Compromised Hindgut Microbial Digestion, Rather Than Chemical Digestion in the Foregut, Leads to Decreased Nutrient Digestibility in Pigs Fed Low-Protein Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Pigs
2.2. Experimental Design and Sample Collection for the In Vivo Trial
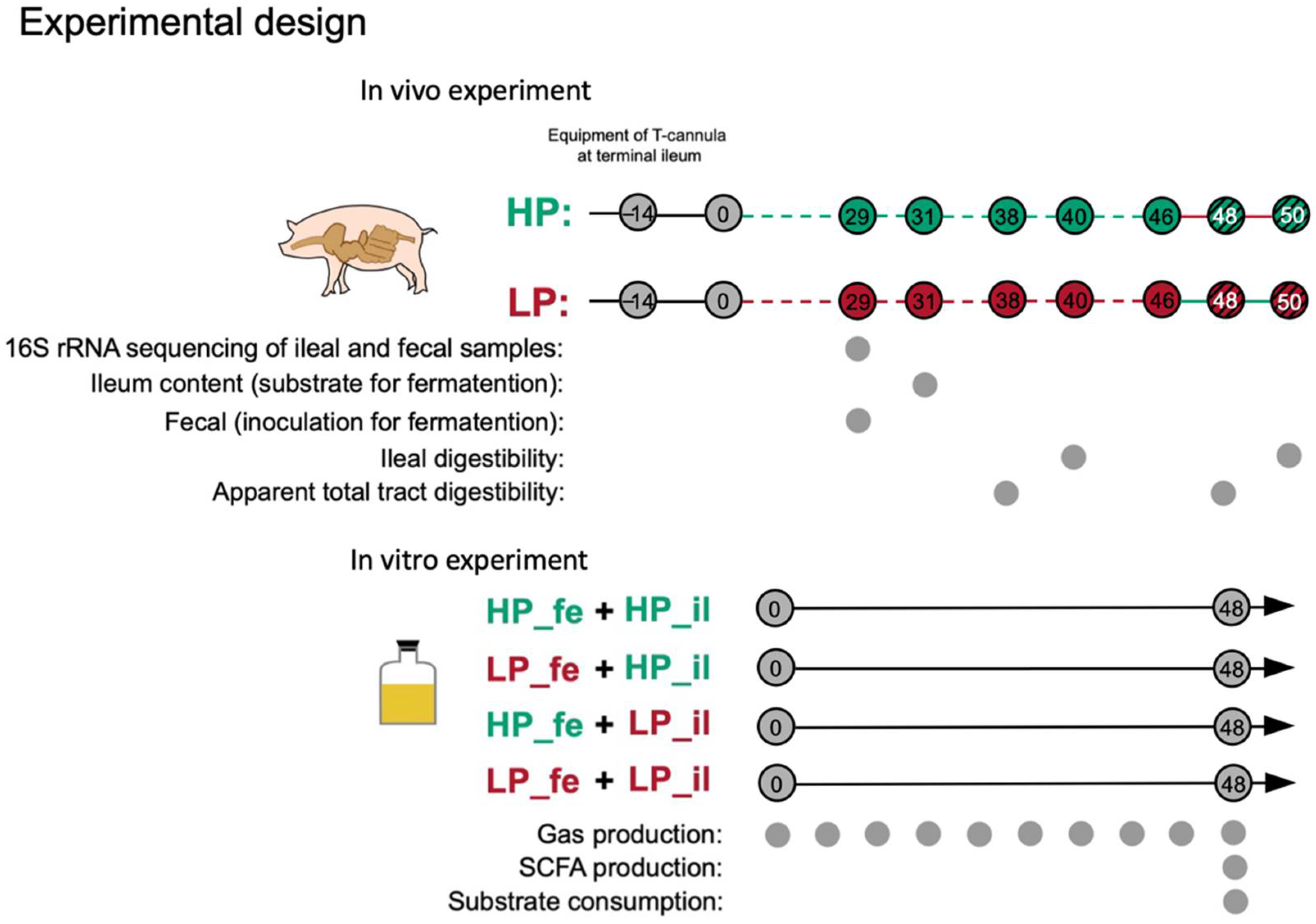
2.3. In Vitro Fermentation Assay
2.4. Chemical Analyses
2.5. Short-Chain Fatty Acid Analyses
2.6. Bacterial Community Structure
2.7. Calculations
2.7.1. Nutrient Digestibility
2.7.2. Gas Production Profiles
2.8. Statistical Analysis
3. Results
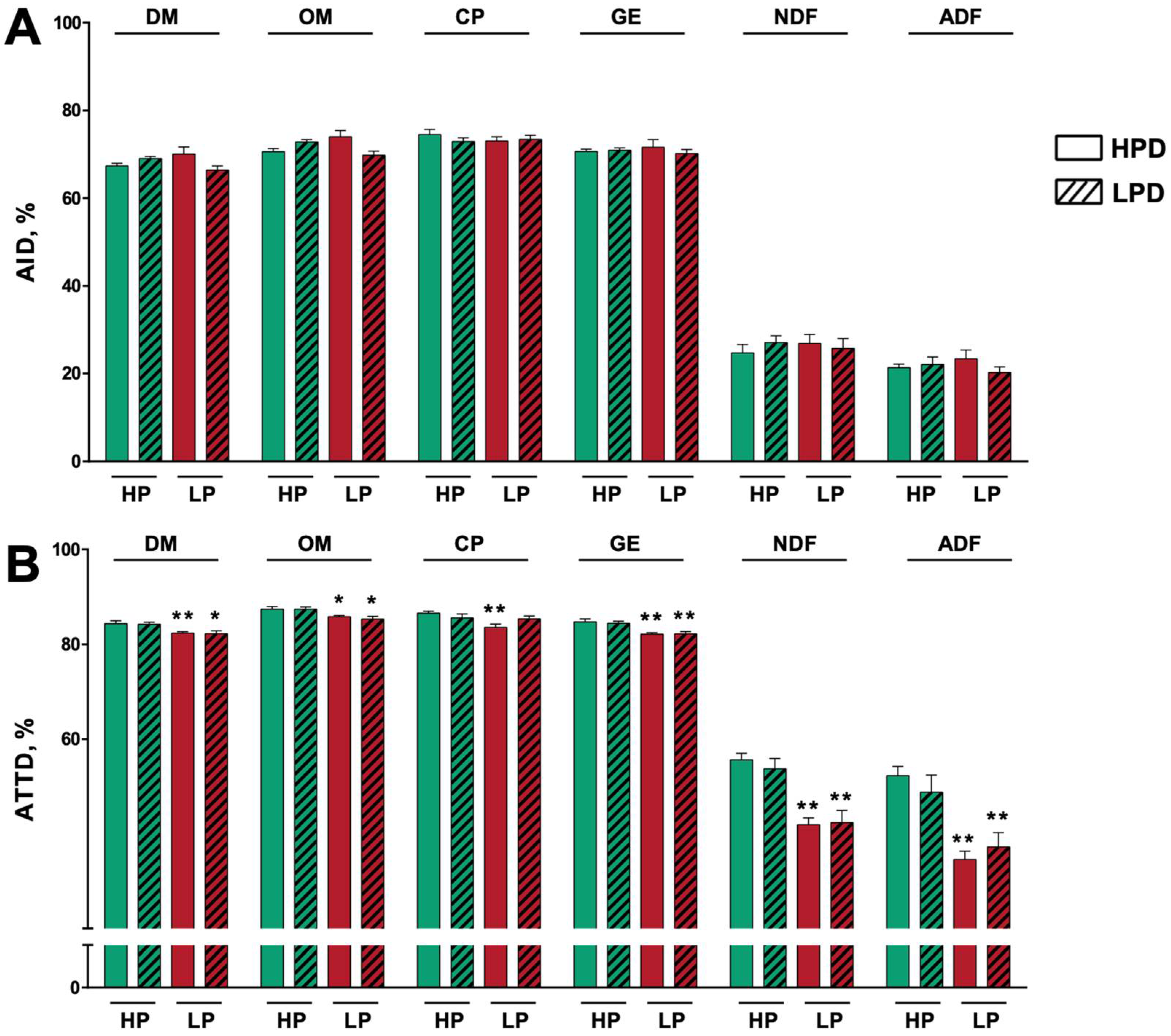
3.1. In Vivo Digestibility
3.2. In vitro Fermentation Digestibility
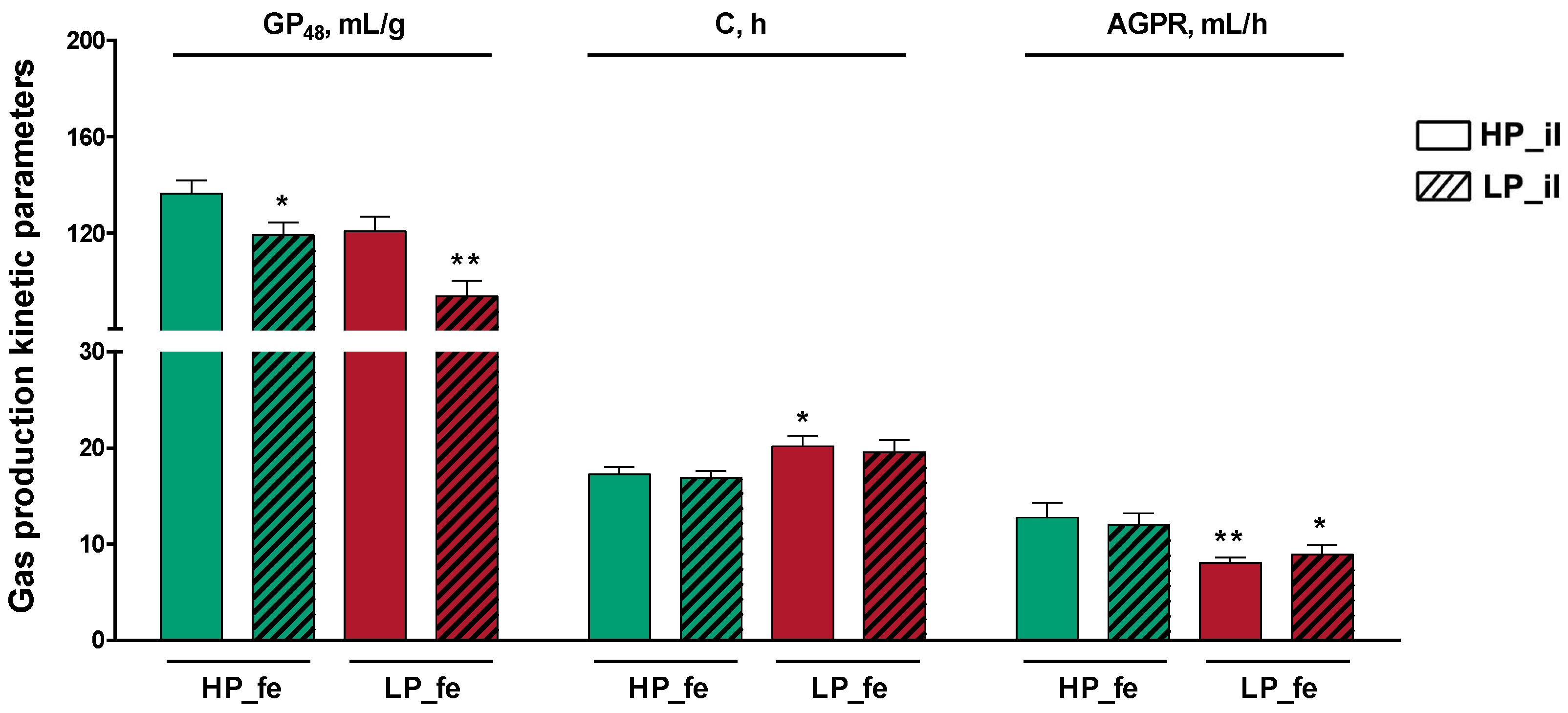
3.3. Gas Production
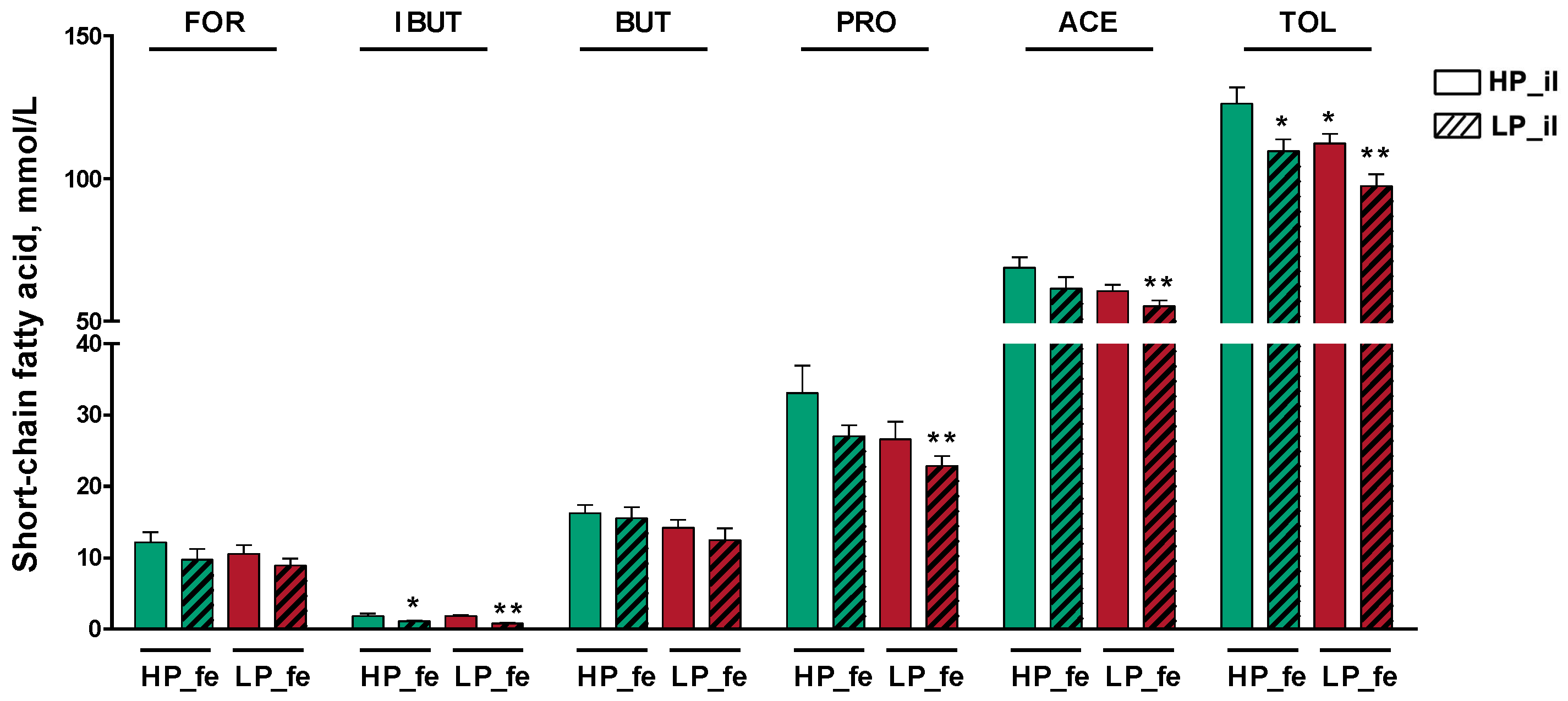
3.4. SCFA Concentrations in the Fermentation Supernatants
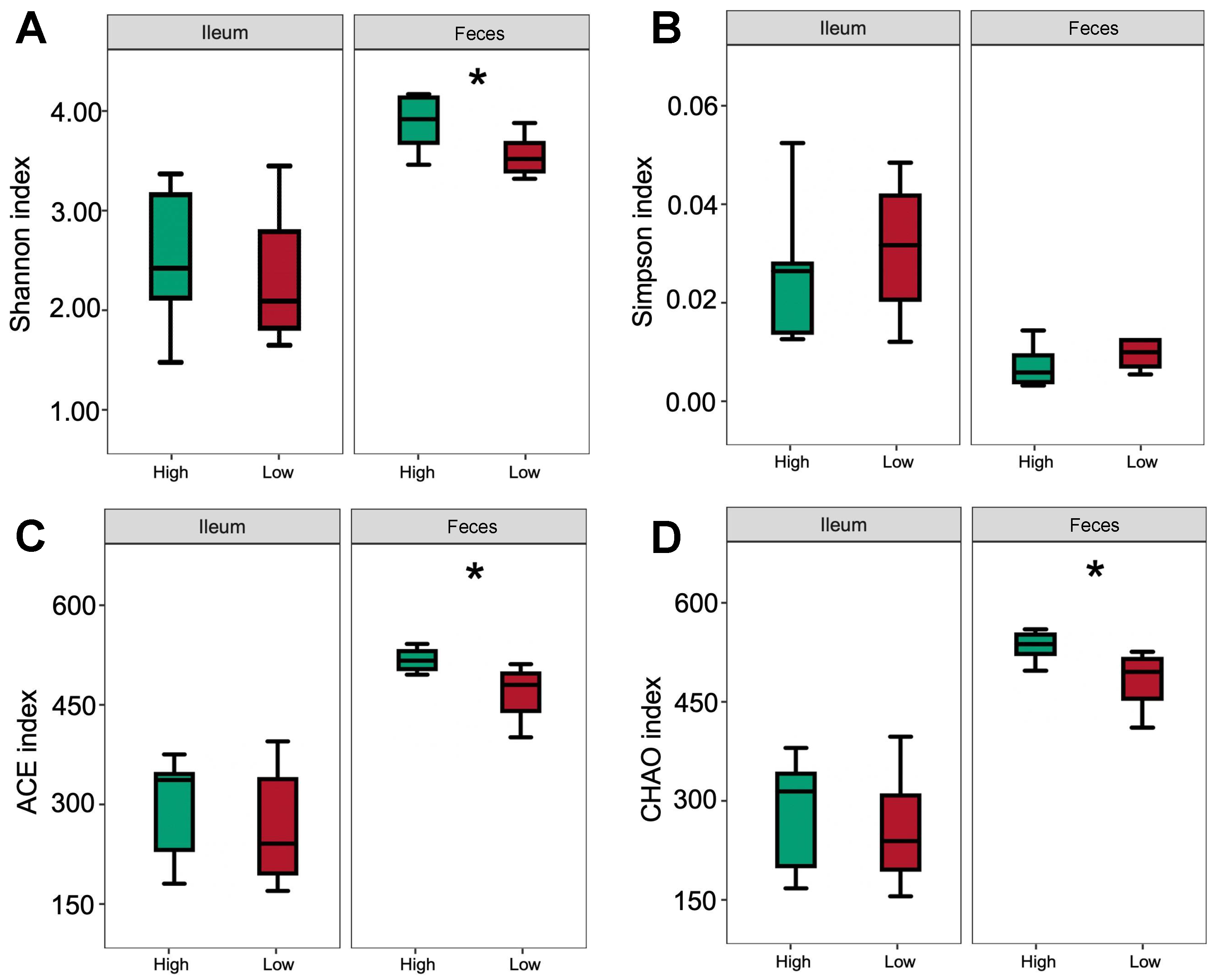
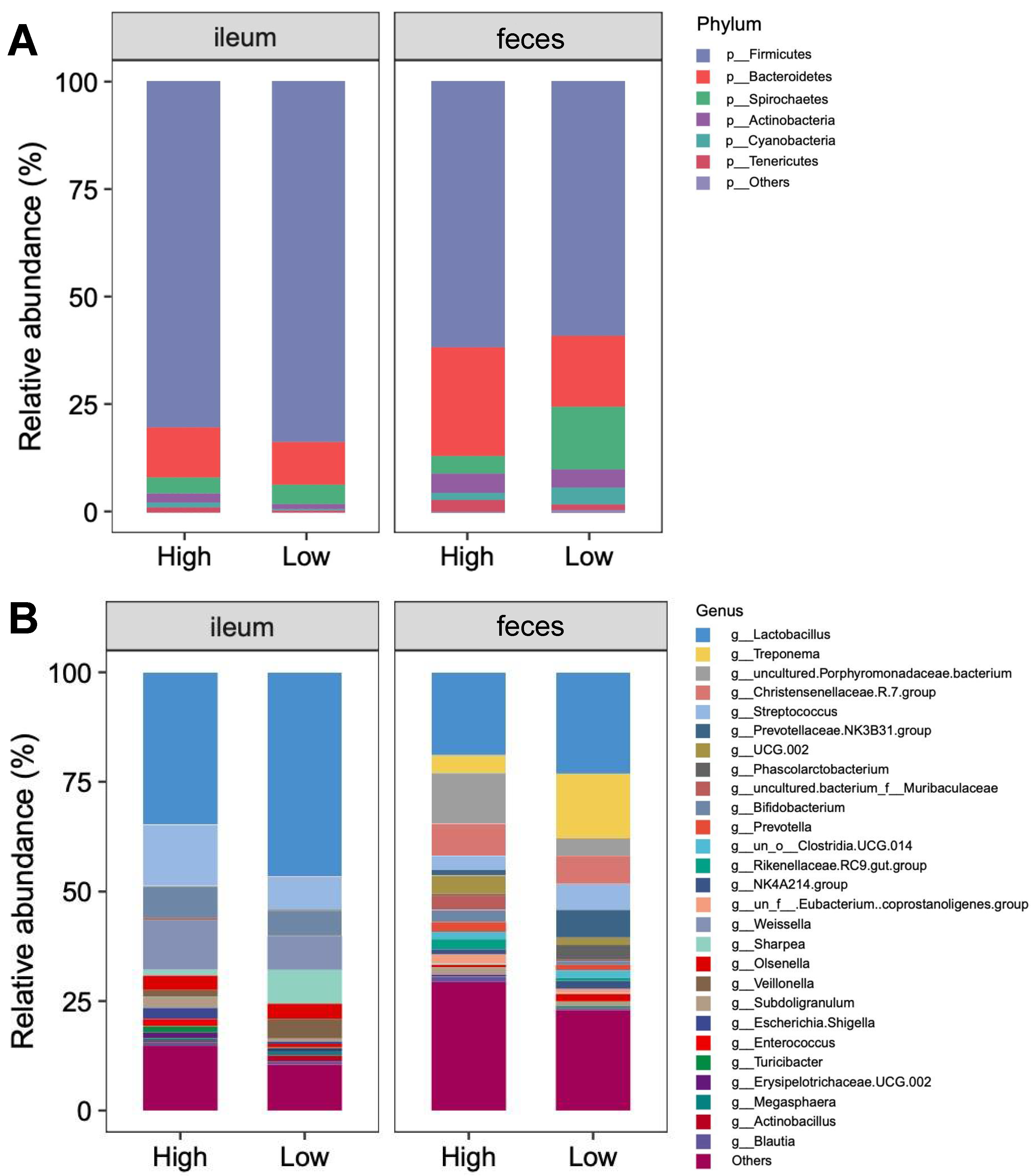
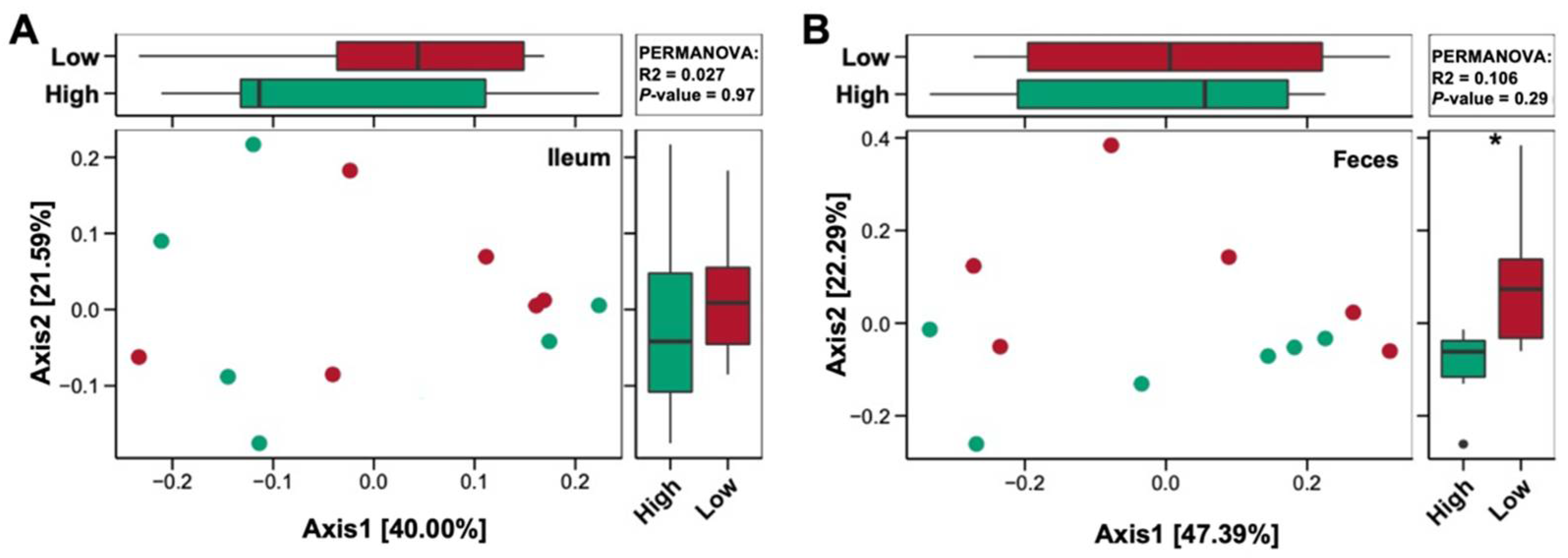
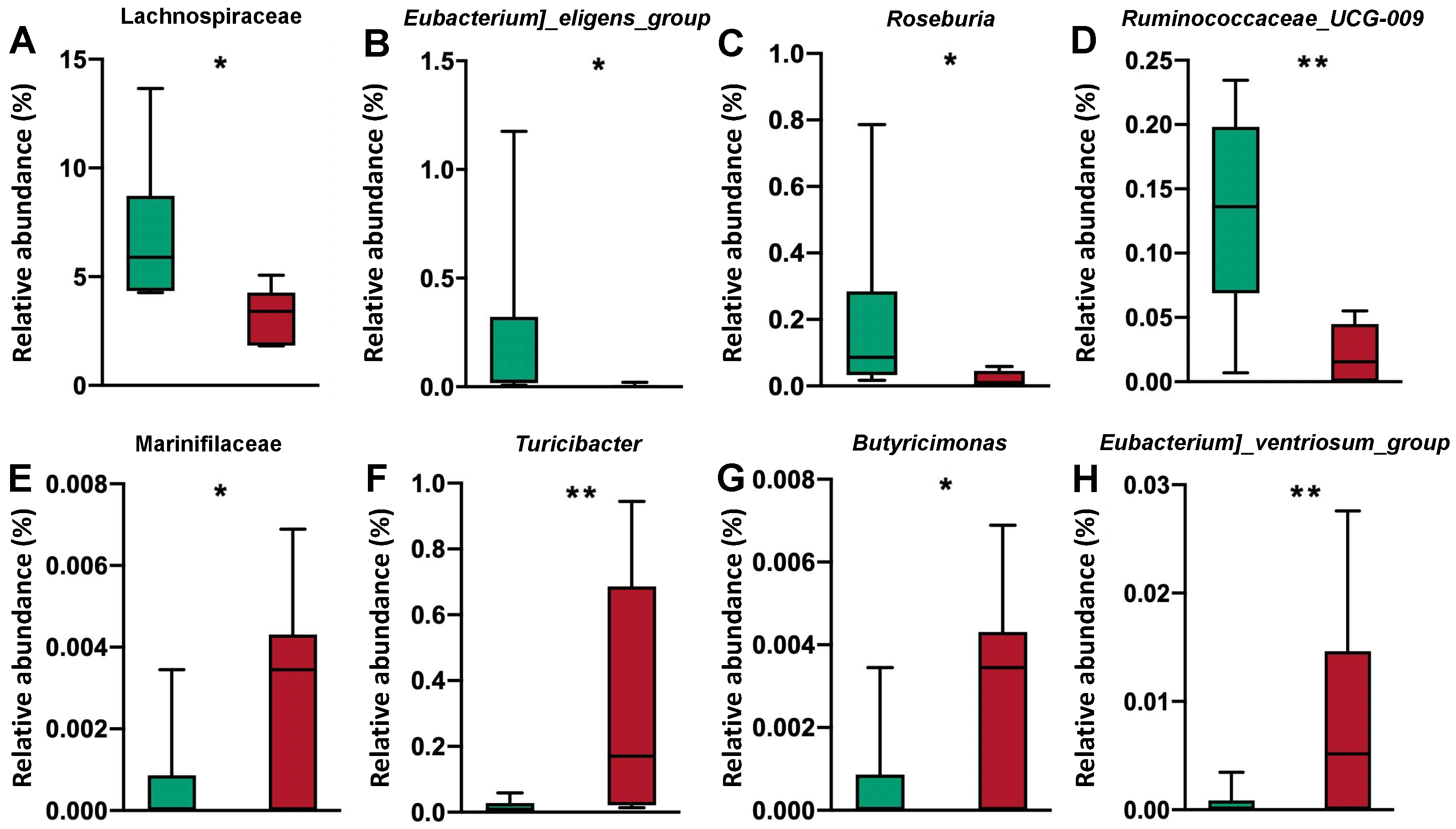
3.5. Bacterial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, H.; Hu, J.; Zuo, S.; Zhang, S.; Li, M.; Nie, S. In vitro gastrointestinal digestion and fermentation models and their applications in food carbohydrates. Crit. Rev. Food. Sci. 2021, 62, 5349–5371. [Google Scholar] [CrossRef] [PubMed]
- Schop, M. Modelling Digestion Kinetics in Pigs: Predicting Nutrient Absorption based on Diet and Ingredient Properties. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2020. [Google Scholar]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Gómez, R.S.; Diedrichsen, R.M. