Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Body Composition Detection
2.4. Detection of Oxidative Stress-Associated Biological Indicators
2.5. Western Blot Analysis
2.6. Statistics Analysis
3. Results
3.1. NT Supplement Improved Dietary Intake and Body Weight in Aging SAMP8 Mice
3.2. NT Supplement Improved BAT Mass and Decreased the Decline in Total Fat Mass
3.3. NT Supplement Promoted Antioxidant-Associated Biological Indicators in BAT
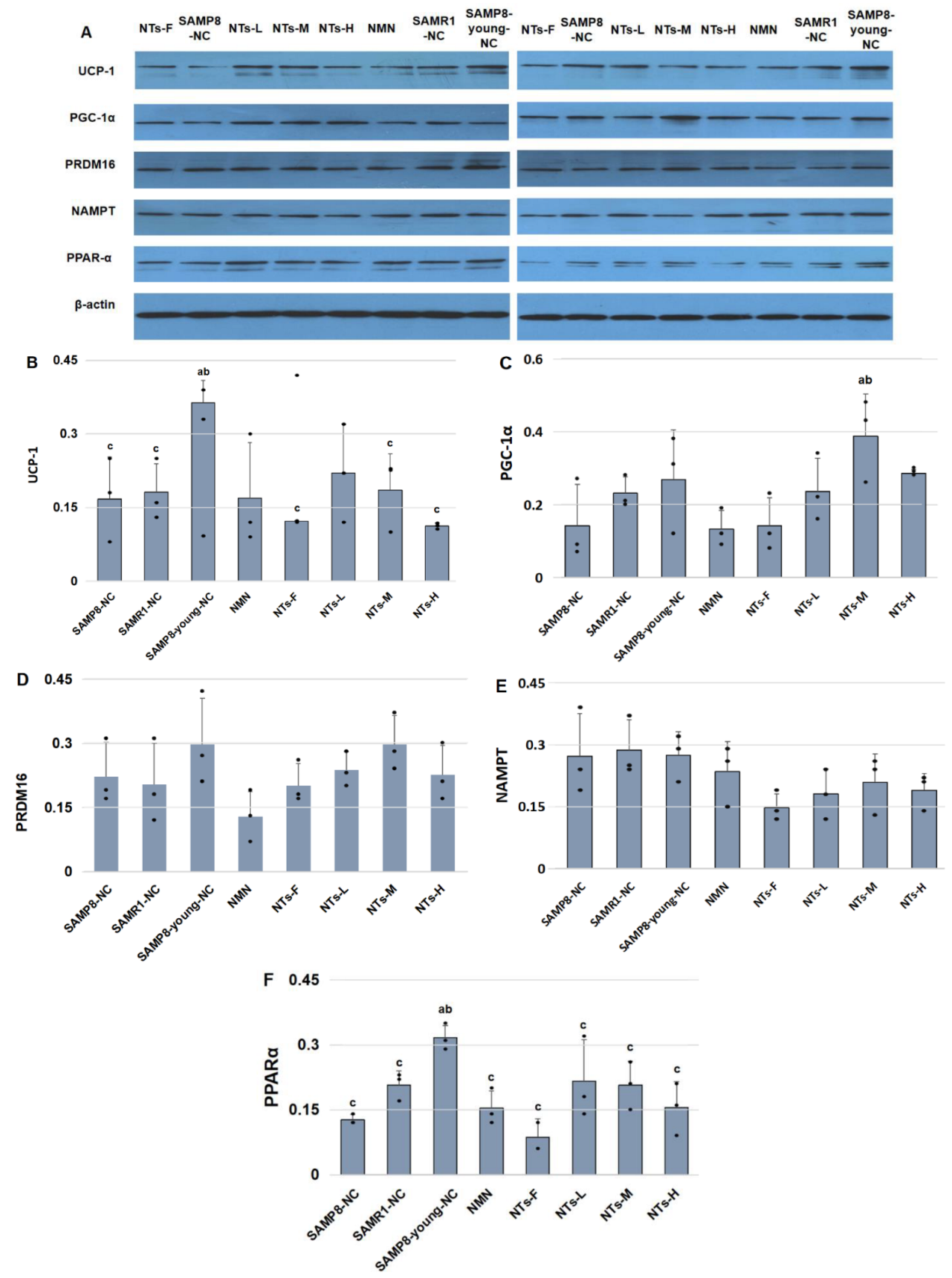
3.4. NTs Increased Brown Adipocyte Markers in BAT
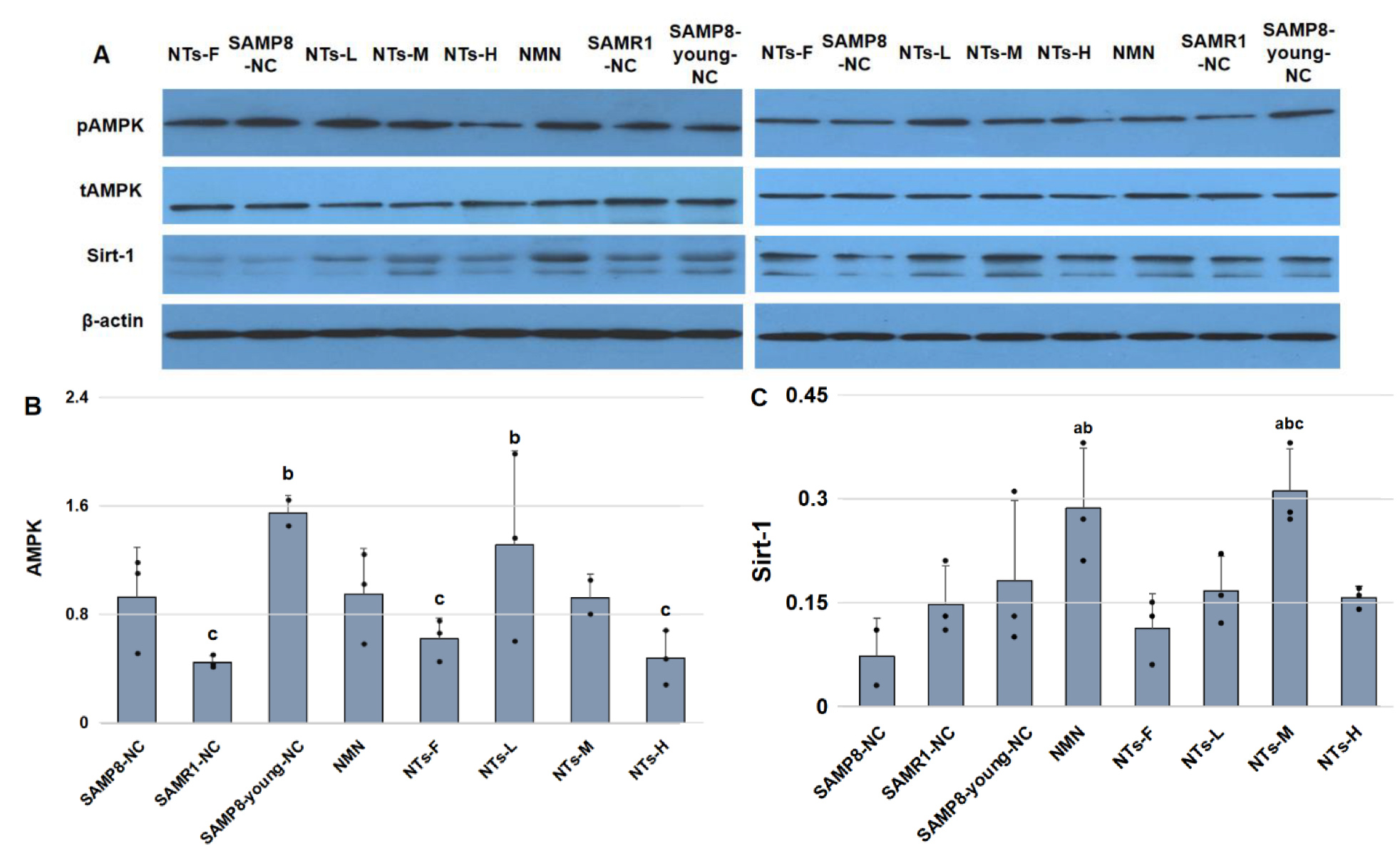
3.5. NTs Enhanced Sirt-1 Protein Level and Might Have Activated AMPK in BAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Wu, K.K.; Jiang, X.; Xu, A.; Cheng, K.K. The role of adipose tissue senescence in obesity- and aging-related metabolic disorders. Clin. Sci. 2020, 134, 315–330. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to aging. Age 2016, 38, 23. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.M.; Sanchez-Gurmaches, J.; Guertin, D.A. Brown Adipose Tissue Development and Metabolism. Handb. Exp. Pharmacol. 2019, 251, 3–36. [Google Scholar]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef] [Green Version]
- Graja, A.; Gohlke, S.; Schulz, T.J. Aging of Brown and Beige/Brite Adipose Tissue. Handb. Exp. Pharmacol. 2019, 251, 55–72. [Google Scholar]
- Bargut, T.C.L.; Silva-e-Silva, A.C.A.G.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Y.; Wu, H.; Sun, F.; Li, Y.; He, H.; Wang, B.; Lu, Z.; Hu, Y.; Wei, X.; et al. Inhibition of Mitochondrial Calcium Overload by SIRT3 Prevents Obesity- or Age-Related Whitening of Brown Adipose Tissue. Diabetes 2020, 69, 165–180. [Google Scholar] [CrossRef]
- Pan, R.; Chen, Y. Management of Oxidative Stress: Crosstalk Between Brown/Beige Adipose Tissues and Skeletal Muscles. Front. Physiol. 2021, 12, 712372. [Google Scholar] [CrossRef]
