Nutr. Res. 2014, 34, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Wang, F.; Sah, B.K.; Jiang, J.; Ni, Z.; Wang, J.; Sun, X. Homocysteine and the risk of age-related macular degeneration: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10585. [Google Scholar] [CrossRef]
- Pinna, A.; Zaccheddu, F.; Boscia, F.; Carru, C.; Solinas, G. Homocysteine and risk of age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2018, 96, e269–e276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat. Res. 2012, 733, 21–33. [Google Scholar] [CrossRef]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The impact of metabolism on DNA methylation. Hum. Mol. Genet. 2005, 14, R139–R147. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Chiuve, S.E.; Ridker, P.M.; Gaziano, J.M. Prospective study of plasma homocysteine, its dietary determinants, and risk of age-related macular degeneration in men. Ophthalmic Epidemiol. 2018, 25, 79–88. [Google Scholar] [CrossRef]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: A prospective cohort study. Am. J. Clin. Nutr. 2016, 103, 1135–1144. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Russell, J.; Cosatto, V.; Burlutsky, G.; Mitchell, P. Intake of key micronutrients and food groups in patients with late-stage age-related macular degeneration compared with age-sex-matched controls. Br. J. Ophthalmol. 2017, 101, 1027–1031. [Google Scholar] [CrossRef]
- Christen, W.G.; Glynn, R.J.; Chew, E.Y.; Albert, C.M.; Manson, J.E. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: The Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch. Intern. Med. 2009, 169, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, B.; Flood, V.M.; Rochtchina, E.; Wang, J.J.; Mitchell, P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am. J. Clin. Nutr. 2013, 98, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamburoglu, G.; Gumus, K.; Kadayifcilar, S.; Eldem, B. Plasma homocysteine, vitamin B12 and folate levels in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Korobelnik, J.F.; Barberger-Gateau, P.; Delyfer, M.N.; Rougier, M.B.; Le Goff, M.; Malet, F.; Colin, J.; Dartigues, J.F. Nutrition and age-related eye diseases: The Alienor (Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes) Study. J. Nutr. Health Aging 2010, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Group, C.S. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R., Jr. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef]
