Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Tissue Collection and DNA Extraction
2.4. 16S rRNA Sequencing
2.5. Bioinformatics and Taxonomic Assignment
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of Study Participants
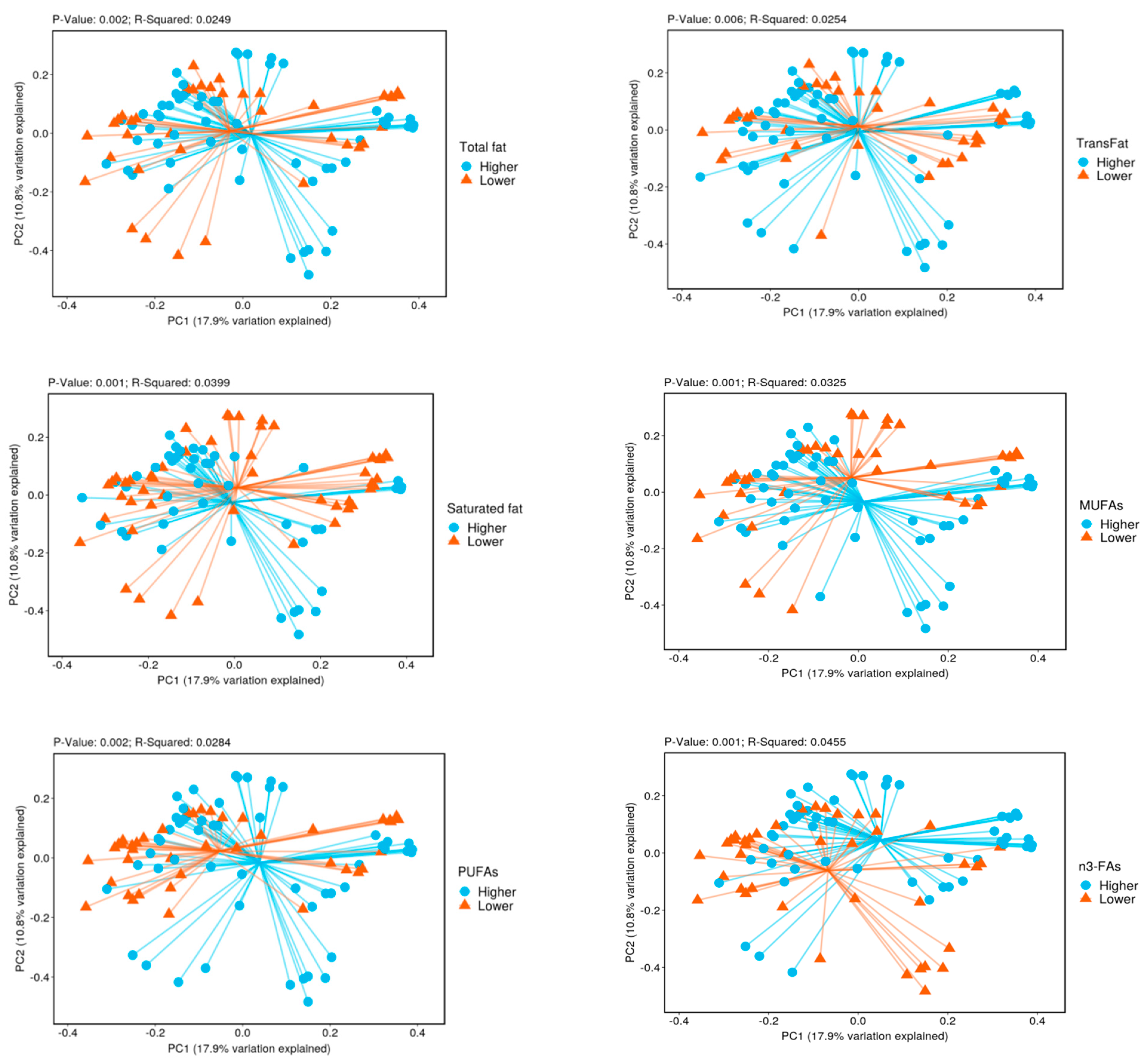
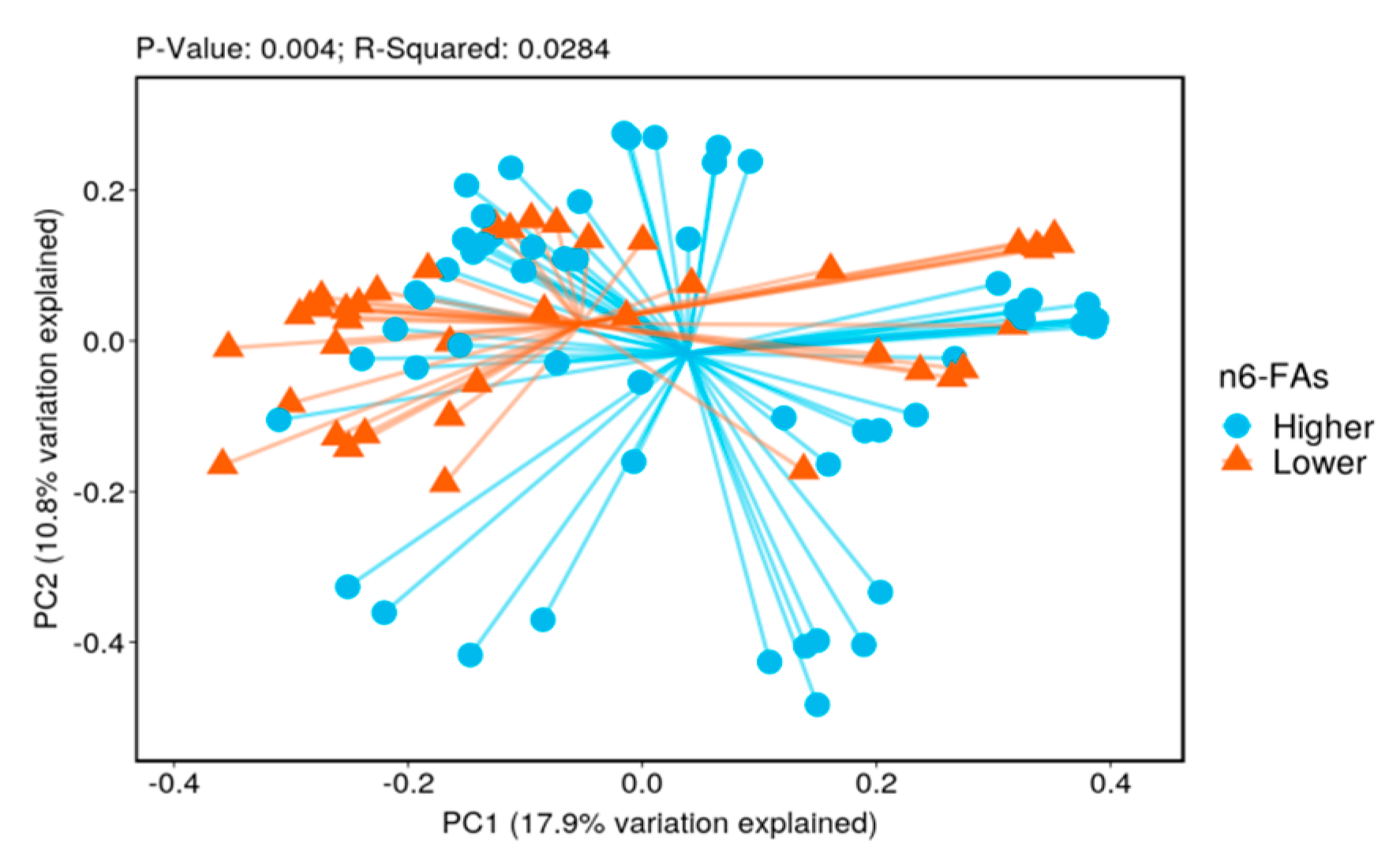
3.2. Biodiversity
3.3. Taxonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heron, M.; Anderson, R.N. Changes in the Leading Cause of Death: Recent Patterns in Heart Disease and Cancer Mortality. NCHS Data Brief 2016, 254, 1–8. [Google Scholar]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.D.; Li, Y.; Chiuve, S.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Zhuang, P.; Zhang, Y.; He, W.; Chen, X.; Chen, J.; He, L.; Mao, L.; Wu, F.; Jiao, J. Dietary Fats in Relation to Total and Cause-Specific Mortality in a Prospective Cohort of 521,120 Individuals with 16 Years of Follow-Up. Circ. Res. 2019, 124, 757–768. [Google Scholar] [CrossRef]
- Van Horn, L.; Carson, J.A.S.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef]
- Wang, D.D.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats With Mortality-Reply. JAMA Intern. Med. 2016, 176, 1879–1880. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Micha, R.; Mozaffarian, D. Dietary fats and cardiometabolic disease: Mechanisms and effects on risk factors and outcomes. Nat. Rev. Cardiol. 2019, 16, 581–601. [Google Scholar] [CrossRef]
- Cao, X.-J.; Zhang, M.-J.; Zhang, L.-L.; Yu, K.; Xiang, Y.; Ding, X.; Fan, J.; Li, J.-C.; Wang, Q.-S. TLR4 mediates high-fat diet induced physiological changes in mice via attenuating PPARγ/ABCG1 signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 1356–1363. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PloS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.-Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Renz, H.; Jenmalm, M.C.; Kozyrskyj, A.L.; Allen, K.J.; Vuillermin, P.; Prescott, S.L.; in-FLAME Microbiome Interest Group. The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. J. Allergy Clin. Immunol. 2015, 135, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biragyn, A.; Ferrucci, L. Gut dysbiosis: A potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Gibiino, G.; De Siena, M.; Sbrancia, M.; Binda, C.; Sambri, V.; Gasbarrini, A.; Fabbri, C. Dietary Habits and Gut Microbiota in Healthy Adults: Focusing on the Right Diet. A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6728. [Google Scholar] [CrossRef]
