Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals, Diets and Experimental Design
2.3. Biochemical Analysis in Serum
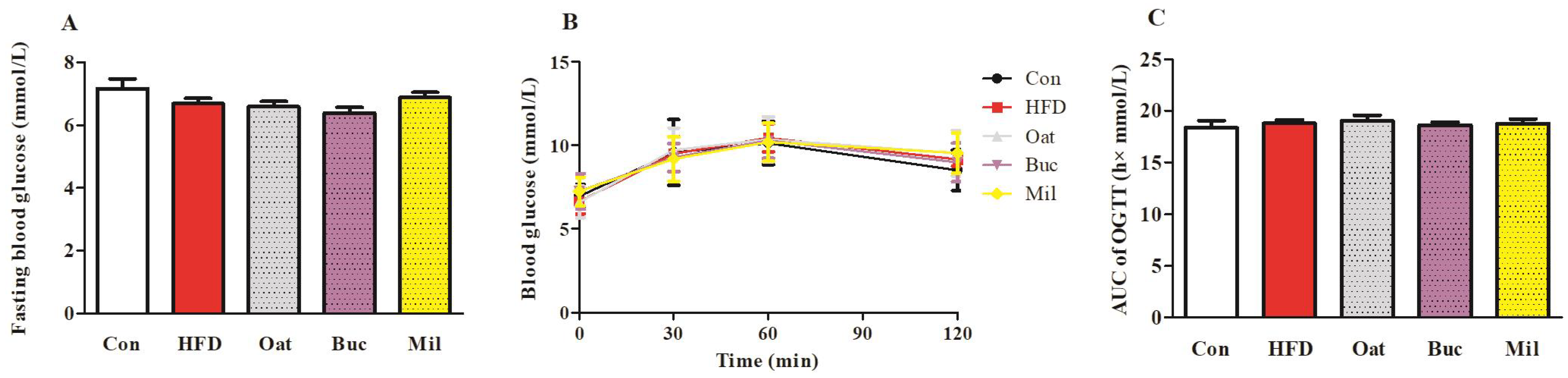
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Histological Analysis
2.6. Western Blot Analysis
2.7. Analysis of Cytokines and Antioxidant Status in Serum
2.8. Determination of Colonic SCFAs
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results
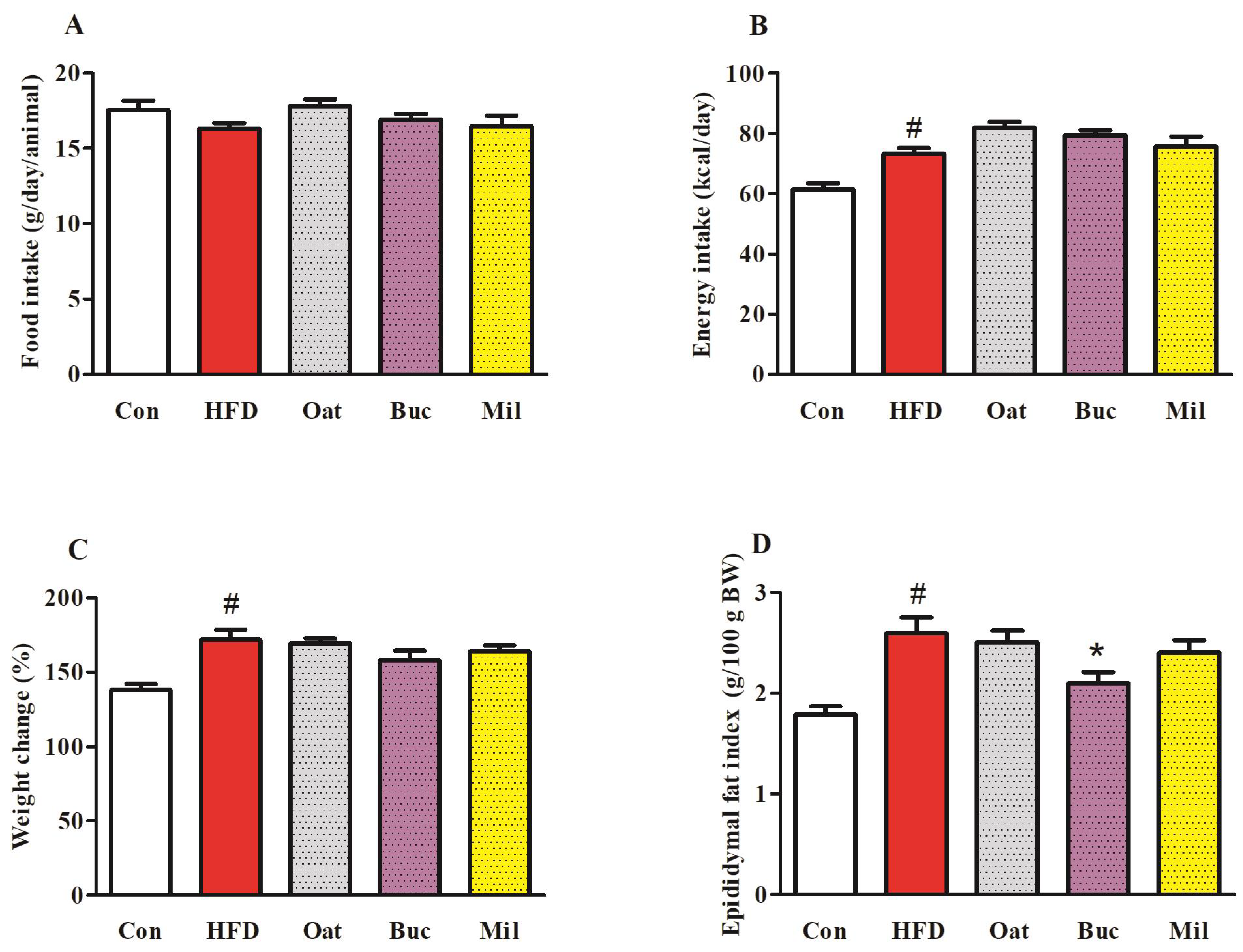
3.1. Food Intake, Energy Intake, Body Weight, and Organ Weights
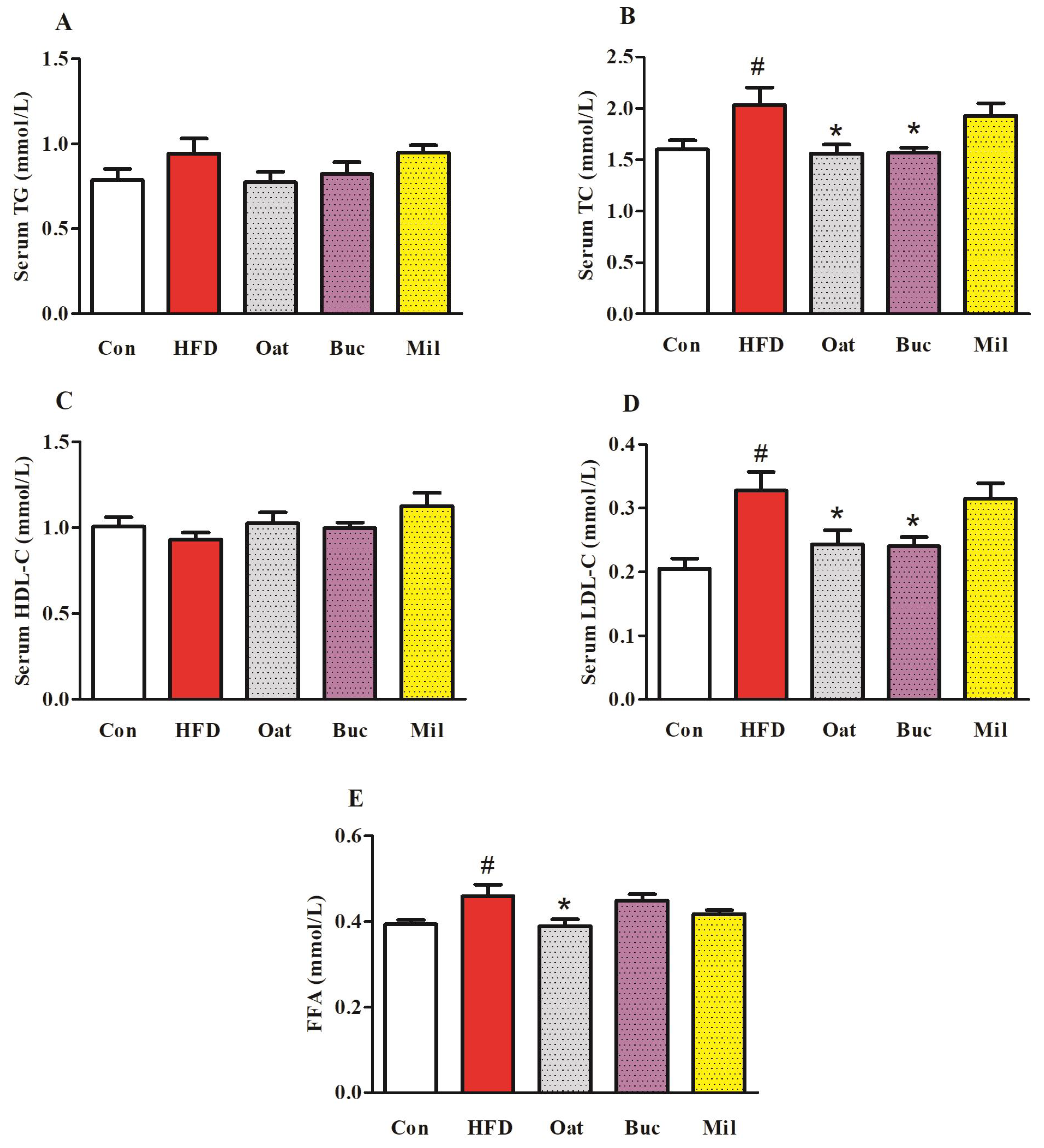
3.2. Lipid Metabolism-Related Parameters in Serum, Fasting Blood Glucose, and OGTT
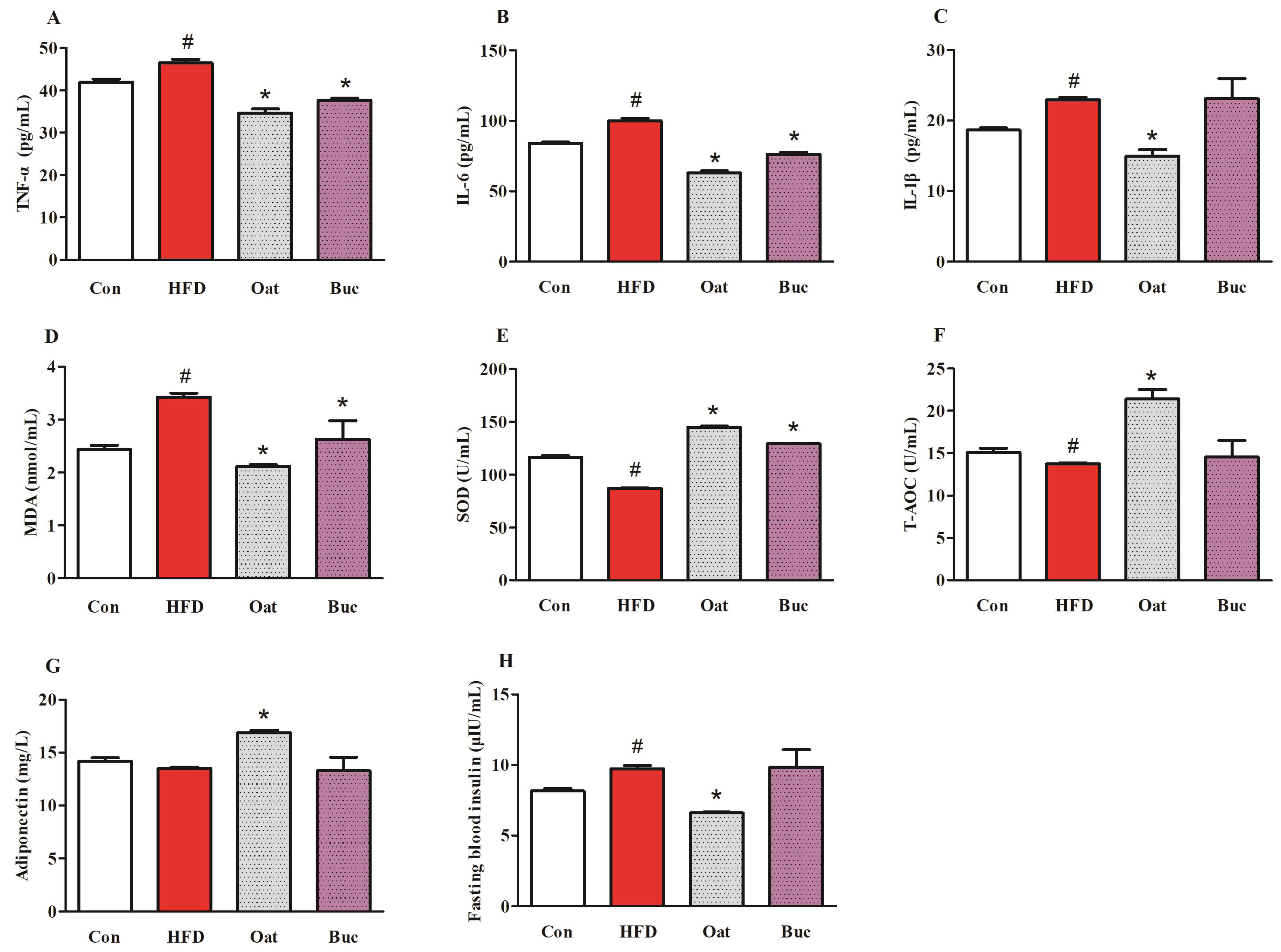
3.3. Pro-Inflammatory Cytokine Levels, Antioxidant Capability, Adiponectin, and Insulin in Serum
3.4. Histological Analysis of the Liver, Pancreas, Epididymal Adipose, and Colon Tissues
3.5. Expression of Proteins Involved in Lipid Metabolism
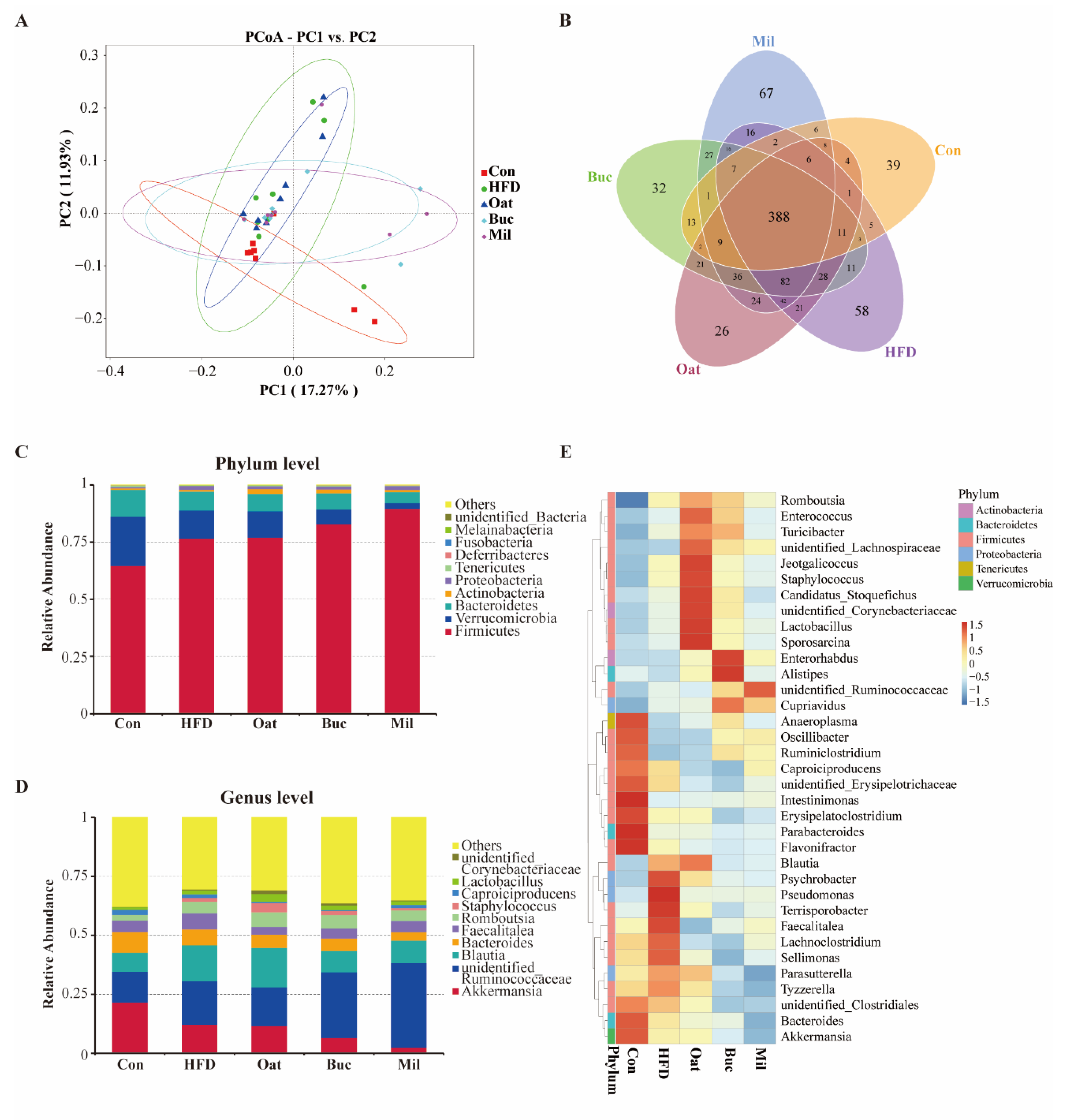
3.6. Coarse Cereals Supplementation Modulated the Gut Microbiota
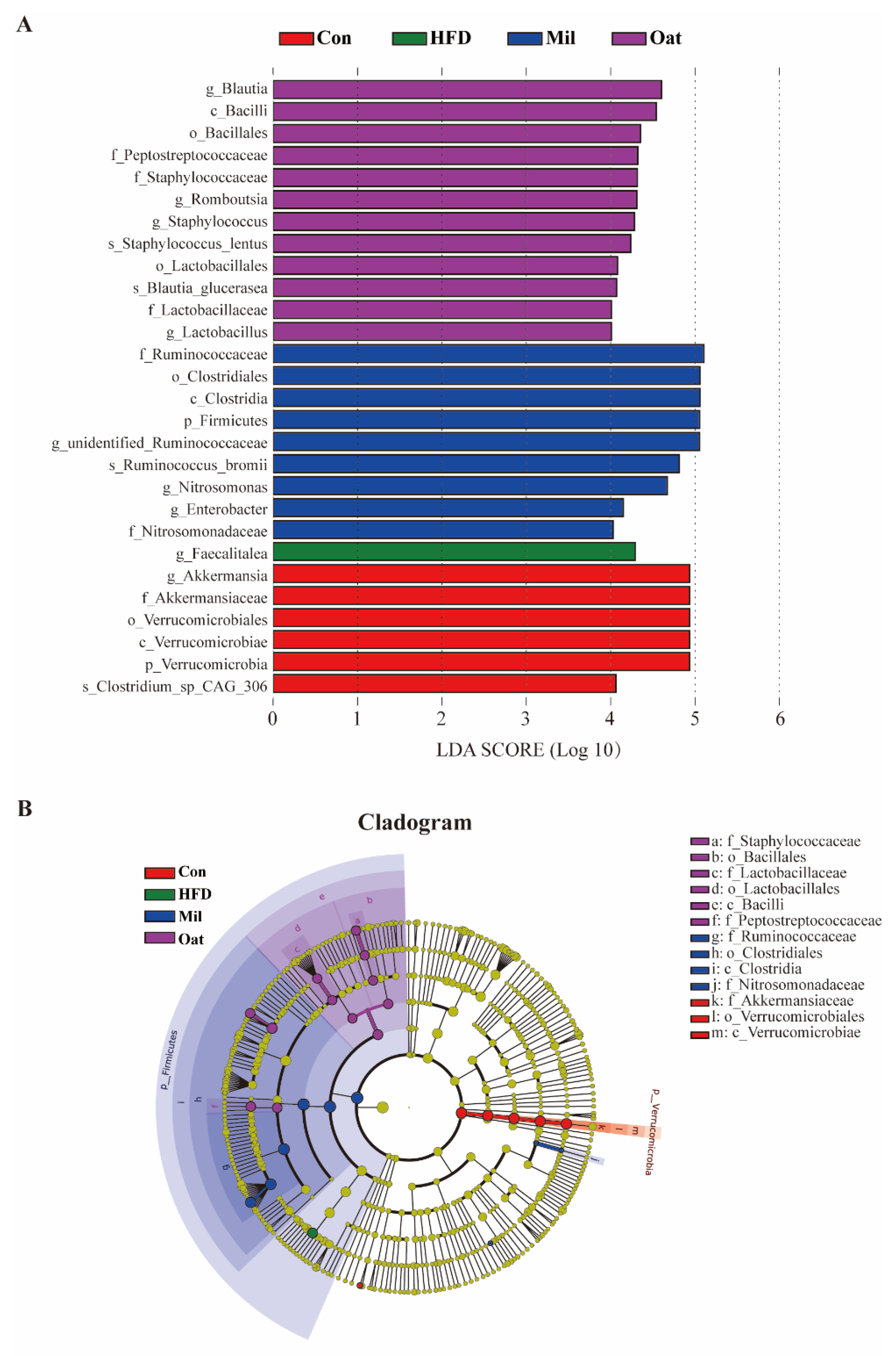
3.7. Coarse Cereals Supplementation Regulated the Specific Bacteria
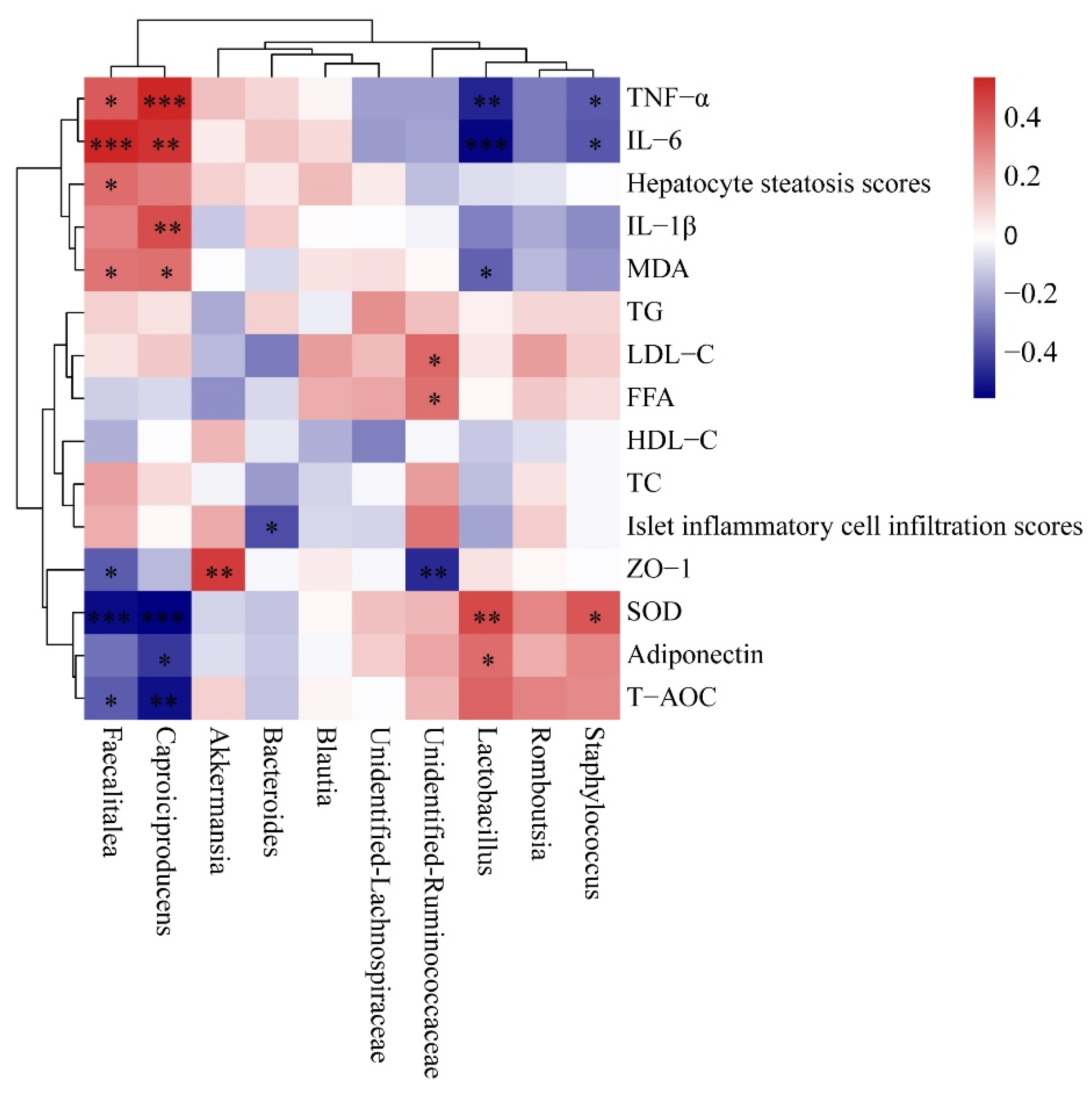
3.8. Correlation between Gut Microbiota and Pro-Inflammatory Cytokine, Antioxidant Capability and Lipid Metabolism-Related Indices
3.9. Coarse Cereals Supplementation Regulated the SCFAs Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhao, L.; Gao, L.; Pan, A.; Xue, H. Obesity in China 3 Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 446–461. