Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Hemodialysis Settings
2.3. Sample Collection and Laboratory Measurements
2.4. Comparison of Daily Losses between Hemodialysis Patients and Controls
2.5. Intradialytic Changes in Amino Acid Concentrations in Hemodialysis Patients
2.6. Single Dialysis Losses, Dialytic Clearance, and Fractional Clearance in Hemodialysis Patients
2.7. Assessment of Severe Fatigue
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of Amino Acid Concentrations between Hemodialysis Patients and Controls
3.3. Daily Amino Acid Losses in Hemodialysis Patients and Controls
3.4. Determinants of Total Amino Acid Losses in Hemodialysis Patients
3.5. Intradialytic Changes in Plasma Concentrations in Hemodialysis Patients
3.6. Single Hemodialysis Amino Acid Losses, Dialytic Amino Acid Clearances, and Relative Contribution of Dialysis and Urine to Daily Losses
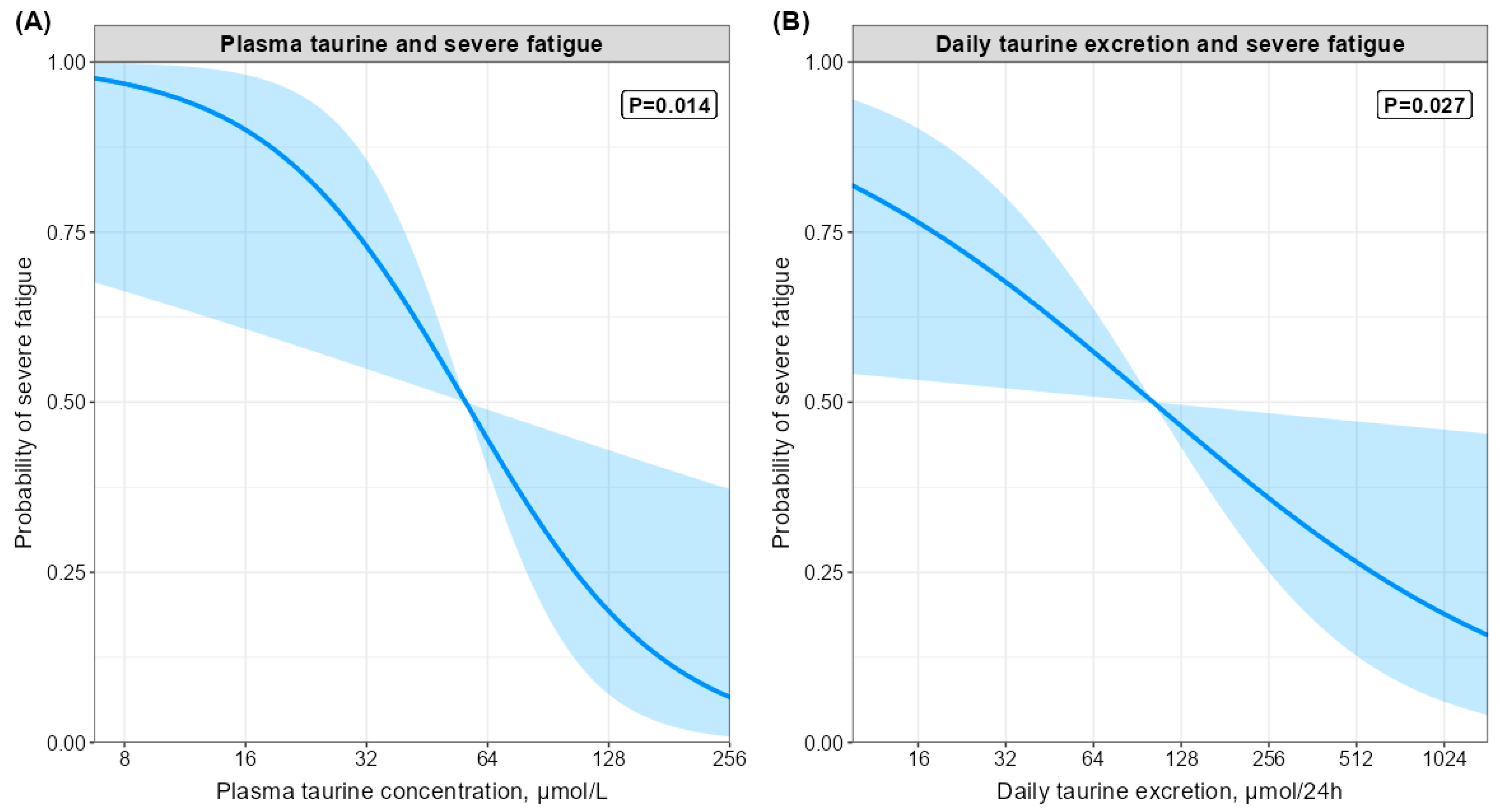
3.7. Associations of Plasma Amino Acid Concentrations with Severe Fatigue
3.8. Associations of Daily Amino Acids Losses and Protein Intake with Severe Fatigue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A Proposed Nomenclature and Diagnostic Criteria for Protein-Energy Wasting in Acute and Chronic Kidney Disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; Terwee, P.; Teta, D.; et al. Prevention and Treatment of Protein Energy Wasting in Chronic Kidney Disease Patients: A Consensus Statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, F.K.; Smeets, J.S.J.; Broers, N.J.H.; van Kranenburg, J.M.X.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J.C. End-Stage Renal Disease Patients Lose a Substantial Amount of Amino Acids during Hemodialysis. J. Nutr. 2020, 150, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.W.; Yang, J.O.; Lee, E.Y.; Lee, E.M.; Choi, J.S.; Hong, S.Y. The Effect of Dialysis Membrane Flux on Amino Acid Loss in Hemodialysis Patients. J. Korean Med. Sci. 2007, 22, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Ikizler, T.A.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino Acid and Albumin Losses during Hemodialysis. Kidney Int. 1994, 46, 830–837. Available online: https://www.kidney-international.org/article/S0085-2538(15)58620-1/pdf (accessed on 1 May 2022). [CrossRef] [Green Version]
- Navarro, J.F.; Mora, C.; Leon, C.; Rio, R.M.-D.; Macia, M.L.; Gallego, E.; Chahin, J.; Mendez, M.L.; Rivero, A.; Garcia, J. Amino Acid Losses during Hemodialysis with Polyacrylonitrile Membranes: Effect of Intradialytic Amino Acid Supplementation on Plasma Amino Acid Concentrations and Nutritional Variables in Nondiabetic Patients. Am. J. Clin. Nutr. 2000, 71, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.F.; Marcen, R.; Teruel, J.L.; del Rio, R.M.; Gamez, C.; Mora, C.; Ortuno, J. Effect of Different Membranes on Amino-Acid Losses during Haemodialysis. Nephrol. Dial. Transplant. 1998, 13, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Tepper, T.; van der Hem, G.K.; Tuma, G.J.; Arisz, L.; Donker, A.J. Loss of Amino Acids during Hemodialysis: Quantitative and Qualitative Investigations. Clin. Nephrol. 1978, 10, 16–20. [Google Scholar]