- Graja, A.; Schulz, T.J. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology 2015, 61, 211–217. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Aging Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.D. Dietary nucleotides: Cellular immune, intestinal and hepatic system effects. J. Nutr. 1994, 124 (Suppl. S1), 144s–148s. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yi, L.; Xu, W.; Zhou, H.; Zhang, Y.; Zhang, W.; Mai, K. Effects of dietary nucleotides on growth, non-specific immune response and disease resistance of sea cucumber Apostichopus japonicas. Fish Shellfish Immunol. 2015, 47, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.Y.; Wang, Q.; Li, G.; Fan, Z.; Wang, H.; Wu, X. Dietary supplement with nucleotides in the form of uridine monophosphate or uridine stimulate intestinal development and promote nucleotide transport in weaned piglets. J. Sci. Food Agric. 2019, 99, 6108–6113. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Wang, N.; Xu, M.; Mao, R.; Li, Y. Dietary Nucleotides Supplementation and Liver Injury in Alcohol-Treated Rats: A Metabolomics Investigation. Molecules 2016, 21, 435. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Wu, Z.; Xu, L.; Fang, Y.; Xu, Y. Maternal supplementation of nucleotides improves the behavioral development of prenatal ethanol-exposed mice. Cogn. Affect. Behav. Neurosci. 2014, 14, 879–890. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Guo, Q.; Wang, S.; Zhao, M.; Zhang, Z.; Wang, J.; Li, Y. Dietary nucleotides extend the life span in Sprague Dawley rats. J. Nutr. Health Aging 2013, 17, 223–229. [Google Scholar] [CrossRef]
- Siahanidou, T.; Mandyla, H.; Papassotiriou, I.; Anagnostakis, D. Serum lipids in preterm infants fed a formula supplemented with nucleotides. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Kiss, T.; Nyúl-Tóth, Á.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovas-cular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 2020, 42, 527–546. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Uejima, Y.; Fukuchi, Y.; Nagase, T.; Tabata, R.; Orimo, H. A new murine model of aging lung: The senescence accelerated mouse (SAM)-P. Mech. Aging Dev. 1991, 61, 223–236. [Google Scholar] [CrossRef]