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, Y.; Zhang, X.; Weng, P.; Liu, L.; Zhang, R.; Wu, Z. The gut microbiota-brain axis: Role of the gut microbial metabolites of dietary food in obesity. Food Res. Int. 2022, 153, 110971. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Modulation of gut health using probiotics: The role of probiotic effector molecules. J. Future Foods 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; Asúnsolo, Á.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, X.; Huang, J. Diet Effects in Gut Microbiome and Obesity. J. Food Sci. 2014, 79, R442–R451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes, Cardiovascular Disease, and Weight Gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef]
- Wu, W.; Qiu, J.; Wang, A.; Li, Z. Impact of whole cereals and processing on type 2 diabetes mellitus: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1447–1474. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Wang, Y.; Song, G.; Sun, H.; Pang, S.; Li, A. Effects of four coarse cereals on blood glucose levels in rats with STZ-induced hyperglycemia. Food Agric. Immunol. 2019, 30, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Wang, L.; Chen, Z.; Hou, D.; Xue, Y.; Diao, X.; Shen, Q. Foxtail Millet Improves Blood Glucose Metabolism in Diabetic Rats through PI3K/AKT and NF-kappa B Signaling Pathways Mediated by Gut Microbiota. Nutrients 2021, 13, 1837. [Google Scholar] [CrossRef]
- Huang, Z.R.; Deng, J.C.; Li, Q.Y.; Cao, Y.J.; Lin, Y.C.; Bai, W.D.; Liu, B.; Rao, P.F.; Ni, L.; Lv, X.C. Protective Mechanism of Common Buckwheat (Fagopyrum esculentum Moench.) against Nonalcoholic Fatty Liver Disease Associated with Dyslipidemia in Mice Fed a High-Fat and High-Cholesterol Diet. J. Agric. Food Chem. 2020, 68, 6530–6543. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.; Chen, Z.; Zhang, M.; Hou, D.; Xue, Y.; Diao, X.; Liu, R.; Shen, Q. Foxtail millet supplementation improves glucose metabolism and gut microbiota in rats with high-fat diet/streptozotocin-induced diabetes. Food Sci. Hum. Wellness 2021, 11, 119–128. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Han, Y.; Zhao, J.; Han, F.; Song, G.; Jiang, P.; Miao, H. Effects of Whole-Grain Rice and Wheat on Composition of Gut Microbiota and Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2018, 66, 6326–6335. [Google Scholar] [CrossRef]
- Ren, G.; Fan, X.; Teng, C.; Li, Y.; Everaert, N.; Blecker, C. The Beneficial Effect of Coarse Cereals on Chronic Diseases through Regulating Gut Microbiota. Foods 2021, 10, 2891. [Google Scholar] [CrossRef]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Hu, Y.; Zhao, G.; Tang, Y.; Zou, L. Concise review: Coarse cereals exert multiple beneficial effects on human health. Food Chem. 2020, 325, 126761. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Sajid, A.; Li, Z. Whole grain cereals: The potential roles of functional components in human health. Crit. Rev. Food Sci. Nutr. 2021, 1928596. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Li, Y.; Strappe, P.; Blanchard, C. Characterization of fecal fat composition and gut derived fecal microbiota in high-fat diet fed rats following intervention with chito-oligosaccharide and resistant starch complexes. Food Funct. 2017, 8, 4374–4383. [Google Scholar] [CrossRef]