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein.; amino acid-supplemented diets. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Bidner, T.D.; Payne, R.L.; Southern, L.L. Growth performance of 20- to 50-kilogram pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J. Anim. Sci. 2011, 89, 3643–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem. 2018, 240, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet-microbe-host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Kovatcheva-Datchary, P.; Shoaie, S.; Lee, S.; Wahlström, A.; Nookaew, I.; Hallen, A.; Perkins, R.; Nielsen, J.; Bäckhed, F. Simplified intestinal microbiota to study microbe-diet-host interactions in a mouse model. Cell Rep. 2019, 26, 3772–3783. [Google Scholar] [CrossRef] [Green Version]
- Snelling, T.J.; Auffret, M.D.; Duthie, C.A.; Stewart, R.D.; Watson, M.; Dewhurst, R.J.; Roehe, R.; Walker, A.W. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microbiome 2019, 1, 16. [Google Scholar] [CrossRef]
- Cone, J.W.; Rodrigues, M.; Guedes, C.M.; Blok, M.C. Comparison of protein fermentation characteristics in rumen fluid determined with the gas production technique and the Nylon bag technique. Anim. Feed Sci. Technol. 2009, 153, 28–38. [Google Scholar] [CrossRef]
- Rink, F.; Bauer, E.; Eklund, M.; Hartung, K.; Mosenthin, R. Use of an in vitro gas production method to assess differences in microbial fermentation characteristics of feed ingredients and betaine additives for pig nutrition. Anim. Feed Sci. Technol. 2011, 169, 281–285. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 2005, 123, 445–462. [Google Scholar] [CrossRef]