- Wolfson, M.; Jones, M.R.; Kopple, J.D. Amino Acid Losses during Hemodialysis with Infusion of Amino Acids and Glucose. Kidney Int. 1982, 21, 500–506. Available online: https://www.kidney-international.org/article/S0085-2538(15)32687-9/pdf (accessed on 1 May 2022). [CrossRef] [Green Version]
- Yokomatsu, A.; Fujikawa, T.; Toya, Y.; Shino-Kakimoto, M.; Itoh, Y.; Mitsuhashi, H.; Tamura, K.; Hirawa, N.; Yasuda, G.; Umemura, S. Loss of Amino Acids into Dialysate during Hemodialysis Using Hydrophilic and Nonhydrophilic Polyester-Polymer Alloy and Polyacrylonitrile Membrane Dialyzers. Ther. Apher. Dial. 2014, 18, 340–346. [Google Scholar] [CrossRef]
- Ramkumar, N.; Beddhu, S.; Eggers, P.; Pappas, L.M.; Cheung, A.K. Patient Preferences for In-Center Intense Hemodialysis. Hemodial. Int. Int. Symp. Home Hemodial. 2005, 9, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Ozyilmaz, A.; Westerhuis, R.; Ipema, K.J.R.; Bakker, S.J.L.; Franssen, C.F.M. Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients. Nutrients 2018, 10, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, A.; Schutten, J.C.; Kremer, D.; van der Veen, Y.; Groothof, D.; Sotomayor, C.G.; Koops, C.A.; de Blaauw, P.; Kema, I.P.; Westerhuis, R.; et al. Creatine Homeostasis and Protein Energy Wasting in Hemodialysis Patients. J. Transl. Med. 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Huberts, M.; Poppe, E.; Faassen, M.V.; Kema, I.P.; Vogels, S.; Geleijnse, J.M.; Westerhuis, R.; Ipema, K.J.R.; Bakker, S.J.L.; et al. Tryptophan Intake and Tryptophan Losses in Hemodialysis Patients: A Balance Study. Nutrients 2019, 11, 2851. [Google Scholar] [CrossRef] [Green Version]
- Poppe, E.S.J.M.; Polinder-Bos, H.A.; Huberts, M.; Vogels, S.; Ipema, K.J.R.; Gansevoort, R.T.; Westerhuis, R.; Bakker, S.J.L.; Gaillard, C.A.J.M.; Franssen, C.F.M. Creatinine Synthesis Rate and Muscle Strength and Self-Reported Physical Health in Dialysis Patients. Clin. Nutr. 2019, 39, 1600–1607. [Google Scholar] [CrossRef]
- Prinsen, H.C.M.T.; Schiebergen-Bronkhorst, B.G.M.; Roeleveld, M.W.; Jans, J.J.M.; de Sain-van der Velden, M.G.M.; Visser, G.; van Hasselt, P.M.; Verhoeven-Duif, N.M. Rapid Quantification of Underivatized Amino Acids in Plasma by Hydrophilic Interaction Liquid Chromatography (HILIC) Coupled with Tandem Mass-Spectrometry. J. Inherit. Metab. Dis. 2016, 39, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins: An Improved System. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A Method for Estimating Nitrogen Intake of Patients with Chronic Renal Failure. Kidney Int. 1985, 27, 58–65. Available online: https://www.kidney-international.org/article/S0085-2538(15)33348-2/pdf (accessed on 1 May 2022). [CrossRef] [Green Version]
- Daugirdas, J.T. Second Generation Logarithmic Estimates of Single-Pool Variable Volume Kt/V: An Analysis of Error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Bultmann, U.; de Vries, M.; Beurskens, A.J.; Bleijenberg, G.; Vercoulen, J.H.; Kant, I. Measurement of Prolonged Fatigue in the Working Population: Determination of a Cutoff Point for the Checklist Individual Strength. J. Occup. Health Psychol. 2000, 5, 411–416. [Google Scholar] [CrossRef]