- Wang, Y.; Song, G.; Pang, S.; Qi, W. Effects of three kinds of coarse cereals on gut microbiota of rats explored by Illumina NovaSeq sequencing technology. Shipin Kexue/Food Sci. 2021, 42, 100–106. [Google Scholar]
- Zhou, X.-L.; Yan, B.-B.; Xiao, Y.; Zhou, Y.-M.; Liu, T.-Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef]
- Han, F.; Han, F.; Wang, Y.; Fan, L.; Song, G.; Chen, X.; Jiang, P.; Miao, H.; Han, Y. Digestible indispensable amino acid scores of nine cooked cereal grains. Br. J. Nutr. 2019, 121, 30–41. [Google Scholar] [CrossRef]
- Chen, H.H.; Nie, Q.X.; Hu, J.L.; Huang, X.J.; Zhang, K.; Nie, S.P. Glucomannans Alleviated the Progression of Diabetic Kidney Disease by Improving Kidney Metabolic Disturbance. Mol. Nutr. Food Res. 2019, 63, 1801008. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q.; et al. Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in vivo. Nutrients 2016, 8, 245. [Google Scholar] [CrossRef]
- Wang, Y.; An, Y.; Ma, W.; Yu, H.; Lu, Y.; Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Xiao, R. 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflamm. 2020, 17, 199. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Huang, Z.R.; Chen, M.; Guo, W.L.; Li, T.T.; Liu, B.; Bai, W.D.; Ai, L.Z.; Rao, P.F.; Ni, L.; Lv, X.C. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020, 136, 109511. [Google Scholar] [CrossRef]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Huang, K.; Yu, W.; Li, S.; Guan, X.; Liu, J.; Song, H.; Liu, D.; Duan, R. Effect of embryo-remaining oat rice on the lipid profile and intestinal microbiota in high-fat diet fed rats. Food Res. Int. 2020, 129, 108816. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Wang, X.; Ding, C.; Liu, J.; Li, Y.; Li, W.; Sun, Y. High-Salt Diet-Induced Gastritis in C57BL/6 Mice is Associated with Microbial Dysbiosis and Alleviated by a Buckwheat Diet. Mol. Nutr. Food Res. 2020, 64, e1900965. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, S.; Xia, Y.; Huang, J.; Ye, J.; Xuan, Z.; Li, P.; Du, B. Probiotic-fermented black tartary buckwheat alleviates hyperlipidemia and gut microbiota dysbiosis in rats fed with a high-fat diet. Food Funct. 2021, 12, 6045–6057. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Liu, S.; Lv, H.; Hu, Y.; Wang, Y.; Li, Z.; Wang, J.; Ji, X.; Ma, H.; et al. Dietary Supplementation of Foxtail Millet Ameliorates Colitis-Associated Colorectal Cancer in Mice via Activation of Gut Receptors and Suppression of the STAT3 Pathway. Nutrients 2020, 12, 2367. [Google Scholar] [CrossRef]
- Gong, L.; Wang, T.; Sun, C.; Wang, J.; Sun, B. Whole barley prevents obesity and dyslipidemia without the involvement of the gut microbiota in germ free C57BL/6J obese mice. Food Funct. 2019, 10, 7498–7508. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, N.; Zhang, J.; Wang, H.; Tao, T.; Pei, F.; Hu, Q. Dietary intake of mixture coarse cereals prevents obesity by altering the gut microbiota in high-fat diet fed mice. Food Chem. Toxicol. 2020, 147, 111901. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: By changing gut barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef]