- Losno, E.; Sieferle, K.; Perez-Cueto, F.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef]
- Mokkala, K.; Houttu, N.; Cansev, T.; Laitinen, K. Interactions of dietary fat with the gut microbiota: Evaluation of mechanisms and metabolic consequences. Clin. Nutr. 2020, 39, 994–1018. [Google Scholar] [CrossRef]
- Reese, A.T.; Carmody, R.N. Thinking Outside the Cereal Box: Noncarbohydrate Routes for Dietary Manipulation of the Gut Microbiota. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer—Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ajami, N.J.; El-Serag, H.B.; Hair, C.; Graham, D.Y.; White, D.; Chen, L.; Wang, Z.; Plew, S.; Kramer, J.; et al. Dietary quality and the colonic mucosa–associated gut microbiome in humans. Am. J. Clin. Nutr. 2019, 110, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Gurwara, S.; Ajami, N.J.; Jang, A.; Hessel, F.C.; Chen, L.; Plew, S.; Wang, Z.; Graham, D.Y.; Hair, C.; White, D.L.; et al. Dietary Nutrients Involved in One-Carbon Metabolism and Colonic Mucosa-Associated Gut Microbiome in Individuals with an Endoscopically Normal Colon. Nutrients 2019, 11, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires the eating at America’s table study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- A framework for human microbiome research. Nature 2012, 486, 215–221. [CrossRef] [PubMed] [Green Version]
- Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klement, R.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Liaset, B.; Kristiansen, K. Links between Dietary Protein Sources, the Gut Microbiota, and Obesity. Front. Physiol. 2017, 8, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Yekutieli, D. Quantitative Trait Loci Analysis Using the False Discovery Rate. Genetics 2005, 171, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Rosso, G.B.; Chung, C.K.K.; Bäriswyl, L.; Rodriguez, M.P.; Kim, B.S.; Engel, P.; Noti, M. High dietary fat intake induces a microbiota signature that promotes food allergy. J. Allergy Clin. Immunol. 2019, 144, 157–170.e8. [Google Scholar] [CrossRef] [Green Version]