- Liu, H.W.; Chan, Y.C.; Wei, C.C.; Chen, Y.A.; Wang, M.F.; Chang, S.J. An alternative model for studying age-associated metabolic complications: Senescence-accelerated mouse prone 8. Exp. Gerontol. 2017, 99, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Ren, G.; Peliciari-Garcia, R.A.; Mia, S.; McGinnis, G.R.; Davis, J.; Gamble, K.L.; Kim, J.A.; Young, M.E. Diurnal, metabolic and thermogenic alterations in a murine model of accelerated aging. Chronobiol. Int. 2020, 37, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Franczyk, M.P.; Chondronikola, M.; Qi, N.; Gunawardana, S.C.; Stromsdorfer, K.L.; Porter, L.C.; Wozniak, D.F.; Sasaki, Y.; Rensing, N.; et al. Adipose tissue NAD(+) biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 23822–23828. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Saraf, M.K.; Annamalai, P.; Yfanti, C.; Chao, T.; Wong, D.; Shinoda, K.; et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016, 23, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Tint, M.T.; Michael, N.; Sadananthan, S.A.; Huang, J.Y.; Khoo, C.M.; Godfrey, K.M.; Shek, L.P.C.; Lek, N.; Tan, K.H.; Yap, F.; et al. Brown Adipose Tissue, Adiposity, and Metabolic Profile in Preschool Children. J. Clin. Endocrinol. Metab. 2021, 106, 2901–2914. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Li, Y.; Wang, J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Xiao, W.; You, L.; Zhang, F.; Cao, X.; Feng, J.; Shen, D.; Li, Y.; Wang, Y.; Ji, C.; et al. Age-induced oxidative stress impairs adipogenesis and thermogenesis in brown fat. FEBS J. 2019, 286, 2753–2768. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, X.Y.; Wang, G.Y.; Wang, C.M.; Zhao, Z.J. Food restriction attenuates oxidative stress in brown adipose tissue of striped hamsters acclimated to a warm temperature. J. Therm. Biol. 2016, 58, 72–79. [Google Scholar] [CrossRef]