- Bai, S.; Cao, Z.J.; Cao, B.B.; Yang, H.J.; Li, S.L.; Liu, J.X. Effects of different forage combinations in total mixed rations on in vitro gas production kinetics, ruminal and milk fatty acid profiles of lactating cows. Anim. Sci. J. 2018, 89, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Dunkley, K.D.; Dunkley, C.S.; Njongmeta, N.L.; Callaway, T.R.; Hume, M.E.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 2007, 86, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Zhou, J.Y.; Wang, G.; Cai, S.; Zeng, X.F.; Qiao, S.Y. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Han, S.J.; Zhou, J.Y.; Li, P.L.; Wang, G.; Yu, H.T.; Cai, S.; Zeng, X.; Johnston, L.J.; Levesque, C.L.; et al. Effects of dietary crude protein level and N-carbamylglutamate supplementation on nutrient digestibility and digestive enzyme activity of jejunum in growing pigs. J. Anim. Sci. 2020, 98, Skaa088. [Google Scholar] [CrossRef]
- Snook, J.T.; Meyer, J.H. Response of digestive enzymes to dietary protein. J. Nutr. 1964, 82, 409–414. [Google Scholar] [CrossRef]
- Corring, T. The adaptation of digestive enzymes to the diet: Its physiological significance. Reprod. Nutr. Dev. 1980, 20, 1217–1235. [Google Scholar] [CrossRef]
- Ndou, S.P.; Kiarie, E.; Nyachoti, C.M. Flaxseed meal and oat hulls supplementation: Impact on predicted production and absorption of volatile fatty acids and energy from hindgut fermentation in growing pigs. J. Anim. Sci. 2019, 97, 302–314. [Google Scholar] [CrossRef]