- Worm-Smeitink, M.; Gielissen, M.; Bloot, L.; van Laarhoven, H.W.M.; van Engelen, B.G.M.; van Riel, P.; Bleijenberg, G.; Nikolaus, S.; Knoop, H. The Assessment of Fatigue: Psychometric Qualities and Norms for the Checklist Individual Strength. J. Psychosom. Res. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Goertz, Y.M.J.; Looijmans, M.; Prins, J.B.; Janssen, D.J.A.; Thong, M.S.Y.; Peters, J.B.; Burtin, C.; Meertens-Kerris, Y.; Coors, A.; Muris, J.W.M.; et al. Fatigue in Patients with Chronic Obstructive Pulmonary Disease: Protocol of the Dutch Multicentre, Longitudinal, Observational FAntasTIGUE Study. BMJ Open 2018, 8, e021745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional Assessment of Chronic Fatigue Syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Van Herck, M.; Spruit, M.A.; Burtin, C.; Djamin, R.; Antons, J.; Goertz, Y.M.J.; Ebadi, Z.; Janssen, D.J.A.; Vercoulen, J.H.; Peters, J.B.; et al. Fatigue Is Highly Prevalent in Patients with Asthma and Contributes to the Burden of Disease. J. Clin. Med. 2018, 7, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Sandwijk, M.S.; Al Arashi, D.; van de Hare, F.M.; Rolien van der Torren, J.M.; Kersten, M.J.; Bijlsma, J.A.; ten Berge, I.J.M.; Bemelman, F.J. Fatigue, Anxiety, Depression and Quality of Life in Kidney Transplant Recipients, Haemodialysis Patients, Patients with a Haematological Malignancy and Healthy Controls. Nephrol. Dial. Transplant. 2019, 34, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Pitts, R.F.; MacLeod, M.B. Synthesis of Serine by the Dog Kidney In Vivo. Am. J. Physiol. 1972, 222, 394–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal Metabolism of Amino Acids: Its Role in Interorgan Amino Acid Exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Bergström, J.; Alvestrand, A.; Fürst, P. Plasma and Muscle Free Amino Acids in Maintenance Hemodialysis Patients without Protein Malnutrition. Kidney Int. 1990, 38, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T. The 1986 Borden Award Lecture. The Role of the Kidney in Amino Acid Metabolism and Nutrition. Can. J. Physiol. Pharmacol. 1987, 65, 2355–2362. [Google Scholar] [CrossRef]
- Lichter-Konecki, U.; Hipke, C.M.; Konecki, D.S. Human Phenylalanine Hydroxylase Gene Expression in Kidney and Other Nonhepatic Tissues. Mol. Genet. Metab. 1999, 67, 308–316. [Google Scholar] [CrossRef]
- Møller, N.; Meek, S.; Bigelow, M.; Andrews, J.; Nair, K.S. The Kidney Is an Important Site for in Vivo Phenylalanine-to-Tyrosine Conversion in Adult Humans: A Metabolic Role of the Kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, V.; Tao, X.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, W.H.; Silbernagl, S. Amino Acid Transport by Juxtamedullary Nephrons: Distal Reabsorption and Recycling. Am. J. Physiol.-Ren. Fluid Electrolyte Physiol. 1988, 255, F397–F407. [Google Scholar] [CrossRef]
- Young, G.A. Amino Acids and the Kidney. Amino Acids 1991, 1, 367–403. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Group, N.K.F.K.W. The National Kidney Foundation K/DOQI Clinical Practice Guidelines for Dietary Protein Intake for Chronic Dialysis Patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 38, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. National Kidney Foundation K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 37, S66–S70. [Google Scholar] [CrossRef]