- Bird, A.C.; Bressler, N.M.; Bressler, S.B.; Chisholm, I.H.; Coscas, G.; Davis, M.D.; de Jong, P.T.; Klaver, C.C.; Klein, B.E.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study G.Group. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Feart, C.; Jutand, M.A.; Larrieu, S.; Letenneur, L.; Delcourt, C.; Combe, N.; Barberger-Gateau, P. Energy, macronutrient and fatty acid intake of French elderly community dwellers and association with socio-demographic characteristics: Data from the Bordeaux sample of the Three-City Study. Br. J. Nutr. 2007, 98, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Hercberg, S.; Deheeger, M.; Preziosi, P. Portions Alimentaires. Manuel Photos Pour L’estimation Des Quantités; Diffusion Economica: Paris, France, 2000. [Google Scholar]
- Favier, J.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Répertoire Général Des Aliments. Table de Composition, 2nd ed.; Editions Tec et Doc Lavoisier et INRA éditions: Paris, France, 1995. [Google Scholar]
- Souci, S.; Fachman, W.; Kraut, H. Food Composition and Nutrition Tables; medpharm Scientific publishers: Stuttgart, Germany, 2000; p. 1182. [Google Scholar]
- Renaud, S.; Godsey, F.; Ortchanian, E.; Baudier, F. Table de Composition Des Aliments; ASTRA-CALVE: Courbevoie, France, 1979; p. 105. [Google Scholar]
- ANSES—Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail. Relatif à L’évaluation des Apports en Vitamines et Minéraux Issus de L’alimentation non Enrichie, de L’alimentation. Enrichie et des Compléments Alimentaires dans la Population Française: Estimation des Apports Usuels, des. Prévalences D’inadéquation et des Risques de Dépassement des Limites de Sécurité; ANSES: Maisons-Alfort, France, 2015. [Google Scholar]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffondre, K.; Jager, K.J.; Boucquemont, J.; Stel, V.S.; Heinze, G. Representation of exposures in regression analysis and interpretation of regression coefficients: Basic concepts and pitfalls. Nephrol. Dial. Transplant. 2014, 29, 1806–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eilers, P.H.C.; Marx, B.D. Flexible smoothing with B-splines and penalties. Stat. Sci. 1996, 11, 89–121. [Google Scholar] [CrossRef]
- De Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Lui, A.; Lumeng, L.; Aronoff, G.R.; Li, T.K. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: Metabolic balance studies in humans. J. Lab. Clin. Med. 1985, 106, 491–497. [Google Scholar]
- Stabler, S.P. Screening the older population for cobalamin (vitamin B12) deficiency. J. Am. Geriatr. Soc. 1995, 43, 1290–1297. [Google Scholar] [CrossRef]
- Heuberger, R.A.; Fisher, A.I.; Jacques, P.F.; Klein, R.; Klein, B.E.; Palta, M.; Mares-Perlman, J.A. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 2002, 76, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochtchina, E.; Wang, J.J.; Flood, V.M.; Mitchell, P. Elevated serum homocysteine, low serum vitamin B12, folate, and age-related macular degeneration: The Blue Mountains Eye Study. Am. J. Ophthalmol. 2007, 143, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Swietochowska, E.; Wielkoszynski, T.; Marek, B.; Kos-Kudla, B.; Szapska, B.; Kajdaniuk, D.; Glogowska-Szelag, J.; Sieminska, L.; Ostrowska, Z.; et al. Homocysteine, vitamin B12, and folic acid in age-related macular degeneration. Eur. J. Ophthalmol. 2005, 15, 764–767. [Google Scholar] [CrossRef]