- Lyu, X.; Cui, K.; Qi, M.; Wang, S.; Diao, Q.; Zhang, N. Ruminal microbiota and fermentation in response to dietary protein and energy levels in weaned lambs. Animals 2020, 10, 109. [Google Scholar]
- Tadele, Y.; Amha, N. Use of different non protein nitrogen sources in ruminant nutrition: A review. Adv. Life Sci. Technol. 2015, 29, 100–105. [Google Scholar]
- Pathak, A.K. Various factors affecting microbial protein synthesis in the rumen. Vet. World 2008, 1, 186–189. [Google Scholar]
- Broderick, G.A. Effects of varying dietary protein and energy levels on the production of lactating dairy cows. J. Dairy Sci. 2003, 86, 1370–1381. [Google Scholar] [CrossRef]
- Rist, V.T.S.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zhao, J.; Tao, S.; Zhou, X.; Pi, Y.; Gerrits, W.J. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Sci. Food. Agri. 2020, 100, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Branch, W.J. Fermentation and the Production of Short-Chain Fatty Acids in the Human Large Intestine; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Bergman, E.N. Energy contribution of volatile fatty-acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, N.I. The contribution of the large-intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.L.; Abraham, A.G.; Rumbo, M. Is lactate an undervalued functional component of fermented food products? Front. Microbiol. 2015, 6, 629. [Google Scholar] [CrossRef]
- Krajmalnikü-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef] [PubMed]