- Gomes Neto, A.W.; Boslooper-Meulenbelt, K.; Geelink, M.; van Vliet, I.M.Y.; Post, A.; Joustra, M.L.; Knoop, H.; Berger, S.P.; Navis, G.J.; Bakker, S.J.L. Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients. Nutrients 2020, 12, 2451. [Google Scholar] [CrossRef]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased Plasma Proline Concentrations Are Associated with Sarcopenia in the Elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef] [Green Version]
- Fukai, K.; Harada, S.; Iida, M.; Kurihara, A.; Takeuchi, A.; Kuwabara, K.; Sugiyama, D.; Okamura, T.; Akiyama, M.; Nishiwaki, Y.; et al. Metabolic Profiling of Total Physical Activity and Sedentary Behavior in Community-Dwelling Men. PLoS ONE 2016, 11, e0164877. [Google Scholar] [CrossRef] [Green Version]
- Ubhi, B.K.; Cheng, K.K.; Dong, J.; Janowitz, T.; Jodrell, D.; Tal-Singer, R.; MacNee, W.; Lomas, D.A.; Riley, J.H.; Griffin, J.L.; et al. Targeted Metabolomics Identifies Perturbations in Amino Acid Metabolism That Sub-Classify Patients with COPD. Mol. BioSystems 2012, 8, 3125–3133. [Google Scholar] [CrossRef]
- Bouckenooghe, T.; Remacle, C.; Reusens, B. Is Taurine a Functional Nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Gaull, G.E. Taurine in Pediatric Nutrition: Review and Update. Pediatrics 1989, 83, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, S.A.; Grosvenor, M.; Kopple, J.D. The Taurine Content of Common Foodstuffs. JPEN J. Parenter. Enter. Nutr. 1990, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, R.; Camilo, M.E. Taurine: A Conditionally Essential Amino Acid in Humans? An Overview in Health and Disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar]
- Suliman, M.E.; Anderstam, B.; Bergström, J. Evidence of Taurine Depletion and Accumulation of Cysteinesulfinic Acid in Chronic Dialysis Patients. Kidney Int. 1996, 50, 1713–1717. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.C.; Laidlaw, S.A.; Kopple, J.D. Taurine Levels in Plasma and Blood Cells in Patients Undergoing Routine Maintenance Hemodialysis. Am. J. Kidney Dis. 1991, 18, 74–79. [Google Scholar] [CrossRef]
- Kurtz, J.A.; VanDusseldorp, T.A.; Doyle, J.A.; Otis, J.S. Taurine in Sports and Exercise. J. Int. Soc. Sports Nutr. 2021, 18, 39. [Google Scholar] [CrossRef]
- Lee, H.; Paik, I.; Park, T. Effects of Dietary Supplementation of Taurine, Carnitine or Glutamine on Endurance Exercise Performance and Fatigue Parameters in Athletes. Korean J. Nutr. 2003, 36, 711–719. [Google Scholar]
- Waldron, M.; Patterson, S.D.; Jeffries, O. Oral Taurine Improves Critical Power and Severe-Intensity Exercise Tolerance. Amino Acids 2019, 51, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Balshaw, T.G.; Bampouras, T.M.; Barry, T.J.; Sparks, S.A. The Effect of Acute Taurine Ingestion on 3-Km Running Performance in Trained Middle-Distance Runners. Amino Acids 2013, 44, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Bridge, C.A.; McNaughton, L.R.; Sparks, S.A. The Effect of Acute Taurine Ingestion on 4-Km Time Trial Performance in Trained Cyclists. Amino Acids 2016, 48, 2581–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ra, S.G.; Akazawa, N.; Choi, Y.; Matsubara, T.; Oikawa, S.; Kumaga, H.; Tanahashi, K.; Ohmori, H.; Maeda, S. Taurine Supplementation Reduces Eccentric Exercise-Induced Delayed Onset Muscle Soreness in Young Men. Adv. Exp. Med. Biol. 2015, 803, 765–772. [Google Scholar] [PubMed]