- Gao, H.; Song, R.; Li, Y.; Zhang, W.; Wan, Z.; Wang, Y.; Zhang, H.; Han, S. Effects of Oat Fiber Intervention on Cognitive Behavior in LDLR–/–Mice Modeling Atherosclerosis by Targeting the Microbiome-Gut-Brain Axis. J. Agric. Food Chem. 2020, 68, 14480–14491. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Petersen, I.L.; Llopis, M.L. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef] [PubMed]
- Lie, L.; Brown, L.; Forrester, T.E.; Plange-Rhule, J.; Bovet, P.; Lambert, E.V.; Layden, B.T.; Luke, A.; Dugas, L.R. The Association of Dietary Fiber Intake with Cardiometabolic Risk in Four Countries across the Epidemiologic Transition. Nutrients 2018, 10, 628. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A Randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhao, J.; Waleed, A.A.; Wang, J.; Xue, L.; Liu, J.; Wang, Y.; Fan, M.; Qian, H.; Li, Y.; et al. Oat beta-glucan alleviates DSS-induced colitis via regulating gut microbiota metabolism in mice. Food Funct. 2021, 12, 8976–8993. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Lam, K.L.; Li, X.; Kong, A.P.S.; Cheung, P.C.K. Circadian disruption-induced metabolic syndrome in mice is ameliorated by oat beta-glucan mediated by gut microbiota. Carbohydr. Polym. 2021, 267, 118216. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory Function of Buckwheat-Resistant Starch Supplementation on Lipid Profile and Gut Microbiota in Mice Fed with a High-Fat Diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G.; Alsharif, S.; Kuhnle, G.G.C.; Walton, G.; Spencer, J.P.E. Oat bran, but not its isolated bioactive beta-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [Green Version]