- Gagen, E.J.; Padmanabha, J.; Denman, S.E.; McSweeney, C.S. Hydrogenotrophic culture enrichment reveals rumen Lachnospiraceae and Ruminococcaceae acetogens and hydrogen-responsive Bacteroidetes from pasture-fed cattle. FEMS Microbiol. Lett. 2015, 362, fnv104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettle, H.; Louis, P.; Holtrop, G.; Duncan, S.H.; Flint, H.J. Modelling the emergent dynamics and major metabolites of the human colonic microbiota. Environ. Microbiol. 2015, 17, 1615–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trend. Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans, metabolism, potential applications and underlying mechanisms. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Peters, S.G.; Pomare, E.W.; Fisher, C.A. Portal and peripheral blood short chain fatty acids concentrations after caecal lactulose instillation at surgery. Gut 1992, 33, 1249–1252. [Google Scholar] [CrossRef] [Green Version]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 900–910. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatziioanou, D.; Gherghisan-Filip, C.; Saalbach, G.; Horn, N.; Wegmann, U.; Duncan, S.H.; Flint, H.J.; Mayer, M.J.; Narbad, A. Discovery of a novel lantibiotic nisin O from Blautia obeum A2-162, isolated from the human gastrointestinal tract. Microbiology 2017, 163, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Elena, H.; Wayne, Y.; Douglas, R.; Ralf, C.; Riedel, C.U.; Markus, E. Determination of resistant starch assimilating bacteria in fecal samples of mice by in vitro rna-based stable isotope probing. Front. Microbiol. 2017, 8, 1331. [Google Scholar]