- da Silva, L.A.; Tromm, C.B.; Bom, K.F.; Mariano, I.; Pozzi, B.; da Rosa, G.L.; Tuon, T.; da Luz, G.; Vuolo, F.; Petronilho, F.; et al. Effects of Taurine Supplementation Following Eccentric Exercise in Young Adults. Appl. Physiol. Nutr. Metab. 2014, 39, 101–104. [Google Scholar] [CrossRef] [PubMed]
- McLeay, Y.; Stannard, S.; Barnes, M. The Effect of Taurine on the Recovery from Eccentric Exercise-Induced Muscle Damage in Males. Antioxidants 2017, 6, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S. Role of Taurine in the Pathogenesis of Obesity. Mol. Nutr. Food Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef]
- Haidari, F.; Asadi, M.; Mohammadi-Asl, J.; Ahmadi-Angali, K. Evaluation of the Effect of Oral Taurine Supplementation on Fasting Levels of Fibroblast Growth Factors, β-Klotho Co-Receptor, Some Biochemical Indices and Body Composition in Obese Women on a Weight-Loss Diet: A Study Protocol for a Double-Blind, Randomized Controlled Trial. Trials 2019, 20, 315. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, M.B.; Brandao, C.F.C.; Fassini, P.G.; Bianco, T.M.; Batitucci, G.; Galan, B.S.M.; de Carvalho, F.G.; Vieira, T.S.; Ferriolli, E.; Marchini, J.S.; et al. Taurine Supplementation Increases Post-Exercise Lipid Oxidation at Moderate Intensity in Fasted Healthy Males. Nutrients 2020, 12, 1540. [Google Scholar] [CrossRef]
- Picariello, F.; Moss-Morris, R.; Macdougall, I.C.; Chilcot, J. The Role of Psychological Factors in Fatigue among End-Stage Kidney Disease Patients: A Critical Review. Clin. Kidney J. 2017, 10, 79–88. [Google Scholar] [CrossRef] [Green Version]

| Hemodialysis Patients (n = 59) | Controls (n = 33) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 65 ± 15 | 54 ± 10 | <0.001 |
| Sex, n male (%) | 37 (63) | 15 (45) | 0.2 |
| Dialysis-related | |||
| Dialysis vintage, months | 15 (6; 39) | – | – |
| Dialysis sessions, n (%) | |||
| Two sessions per week | 3 (5) | – | – |
| Three sessions per week | 56 (95) | – | – |
| Dialysate volume, L | 135 ± 27 | – | – |
| Ultrafiltration volume, L | 1.9 ± 0.9 | – | – |
| Kt/V | 1.4 ± 0.3 | – | – |
| Residual diuresis, n (%) | 32 (54) | 33 (100) | <0.001 |
| Urinary volume 1, L | 0.9 ± 0.6 | 2.5 ± 0.9 | <0.001 |
| Urinary protein excretion, g/24 h | 0.7 (0.3; 1.3) | ||
| Plasma albumin, g/L | 39 ± 5 | 45 ± 2 | <0.001 |
| Hemoglobin, mmol/L | 6.9 ± 0.7 | 8.8 ± 0.8 | <0.001 |
| Body composition | |||
| Weight predialysis 2, kg | 80 ± 16 | 77 ± 17 | 0.3 |
| Weight postdialysis, kg | 78 ± 16 | – | – |
| Height, m | 1.75 ± 0.09 | 1.72 ± 0.10 | 0.2 |
| BMI 3, kg/m2 | 25.5 ± 4.3 | 25.6 ± 4.3 | 0.4 |
| Protein intake | |||
| Protein intake absolute, g/24 h | 64 ± 21 | 84 ± 21 | <0.001 |
| Protein intake per kg bodyweight, g/kg/24 h | 0.82 ± 0.23 | 1.11 ± 0.21 | <0.001 |
| Metabolite | Predialysis Concentration in Hemodialysis Patients (μmol/L) | Concentration in Healthy Controls (μmol/L) | Proportional Difference * | p-Value |
|---|---|---|---|---|
| Essential | ||||
| Histidine | 69 ± 19 | 74 ± 12 | –7% | 0.2 |
| Isoleucine | 63 ± 23 | 61 ± 18 | +3% | 0.6 |
| Leucine | 103 ± 41 | 110 ± 29 | –6% | 0.4 |
| Lysine | 141 ± 36 | 171 ± 37 | –18% | <0.001 |