- Parks, B.W.; Nam, E.; Org, E.; Kostem, E.; Norheim, F.; Hui, S.T.; Pan, C.; Civelek, M.; Rau, C.D.; Bennett, B.J.; et al. Genetic Control of Obesity and Gut Microbiota Composition in Response to High-Fat, High-Sucrose Diet in Mice. Cell Metab. 2013, 17, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Partula, V.; Mondot, S.; Torres, M.J.; Kesse-Guyot, E.; Deschasaux, M.; Assmann, K.; Latino-Martel, P.; Buscail, C.; Julia, C.; Galan, P.; et al. Associations between usual diet and gut microbiota composition: Results from the Milieu Intérieur cross-sectional study. Am. J. Clin. Nutr. 2019, 109, 1472–1483. [Google Scholar] [CrossRef]
- Dan, Z.; Mao, X.; Liu, Q.; Guo, M.; Zhuang, Y.; Liu, Z.; Chen, K.; Chen, J.; Xu, R.; Tang, J.; et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes 2020, 11, 1246–1267. [Google Scholar] [CrossRef]
- Humbel, F.; Rieder, J.; Franc, Y.; Juillerat, P.; Scharl, M.; Misselwitz, B.; Schreiner, P.; Begré, S.; Rogler, G.; von Känel, R.; et al. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 2020, 18, 2019–2029.e11. [Google Scholar] [CrossRef]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Ajami, N.; Malhotra, S.; Chen, L.; White, D.; Sharafkhaneh, A.; Hoffman, K.; Graham, D.; El-Serag, H.; Petrosino, J.; et al. Habitual Sleep Duration and the Colonic Mucosa-Associated Gut Microbiota in Humans—A Pilot Study. Clocks Sleep 2021, 3, 387–397. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Liu, H.; Zhang, H.; Bao, Y.; Di, J.; Hu, C. The genus Sutterella is a potential contributor to glucose metabolism improvement after Roux-en-Y gastric bypass surgery in T2D. Diabetes Res. Clin. Pract. 2020, 162, 108116. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Centanni, M.; Consolandi, C.; Rampelli, S.; Turroni, S.; Severgnini, M.; Peano, C.; Ghezzo, A.; Scurti, M.; et al. Gut Microbiome in Down Syndrome. PLoS ONE 2014, 9, e112023. [Google Scholar] [CrossRef]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 2017, 66, 1031–1038. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of Novel PCR-Based Methods for Detection, Quantitation, and Phylogenetic Characterization of Sutterella Species in Intestinal Biopsy Samples from Children with Autism and Gastrointestinal Disturbances. mBio 2012, 3, e00261-11. [Google Scholar] [CrossRef] [Green Version]
- Lehr, H.-A.; Sagban, T.A.; Ihling, C.; Zähringer, U.; Hungerer, K.-D.; Blumrich, M.; Reifenberg, K.; Bhakdi, S. Immunopathogenesis of Atherosclerosis. Circulation 2001, 104, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Fusobacterium nucleatum Alters Atherosclerosis Risk Factors and Enhances Inflammatory Markers with an Atheroprotective Immune Response in ApoEnull Mice. PLoS ONE 2015, 10, e0129795. [Google Scholar] [CrossRef] [Green Version]