- Lionaki, E.; Ploumi, C.; Tavernarakis, N. One-Carbon Metabolism: Pulling the Strings behind Aging and Neurodegeneration. Cells 2022, 11, 214. [Google Scholar] [CrossRef]
- Park, L.K.; Friso, S.; Choi, S.W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Antioxidant status and neovascular age-related macular degeneration. Eye Disease Case-Control Study Group. Arch. Ophthalmol. 1993, 111, 104–109. [CrossRef] [PubMed]
- Fletcher, A.E.; Bentham, G.C.; Agnew, M.; Young, I.S.; Augood, C.; Chakravarthy, U.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch. Ophthalmol. 2008, 126, 1396–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W. Nutritional Epidemiolgy, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
| Participants, No (%) | ||||
|---|---|---|---|---|
| Characteristics | Total (n = 861) | Incident AMD Cases (n = 93) | Non-Incident AMD Cases (n = 768) | p-Value a |
| Female, sex | 530 (61.6) | 68 (73.1) | 462 (60.2) | 0.02 |
| Age, mean (SD), y | 74.7 (4.3) | 76.4 (4.5) | 74.5 (4.2) | <0.001 |
| Smoking status, pack years | 0.61 | |||
| Never smoker | 549 (64.4) | 59 (64.1) | 490 (64.5) | |
| <20 | 153 (18.0) | 14 (15.2) | 139 (18.3) | |
| ≥20 | 150 (17.6) | 19 (20.7) | 131 (17.2) | |
| Missing data | 9 | 1 | 8 | |
| Physical activity | 0.28 | |||
| None | 459 (53.3) | 48 (51.6) | 411 (53.5) | |
| Medium | 173 (20.1) | 18 (19.4) | 155 (20.2) | |
| High | 87 (10.1) | 6 (6.5) | 81 (10.5) | |
| No answer | 142 (16.5) | 21 (22.5) | 121 (15.8) | |
| Alcohol consumption, mean (SD), g/day | 12.0 (14.5) | 12.0 (14.3) | 12.0 (14.5) | 0.98 |
| Missing data | 10 | 2 | 8 | |
| Use of AMD supplement | 98 (11.4) | 27 (27.5) | 71 (8.5) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 26.4 (3.9) | 25.8 (3.6) | 26.4 (4.0) | 0.18 |
| Missing data | 5 | 0 | 5 | |
| HDL-Cholesterol, mean (SD), mg/dL | 61.9 (15.6) | 65.8 (16.0) | 61.5 (15.2) | 0.01 |
| Missing data | 46 | 5 | 41 | |
| Genetic risk score, mean (SD) | 0.28 (1.19) | 0.98 (1.35) | 0.19 (1.14) | <0.001 |
| Missing data | 141 | 12 | 129 | |
| Dietary Intake of Vitamins B | Participants, Total No. | ||||
|---|---|---|---|---|---|
| Total (n = 710) | Incident AMD (n = 80) | Non-Incident AMD (n = 630) | HR b (95% CI) | p Value | |
| Vitamin B1, mean (SD), mg/d | 1.04 (0.44) | 1.02 (0.43) | 1.05 (0.45) | 0.97 (0.74–1.27) | 0.83 |