- Zhou, S.S.; Cao, L.L.; Xu, W.D.; Cao, J.; Zhao, Z.J. Effect of temperature on oxidative stress, antioxidant levels and uncoupling protein expression in striped hamsters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 189, 84–90. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.J.; Ishibashi, J.; Wang, W.; Lim, H.W.; Goyama, S.; Sato, T.; Kurokawa, M.; Won, K.J.; Seale, P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014, 19, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 SAMP8-NCs a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef]
- Xu, W.; Yan, J.; Ocak, U.; Lenahan, C.; Shao, A.; Tang, J.; Zhang, J.; Zhang, J.H. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by SAMP8-NCling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics 2021, 11, 522–539. [Google Scholar] [CrossRef]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef]
- Eo, H.; Jeon, Y.J.; Lee, M.; Lim, Y. Brown Alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 349–359. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.T.; Xu, Z.; Liu, H.; Gu, L.Q.; Ye, J.M.; Huang, Z.S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]


| Food | Purified Food | Standard Food |
|---|---|---|
| C | 0 | 95.4 |
| A | 0 | 348 |
| U | 0 | 714.3 |
| G | 0 | 328.5 |
| Total NT content (mg/kg) | 0 | 1486.2 |
| Groups | Feed | Sample Size | Ages at the Endpoint (m) | Survival Animal Numbers |
|---|---|---|---|---|
| SAMP8-young-NC | Standard food | 12 | 6 | 12 |
| SAMP8-NC | Standard food | 15 | 12 | 12 |
| SAMR1-NC | Standard food | 15 | 12 | 13 |
| NMN | Standard food + 0.3 g/kg NMN | 15 | 12 | 12 |
| NTs-F | Purified food, AIN-93M | 15 | 12 | 11 |
| NTs-L | Standard food + 0.3 g/kg NTs | 15 | 12 | 13 |
| NTs-M | Standard food + 0.6 g/kg NTs | 15 | 12 | 13 |
| NTs-H | Standard food + 1.2 g/kg NTs | 15 | 12 | 12 |
| Groups | 3 Months Old | 6 Months Old | 9 Months Old | 12 Months Old |
|---|---|---|---|---|
| SAMP8-NC | 29.84 ± 1.84 | 33.27 ± 2.36 | 31.66 ± 2.59 b | 29.78 ± 3.86 |
| SAMR1-NC | 30.88 ± 1.53 | 35.77 ± 2.73 | 35.78 ± 3.32 a | 32.01 ± 3.63 |
| SAMP8-young-NC | 30.80 ± 1.95 | 35.01 ± 2.02 | ||
| NMN | 30.12 ± 1.77 | 35.32 ± 3.59 | 33.45 ± 2.74 | 31.63 ± 2.80 |
| NTs-F | 30.89 ± 3.62 | 31.89 ± 2.92 b | 31.40 ± 2.98 b | 27.69 ± 3.09 b |