| Methionine | 19 ± 7 | 21 ± 5 | –10% | 0.03 |
| Phenylalanine | 68 ± 22 | 57 ± 9 | +19% | 0.002 |
| Threonine | 87 ± 29 | 115 ± 24 | –24% | <0.001 |
| Tryptophan | 22 (20; 28) | 54 (46; 57) | –59% | <0.001 |
| Valine | 186 ± 59 | 213 ± 51 | –13% | 0.03 |
| Non-essential | ||||
| Alanine | 351 ± 135 | 423 ± 113 | –17% | 0.01 |
| Arginine | 77 ± 22 | 78 ± 17 | –1% | 0.7 |
| Asparagine | 53 ± 16 | 61 ± 11 | –13% | 0.004 |
| Citrulline | 78 (61; 95) | 28 (24; 32) | +179% | <0.001 |
| Glutamic acid | 101 (69; 161) | 57 (45; 81) | +78% | <0.001 |
| Glutamine | 514 ± 111 | 575 ± 87 | –11% | 0.008 |
| Glycine | 260 ± 84 | 202 ± 54 | +29% | <0.001 |
| Ornithine | 63 ± 20 | 59 ± 16 | +7% | 0.4 |
| Proline | 276 (223; 322) | 248 (197; 321) | +11% | 0.3 |
| Serine | 61 ± 19 | 103 ± 19 | –41% | <0.001 |
| Taurine | 58 (41; 87) | 40 (37; 50) | +45% | 0.014 |
| Tyrosine | 45 ± 16 | 62 ± 18 | –27% | <0.001 |
| Total amino acids | ||||
| Total BCAAs | 353 ± 119 | 384 ± 94 | –9% | 0.2 |
| Total essential | 761 ± 200 | 875 ± 155 | –13% | 0.006 |
| Total non-essential | 2052 ± 587 | 1977 ± 328 | +4% | 0.4 |
| Total all amino acids | 2813 ± 693 | 2852 ± 427 | –1% | 0.7 |
| Metabolite | Daily Losses in Hemodialysis Patients (μmol/24 h) | Daily Losses in Controls (μmol/24 h) | Ratio of Hemodialysis to Controls | p-Value |
|---|---|---|---|---|
| Essential | ||||
| Histidine | 862 ± 270 | 698 ± 463 | 1.2 | 0.07 |
| Isoleucine | 799 ± 338 | 12 ± 7 | 67 | <0.001 |
| Leucine | 1382 ± 587 | 67 ± 23 | 21 | <0.001 |
| Lysine | 1505 (1233; 1845) | 209 (157; 288) | 7 | <0.001 |
| Methionine | 172 ± 95 | 5 ± 2 | 34 | <0.001 |
| Phenylalanine | 729 (631; 967) | 68 (52; 103) | 11 | <0.001 |
| Threonine | 1043 (731; 1279) | 105 (81; 161) | 10 | <0.001 |
| Tryptophan | 216 ± 63 | 148 ± 57 | 1.5 | <0.001 |
| Valine | 2017 (1606; 2614) | 42 (35; 54) | 45 | <0.001 |
| Non-essential | ||||
| Alanine | 3926 ± 1548 | 361 ± 219 | 11 | <0.001 |
| Arginine | 826 ± 377 | 34 ± 15 | 24 | <0.001 |
| Asparagine | 747 ± 290 | 122 ± 82 | 6 | <0.001 |
| Citrulline | 463 (347; 570) | 11 (7; 13) | 42 | <0.001 |
| Glutamic acid | 966 ± 559 | 35 ± 33 | 28 | <0.001 |
| Glutamine | 6954 ± 2410 | 449 ± 211 | 15 | <0.001 |
| Glycine | 3502 ± 1271 | 1521 ± 848 | 2 | <0.001 |
| Ornithine | 541 ± 194 | 22 ± 17 | 25 | <0.001 |
| Proline | 3601 ± 1351 | 8 ± 5 | 450 | <0.001 |
| Serine | 754 (572; 1004) | 324 (236; 467) | 2 | <0.001 |
| Taurine | 132 (24; 235) | 339 (204; 668) | 0.4 | <0.001 |
| Tyrosine | 462 ± 192 | 105 ± 68 | 4 | <0.001 |
| Totals | ||||
| Total BCAAs | 4307 ± 1717 | 125 ± 52 | 34 | <0.001 |
| Total essential | 9060 ± 3287 | 1463 ± 862 | 6 | <0.001 |
| Total non-essential | 23,059 ± 7284 | 3837 ± 1939 | 6 | <0.001 |
| Total losses | 32,119 ± 10,221 | 5300 ± 2749 | 6 | <0.001 |
| Metabolite | Predialysis Concentration (μmol/L) | Postdialysis Concentration (μmol/L) | Absolute Change (μmol/L) | Proportional Difference | p-Value |
|---|---|---|---|---|---|
| Essential | |||||
| Histidine | 69 ± 19 | 54 ± 14 | –15 ± 13 | –22% | <0.001 |
| Isoleucine | 63 ± 23 | 74 ± 22 | +11 ± 26 | +17% | 0.002 |
| Leucine | 103 ± 41 | 123 ± 38 | +20 ± 46 | +20% | 0.001 |
| Lysine | 141 ± 36 | 133 ± 37 | –7 ± 38 | –5% | 0.14 |
| Methionine | 19 ± 7 | 19 ± 6 | +1 ± 7 | +5% | 0.46 |