- Newman, M.A.; Petri, R.M.; Dietmar, G.; Qendrim, Z.; Zebeli, B.U. Transglycosylated starch modulates the gut microbiome and expression of genes related to lipid synthesis in liver and adipose tissue of pigs. Front. Microbiol. 2018, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Guan, L.L. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol. 2017, 83, e00061-117. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Ma, J.; Cao, G.; Hu, R.; Zhu, Y.; Li, G.; Zou, H.; Wang, Z.; Peng, Q.; Xue, B.; et al. Comparative study of growth performance, nutrient digestibility, and ruminal and fecal bacterial community between yaks and cattle-yaks raised by stall-feeding. AMB Express 2021, 11, 98. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kakiyama, G.; Savidge, T.; Takei, H.; Kassam, Z.A.; Fagan, A.; Gavis, E.A.; Pandak, W.M.; Nittono, H.; Hylemon, P.B.; et al. Antibioticü-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 2018, 68, 1549–1558. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.F.; Dai, T.Y.; Qi, X.; Wang, Y.; Zheng, T.Z.; Gao, X.Y.; Zhang, Y.J.; Ai, Y.; Ma, L.; et al. Short-chain fatty acids produced by Ruminococcaceae mediate α-linolenic acid promote intestinal stem cells proliferation. Mol. Nutr. Food Res. 2022, 66, 2100408. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zeng, X.; Zhang, T.; Li, P.; Yao, B.; Wang, L.; Qiao, S.; Zeng, X. Effect of antibiotic-free, low-protein diets with specific amino acid compositions on growth and intestinal flora in weaned pigs. Food Funct. 2020, 11, 493–507. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, Q.; Ji, H.; Ning, J.; Li, C.; Zheng, H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim. Et Biophys. Acta Mol. Basis Dis. 2019, 1865, 165541. [Google Scholar] [CrossRef]