| NTs-L | 30.32 ± 2.61 | 33.70 ± 3.93 | 33.51 ± 4.71 | 32.16 ± 4.42 |
| NTs-M | 30.80 ± 2.59 | 36.67 ± 4.53 a | 35.28 ± 4.48 a | 33.28 ± 3.88 a |
| NTs-H | 29.93 ± 1.70 | 36.46 ± 5.19 a | 36.23 ± 4.84 a | 32.50 ± 3.12 |
| Groups | 3 Months Old | 6 Months Old | 9 Months Old | 12 Months Old |
|---|---|---|---|---|
| SAMP8-NC | 35.34 ± 1.54 | 33.14 ± 4.30 | 27.86 ± 4.04 b | 25.04 ± 2.69 |
| SAMR1-NC | 35.63 ± 5.73 | 33.04 ± 2.18 | 32.83 ± 2.91 a | 26.87 ± 3.42 |
| SAMP8-young-NC | 36.69 ± 1.65 | 33.74 ± 4.18 | ||
| NMN | 37.11 ± 6.64 | 33.39 ± 4.14 | 29.45 ± 3.60 b | 28.61 ± 6.15 |
| NTs-F | 34.04 ± 8.81 | 32.17 ± 7.08 | 29.99 ± 3.77 | 29.63 ± 3.97 a |
| NTs-L | 36.68 ± 2.49 | 37.63 ± 4.12 ab | 32.89 ± 4.57 a | 28.41 ± 2.43 |
| NTs-M | 37.75 ± 2.89 | 37.04 ± 4.98 ab | 32.28 ± 4.04 a | 25.77 ± 5.64 |
| NTs-H | 37.74 ± 2.08 | 37.49 ± 4.60 ab | 30.26 ± 2.89 | 28.93 ± 4.94 |
| Groups | 3–6 Months | 6–9 Months | 9–12 Months |
|---|---|---|---|
| SAMP8-NC | 0.52 ± 0.25 | −0.38 ± 0.35 b | −0.36 ± 0.47 |
| SAMR1-NC | 0.71 ± 0.80 | −0.46 ± 0.46 a | −0.31 ± 0.52 |
| SAMP8-young-NC | 0.39 ± 0.47 | ||
| NMN | 0.67 ± 0.50 | −0.73 ± 0.42 a | −0.18 ± 0.58 |
| NTs-F | 0.36 ± 0.86 | 0.00 ± 0.27 | −0.04 ± 0.47 |
| NTs-L | 0.62 ± 0.31 | −0.50 ± 0.35 a | −0.11 ± 0.31 |
| NTs-M | 0.49 ± 0.27 | −0.12 ± 0.49 | −0.27 ± 0.44 |
| NTs-H | 0.53 ± 0.73 | −0.62 ± 0.37 a | −0.08 ± 0.31 |
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, R.; Wei, C.; Xu, M.; Li, Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2022, 14, 2796. https://doi.org/10.3390/nu14142796
Wang X, Liu R, Wei C, Xu M, Li Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients. 2022; 14(14):2796. https://doi.org/10.3390/nu14142796
Chicago/Turabian StyleWang, Xiujuan, Rui Liu, Chan Wei, Meihong Xu, and Yong Li. 2022. "Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice" Nutrients 14, no. 14: 2796. https://doi.org/10.3390/nu14142796
APA StyleWang, X., Liu, R., Wei, C., Xu, M., & Li, Y. (2022). Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients, 14(14), 2796. https://doi.org/10.3390/nu14142796