| Phenylalanine | 68 ± 22 | 65 ± 13 | –3 ± 18 | –4% | 0.27 |
| Threonine | 87 ± 29 | 80 ± 22 | –7 ± 22 | –8% | 0.02 |
| Tryptophan | 22 (20; 28) | 27 (24; 34) | +5 (0; 15) | +23% | <0.001 |
| Valine | 186 ± 59 | 146 ± 37 | –38 ± 52 | –20% | <0.001 |
| Non-essential | |||||
| Alanine | 351 ± 135 | 251 ± 85 | –99 ± 110 | –28% | <0.001 |
| Arginine | 77 ± 22 | 60 ± 19 | –17 ± 23 | –22% | <0.001 |
| Asparagine | 53 ± 16 | 50 ± 15 | –3 ± 14 | –6% | 0.10 |
| Citrulline | 78 (61; 95) | 34 (27; 46) | –42 (27; 50) | –54% | <0.001 |
| Glutamic acid | 101 (69; 161) | 108 (64; 138) | +2 (–26; 14) | +2% | 0.78 |
| Glutamine | 514 ± 111 | 457 ± 96 | –58 ± 84 | –11% | <0.001 |
| Glycine | 260 ± 84 | 203 ± 55 | –57 ± 57 | –22% | <0.001 |
| Ornithine | 63 ± 20 | 51 ± 12 | –12 ± 17 | –19% | <0.001 |
| Proline | 276 (223; 322) | 230 (212; 280) | –33 (– 83; 16) | –12% | 0.002 |
| Serine | 61 ± 19 | 60 ± 18 | –1 ± 13 | –2% | 0.54 |
| Taurine | 58 (41 − 87) | 35 (23; 53) | –17 (–4; –39) | –29% | <0.001 |
| Tyrosine | 45 ± 16 | 45 ± 12 | –0 ± 16 | –0% | 0.97 |
| Total amino acids | |||||
| Total BCAAs | 353 ± 119 | 343 ± 94 | –7 ± 119 | –3% | 0.7 |
| Total essential | 761 ± 200 | 724 ± 170 | –34 ± 206 | –5% | 0.2 |
| Total non-essential | 2052 ± 587 | 1664 ± 309 | –384 ± 510 | –19% | <0.001 |
| Total amino acids | 2813 ± 693 | 2388 ± 442 | –418 ± 637 | –15% | <0.001 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | p-Value | Odds Ratio (95% Confidence Interval) | p-Value | |
| Essential | ||||
| Histidine | 1.21 (0.66; 2.38) | 0.5 | 1.97 (0.91; 4.97) | 0.1 |
| Isoleucine | 0.83 (0.42; 1.53) | 0.6 | 0.88 (0.43; 1.71) | 0.7 |
| Leucine | 0.75 (0.38; 1.37) | 0.4 | 0.77 (0.37; 1.50) | 0.5 |
| Lysine | 0.80 (0.41; 1.46) | 0.5 | 0.78 (0.36; 1.51) | 0.5 |
| Methionine | 0.80 (0.41; 1.47) | 0.5 | 0.98 (0.47; 2.01) | 0.9 |
| Phenylalanine | 0.95 (0.46; 1.85) | 0.9 | 1.05 (0.48; 2.20) | 0.9 |
| Threonine | 0.88 (0.48; 1.61) | 0.7 | 1.05 (0.53; 2.15) | 0.9 |
| Tryptophan | 1.52 (0.81; 3.00) | 0.2 | 1.70 (0.87; 3.66) | 0.1 |
| Valine | 0.77 (0.41; 1.41) | 0.4 | 0.79 (0.39; 1.53) | 0.5 |
| Non-essential | ||||
| Alanine | 1.42 (0.80; 2.61) | 0.2 | 1.44 (0.80; 2.73) | 0.2 |
| Arginine | 0.63 (0.30; 1.21) | 0.2 | 0.66 (0.28; 1.36) | 0.3 |
| Asparagine | 0.70 (0.35; 1.29) | 0.3 | 0.85 (0.38; 1.78) | 0.7 |
| Citrulline | 0.92 (0.50; 1.66) | 0.8 | 1.14 (0.58; 2.30) | 0.7 |
| Glutamic acid | 1.03 (0.50; 1.84) | 0.9 | 0.89 (0.40; 1.66) | 0.7 |
| Glutamine | 0.99 (0.54; 1.80) | 0.9 | 1.36 (0.67; 2.84) | 0.4 |
| Glycine | 0.76 (0.39; 1.39) | 0.4 | 0.92 (0.43; 1.87) | 0.8 |
| Ornithine | 0.84 (0.44; 1.53) | 0.6 | 0.91 (0.46; 1.74) | 0.8 |
| Proline | 2.55 (1.17; 7.12) | 0.042 | 2.97 (1.27; 9.26) | 0.032 |
| Serine | 0.60 (0.30; 1.11) | 0.1 | 0.71 (0.33; 1.39) | 0.3 |
| Taurine * | 0.41 (0.17; 0.84) | 0.027 | 0.30 (0.10; 0.70) | 0.014 |
| Tyrosine | 0.98 (0.51; 1.86) | 0.9 | 1.18 (0.57; 2.50) | 0.7 |
| Average amino acids | ||||
| BCAAs | 0.77 (0.40; 1.41) | 0.4 | 0.80 (0.39; 1.54) | 0.5 |
| Essential | 0.83 (0.44; 1.51) | 0.6 | 0.92 (0.47; 1.75) | 0.8 |
| Non-essential | 1.05 (0.57; 1.89) | 0.5 | 1.15 (0.63; 2.16) | 0.6 |
| Total amino acids | 0.99 (0.54; 1.78) | 0.9 | 1.10 (0.60; 2.08) | 0.7 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Daily Amino Acid Losses | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Essential | ||||