- Jiang, Q.; He, X.; Zou, Y.; Ding, Y.; Li, H.; Chen, H. Altered gut microbiome promotes proteinuria in mice induced by Adriamycin. AMB Express 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeineldin, M.; Aldridge, B.; Lowe, J. Dysbiosis of the fecal microbiota in feedlot cattle with hemorrhagic diarrhea. Microb. Pathog. 2018, 115, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Cai, R.; Wang, Q.; Shi, L.; Li, C.; Yan, H. Dynamic change of gut microbiota during porcine epidemic diarrhea virus infection in suckling piglets. Front. Microbiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Item | HP | LP |
|---|---|---|
| Ingredient, % | ||
| Corn | 58.60 | 71.70 |
| Soybean meal | 36.50 | 15.40 |
| Wheat bran | 1.50 | 8.90 |
| Soybean oil | 0.80 | - |
| Limestone | 0.86 | 0.92 |
| Dicalcium phosphate | 0.90 | 1.05 |
| Salt | 0.30 | 0.30 |
| L-Lysine·HCl | 0.03 | 0.59 |
| L-Threonine | 0.01 | 0.24 |
| DL-Methionine | - | 0.17 |
| L-Tryptophan | - | 0.09 |
| L-Valine | - | 0.14 |
| Premix 1 | 0.50 | 0.50 |
| Analyzed nutrient level | ||
| Dry matter, % | 90.20 | 88.97 |
| Gross energy, MJ/kg | 16.85 | 16.27 |
| Crude protein, % | 20.54 | 15.29 |
| Ether extract, % | 2.99 | 2.69 |
| Ash, % | 5.11 | 4.53 |
| Acid detergent fiber, % | 3.89 | 3.96 |
| Neutral detergent fiber, % | 11.35 | 12.03 |
| Calculated nutrient level | ||
| Net energy, MJ/kg | 10.04 | 10.04 |
| SID Lysine, % | 1.00 | 1.00 |
| SID Methionine + cysteine, % | 0.59 | 0.59 |