- Ashley, D.; Marasini, D.; Brownmiller, C.; Lee, J.A.; Carbonero, F.; Lee, S.O. Impact of Grain Sorghum Polyphenols on Microbiota of Normal Weight and Overweight/Obese Subjects during In Vitro Fecal Fermentation. Nutrients 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.A.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Zhang, C.; Shi, J.; Li, H.; Li, Z. Inhibitory Effects of Bound Polyphenol from Foxtail Millet Bran on Colitis-Associated Carcinogenesis by the Restoration of Gut Microbiota in a Mice Model. J. Agric. Food Chem. 2020, 68, 3506–3517. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shan, S.; Li, H.; Shi, J.; Hao, R.; Yang, R.; Li, Z. Millet shell polyphenols prevent atherosclerosis by protecting the gut barrier and remodeling the gut microbiota in ApoE−/− mice. Food Funct. 2021, 12, 7298–7309. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [Green Version]

| Con | HFD | HFD + Oat | HFD + Buc | HFD + Mil | |
|---|---|---|---|---|---|
| Oats | 0 | 0 | 220 | 0 | 0 |
| Tartary buckwheat | 0 | 0 | 0 | 220 | 0 |
| Foxtail millet | 0 | 0 | 0 | 0 | 220 |
| Casein | 190 | 242 | 206 | 202 | 213 |
| Corn starch | 504 | 193 | 25 | 38 | 11 |
| Dextrin | 81 | 103 | 103 | 103 | 103 |
| Sucrose | 95 | 121 | 121 | 121 | 121 |
| Soybean oil | 24 | 30 | 30 | 30 | 30 |
| Lard | 16 | 195 | 183 | 187 | 186 |
| Cellulose | 48 | 61 | 51 | 41 | 56 |
| Mineral mixture | 27 | 36 | 35 | 34 | 35 |
| Vitamin mixture | 10 | 12 | 12 | 12 | 12 |
| L-Cystine | 3 | 4 | 4 | 4 | 4 |
| Choline chloride | 2 | 3 | 3 | 3 | 3 |
| TBHQ | 0.008 | 0.045 | 0.045 | 0.045 | 0.045 |
| Total | 1000 | 1000 | 993 | 995 | 994 |
| Content of coarse cereals/% | 0 | 0 | 22.2 | 22.1 | 22.1 |
| Energy density (Kcal/g) | 3.5 | 4.5 | 4.6 | 4.7 | 4.6 |