| Vitamin B2, mean (SD), mg/d | 1.60 (0.78) | 1.52 (0.50) | 1.61 (0.80) | 0.78 (0.56–1.10) | 0.15 |
| Vitamin B3, mean (SD), mg/d | 14.69 (7.01) | 14.18 (6.34) | 14.76 (7.09) | 0.92 (0.70–1.21) | 0.56 |
| Vitamin B5, mean (SD), mg/d | 4.20 (1.84) | 3.88 (1.50) | 4.24 (1.88) | 0.72 (0.53–0.99) | 0.049 |
| Vitamin B6, mean (SD), mg/d | 1.47 (0.60) | 1.41 (0.57) | 1.48 (0.60) | 0.90 (0.81–0.99) | 0.049 |
| Folate, mean (SD), µg/d | 290 (143) | 302 (181) | 288 (138) | 1.02 (0.82–1.28) | 0.83 |
| Vitamin B12, mean (SD), µg/d | 6.10 (12.78) | 4.93 (8.05) | 6.24 (13.25) | 0.77 (0.51–1.17) | 0.22 |
| Serum Level of Vitamins B | Total | Incident AMD | Non-Incident AMD | HR (95% CI) | p Value | |||
|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD) or (%) | No. | Mean (SD) or (%) | No. | Mean (SD) or (%) | |||
| Vitamin B6, nmol/L | 645 | 41.92 (36.41) | 67 | 37.37 (27.73) | 578 | 42.45 (37.27) | 0.86 (0.59–1.25) a | 0.43 |
| Deficient <20 nmol/L | 81 | (12.6) | 13 | (19.4) | 68 | (11.8) | Reference | |
| Normal ≥20 nmol/L | 564 | (87.4) | 54 | (81.6) | 510 | (88.2) | 0.64 (0.5–1.20) a | 0.16 |
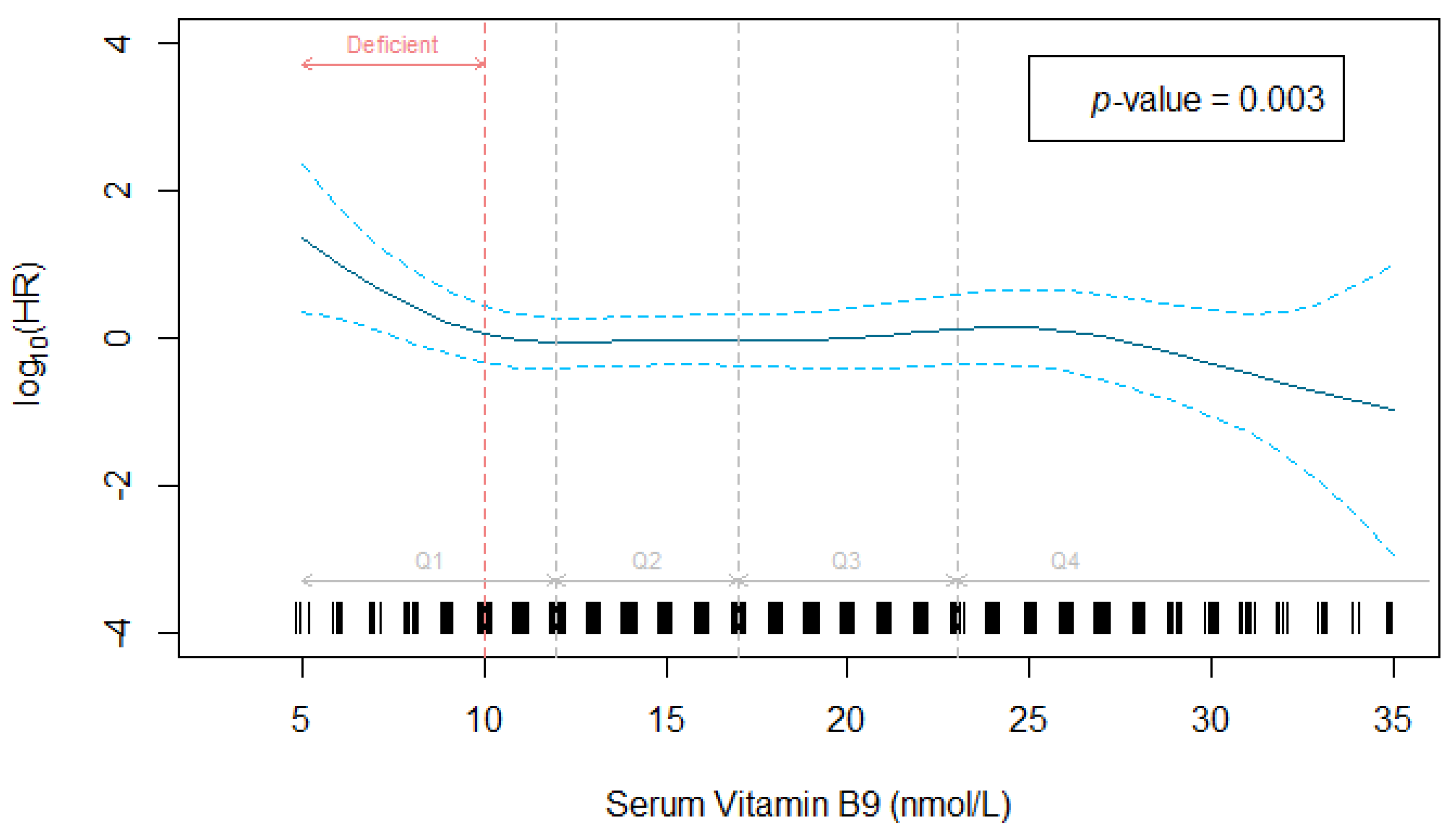
| Folate, nmol/L | 654 | 19.02 (9.97) | 69 | 16.58 (7.09) | 585 | 19.30 (10.22) | ND c | |
| Deficient <10 nmol/L | 50 | (7.6) | 11 | (15.9) | 39 | (6.7) | Reference | |
| Normal ≥10 nmol/L | 604 | (92.4) | 58 | (84.1) | 546 | (93.3) | 0.49 (0.25–0.95) b | 0.036 |
| Vitamin B12, pmol/L | 648 | 374 (431) | 70 | 417 (545) | 578 | 369 (416) | 1.06 (0.89–1.27) a | 0.51 |
| Deficient <185 pmol/L | 66 | (10.2) | 5 | (7.1) | 61 | (10.5) | Reference | |
| Normal ≥185 pmol/L | 582 | (89.8) | 65 | (92.9) | 517 | (89.5) | 1.61 (0.64–4.06) a | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merle, B.M.J.; Barthes, S.; Féart, C.; Cougnard-Grégoire, A.; Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Delcourt, C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2022, 14, 2821. https://doi.org/10.3390/nu14142821
Merle BMJ, Barthes S, Féart C, Cougnard-Grégoire A, Korobelnik J-F, Rougier M-B, Delyfer M-N, Delcourt C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients. 2022; 14(14):2821. https://doi.org/10.3390/nu14142821
Chicago/Turabian StyleMerle, Bénédicte M. J., Stéphanie Barthes, Catherine Féart, Audrey Cougnard-Grégoire, Jean-François Korobelnik, Marie-Bénédicte Rougier, Marie-Noëlle Delyfer, and Cécile Delcourt. 2022. "B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study" Nutrients 14, no. 14: 2821. https://doi.org/10.3390/nu14142821
APA StyleMerle, B. M. J., Barthes, S., Féart, C., Cougnard-Grégoire, A., Korobelnik, J.-F., Rougier, M.-B., Delyfer, M.-N., & Delcourt, C. (2022). B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients, 14(14), 2821. https://doi.org/10.3390/nu14142821