| SID Threonine, % | 0.63 | 0.63 |
| SID Tryptophan, % | 0.21 | 0.21 |
| SID Valine, % | 0.79 | 0.66 |
| Diet | DM, % | OM, % | CP, % | GE, kcal/kg | NDF, % | ADF, % |
|---|---|---|---|---|---|---|
| HP | 91.5 | 86.0 | 16.5 | 3651 | 26.5 | 9.5 |
| LP | 91.8 | 86.6 | 14.1 | 3772 | 30.5 | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, Y.; Wang, L.; Tu, J.; Yang, L.; Yang, G.; Zeng, X.; Qiao, S. Compromised Hindgut Microbial Digestion, Rather Than Chemical Digestion in the Foregut, Leads to Decreased Nutrient Digestibility in Pigs Fed Low-Protein Diets. Nutrients 2022, 14, 2793. https://doi.org/10.3390/nu14142793
Zhou J, Wang Y, Wang L, Tu J, Yang L, Yang G, Zeng X, Qiao S. Compromised Hindgut Microbial Digestion, Rather Than Chemical Digestion in the Foregut, Leads to Decreased Nutrient Digestibility in Pigs Fed Low-Protein Diets. Nutrients. 2022; 14(14):2793. https://doi.org/10.3390/nu14142793
Chicago/Turabian StyleZhou, Junyan, Yuming Wang, Lu Wang, Jiayu Tu, Lijie Yang, Guangxin Yang, Xiangfang Zeng, and Shiyan Qiao. 2022. "Compromised Hindgut Microbial Digestion, Rather Than Chemical Digestion in the Foregut, Leads to Decreased Nutrient Digestibility in Pigs Fed Low-Protein Diets" Nutrients 14, no. 14: 2793. https://doi.org/10.3390/nu14142793
APA StyleZhou, J., Wang, Y., Wang, L., Tu, J., Yang, L., Yang, G., Zeng, X., & Qiao, S. (2022). Compromised Hindgut Microbial Digestion, Rather Than Chemical Digestion in the Foregut, Leads to Decreased Nutrient Digestibility in Pigs Fed Low-Protein Diets. Nutrients, 14(14), 2793. https://doi.org/10.3390/nu14142793