| Histidine | 0.93 (0.47; 1.81) | 0.8 | 0.95 (0.46; 1.88) | 0.9 |
| Isoleucine | 0.75 (0.38; 1.37) | 0.4 | 0.62 (0.28; 1.21) | 0.2 |
| Leucine | 0.68 (0.34; 1.27) | 0.2 | 0.54 (0.24; 1.09) | 0.1 |
| Lysine | 0.79 (0.40; 1.49) | 0.5 | 0.60 (0.26; 1.24) | 0.2 |
| Methionine | 0.70 (0.35; 1.31) | 0.3 | 0.71 (0.34; 1.36) | 0.3 |
| Phenylalanine | 0.90 (0.45; 1.64) | 0.7 | 0.80 (0.37; 1.51) | 0.5 |
| Threonine | 0.88 (0.45; 1.68) | 0.7 | 0.82 (0.40; 1.64) | 0.6 |
| Tryptophan * | 1.31 (0.40; 5.00) | 0.6 | 1.18 (0.35; 4.55) | 0.8 |
| Valine | 0.74 (0.38; 1.38) | 0.4 | 0.60 (0.27; 1.19) | 0.2 |
| Non-essential | ||||
| Alanine | 1.26 (0.68; 2.37) | 0.5 | 1.20 (0.61; 2.38) | 0.6 |
| Arginine | 0.86 (0.46; 1.62) | 0.6 | 0.82 (0.40; 1.64) | 0.6 |
| Asparagine | 0.74 (0.36; 1.47) | 0.4 | 0.77 (0.34; 1.61) | 0.5 |
| Citrulline | 0.84 (0.46; 1.50) | 0.6 | 0.81 (0.44; 1.46) | 0.5 |
| Glutamic acid | 0.84 (0.44; 1.53) | 0.6 | 0.41 (0.15; 0.97) | 0.06 |
| Glutamine | 0.99 (0.52; 1.89) | 0.9 | 1.01 (0.51; 1.98) | 0.9 |
| Glycine | 0.67 (0.33; 1.28) | 0.2 | 0.62 (0.27; 1.34) | 0.2 |
| Ornithine | 0.79 (0.40; 1.50) | 0.5 | 0.71 (0.33; 1.40) | 0.3 |
| Proline | 1.46 (0.78; 2.83) | 0.2 | 1.50 (0.77; 3.04) | 0.2 |
| Serine | 0.89 (0.47; 1.63) | 0.7 | 0.97 (0.49; 1.85) | 0.9 |
| Taurine * | 0.69 (0.47; 0.97) | 0.043 | 0.64 (0.42; 0.93) | 0.027 |
| Tyrosine | 0.83 (0.44; 1.47) | 0.5 | 0.78 (0.42; 1.41) | 0.4 |
| Average amino acids | ||||
| BCAAs | 0.72 (0.36; 1.33) | 0.3 | 0.58 (0.26; 1.15) | 0.1 |
| Essential | 0.77 (0.39; 1.44) | 0.4 | 0.65 (0.30; 1.28) | 0.2 |
| Non-essential | 0.98 (0.51; 1.84) | 0.9 | 0.94 (0.47; 1.86) | 0.9 |
| Total amino acids | 0.91 (0.47; 1.70) | 0.8 | 0.84 (0.42; 1.64) | 0.6 |
| Dietary intake | ||||
| Protein intake | 0.18 (0.04; 0.50) | 0.003 | 0.18 (0.04; 0.54) | 0.007 |
| Protein intake per kg bodyweight | 0.22 (0.07; 0.53) | 0.003 | 0.23 (0.07; 0.57) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Post, A.; Kremer, D.; Groothof, D.; van der Veen, Y.; de Blaauw, P.; van der Krogt, J.; Kema, I.P.; Westerhuis, R.; Heiner-Fokkema, M.R.; Bakker, S.J.L.; et al. Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients. Nutrients 2022, 14, 2810. https://doi.org/10.3390/nu14142810
Post A, Kremer D, Groothof D, van der Veen Y, de Blaauw P, van der Krogt J, Kema IP, Westerhuis R, Heiner-Fokkema MR, Bakker SJL, et al. Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients. Nutrients. 2022; 14(14):2810. https://doi.org/10.3390/nu14142810
Chicago/Turabian StylePost, Adrian, Daan Kremer, Dion Groothof, Yvonne van der Veen, Pim de Blaauw, Jennifer van der Krogt, Ido P. Kema, Ralf Westerhuis, M. Rebecca Heiner-Fokkema, Stephan J. L. Bakker, and et al. 2022. "Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients" Nutrients 14, no. 14: 2810. https://doi.org/10.3390/nu14142810
APA StylePost, A., Kremer, D., Groothof, D., van der Veen, Y., de Blaauw, P., van der Krogt, J., Kema, I. P., Westerhuis, R., Heiner-Fokkema, M. R., Bakker, S. J. L., & Franssen, C. F. M. (2022). Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients. Nutrients, 14(14), 2810. https://doi.org/10.3390/nu14142810