| Protein (% energy) | 19 | 19 | 19 | 19 | 19 |
| Carbohydrate (% energy) | 71 | 36 | 36 | 36 | 36 |
| Fat (% energy) | 10 | 45 | 45 | 45 | 45 |
| Con | HFD | Oat | Buc | Mil | |
|---|---|---|---|---|---|
| Acetic acid (μg/g) | 44.22 ± 7.11 | 69.71 ± 18.29 | 76.17 ± 7.685 | 74.89 ± 13.54 | 66.82 ± 10.55 |
| Propionic acid (μg/g) | 22.41 ± 4.69 | 34.56± 8.99 | 41.28 ± 4.72 | 34.50 ± 6.02 | 33.42 ± 4.90 |
| Butyric acid (μg/g) | 16.77 ± 3.01 a | 15.69 ± 1.98 a | 33.92 ± 6.97 b | 27.71 ± 3.49 a,b | 15.96 ± 1.75 a |
| Isobutyric acid (μg/g) | 5.20 ± 0.77 | 7.30 ± 1.25 | 8.56 ± 0.76 | 7.31 ± 0.84 | 6.90 ± 0.89 |
| Isovaleric acid (μg/g) | 4.19± 0.59 | 4.97 ± 0.60 | 5.85 ± 1.05 | 4.60 ± 0.63 | 4.43 ± 0.37 |
| Valeric acid (μg/g) | 5.08 ± 0.86 | 7.52 ± 1.43 | 9.31 ± 1.37 | 7.12 ± 1.03 | 6.74 ± 0.66 |
| Total SCFAs (μg/g) | 104.25 (71.95, 120.45) | 126.82 (72.34, 223.53) | 163.67 (141.09, 206.58) | 159.76 (95.48, 212.05) | 127.03 (99.15, 163.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. https://doi.org/10.3390/nu14132760
Wang Y, Qi W, Guo X, Song G, Pang S, Fang W, Peng Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients. 2022; 14(13):2760. https://doi.org/10.3390/nu14132760
Chicago/Turabian StyleWang, Yong, Wentao Qi, Xiaoxuan Guo, Ge Song, Shaojie Pang, Wei Fang, and Zhenzhen Peng. 2022. "Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats" Nutrients 14, no. 13: 2760. https://doi.org/10.3390/nu14132760
APA StyleWang, Y., Qi, W., Guo, X., Song, G., Pang, S., Fang, W., & Peng, Z. (2022). Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients, 14(13), 2760. https://doi.org/10.3390/nu14132760