- Proença, M.A.; Biselli, J.M.; Succi, M.; Severino, F.E.; Berardinelli, G.N.; Caetano, A.; Reis, R.M.; Hughes, D.J.; Silva, A.E. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J. Gastroenterol. 2018, 24, 5351–5365. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Olaisen, M.; Flatberg, A.; Granlund, A.V.B.; Røyset, E.S.; Martinsen, T.C.; Sandvik, A.K.; Fossmark, R. Bacterial Mucosa-associated Microbiome in Inflamed and Proximal Noninflamed Ileum of Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2020, 27, 12–24. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Yuan, L.; Zhang, J.; Han, L.; Liu, R.; Wu, R.; Shi, Y.; Mushtaq, N.; Ullah, S.; et al. Molecular characterization of gut microbiota in high lipid di-et induced hyperlipidemic rats treated with simvastatin. Int. J. Mol. Med. 2020, 45, 1601–1615. [Google Scholar] [CrossRef]
- Rogosa, M. Acidaminococcus gen. n., Acidaminococcus fermentans sp. n., Anaerobic Gram-negative Diplococci Using Amino Acids as the Sole Energy Source for Growth. J. Bacteriol. 1969, 98, 756–766. [Google Scholar] [CrossRef] [Green Version]
- Burrin, D.G.; Stoll, B. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutr. 2009, 90, 850S–856S. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, M.A.; de Jong, J.; Vaessen, M.J.; van Leeuwen, P.A.; Houdijk, A.P. Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J. Gastroenterol. 2011, 17, 1569–1573. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Gaundal, L.; Myhrstad, M.C.W. Polyunsaturated Fatty Acids and Glycemic Control in Type 2 Diabetes. Nutrients 2019, 11, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, L.E.M.; Koetsier, M.A.; Balvers, M.; Beermann, C.; Stahl, B.; van Tol, E.A.F. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur. J. Nutr. 2008, 47, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liput, K.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
| Characteristics Mean ± Standard Deviation or n (%) | Lower Intake n = 17 | Higher Intake n = 17 | p Value a |
|---|---|---|---|
| Age (years) | 61.1 ± 6.0 | 62.9 ± 5.1 | 0.34 |
| Men, n (%) | 16 (95%) | 17 (100%) | 0.31 |
| Racial group | |||
| White, n (%) | 11 (65%) | 13 (76%) | 0.66 |
| African American, n (%) | 4 (23%) | 2 (12%) | |
| Hispanic, n (%) | 2 (12%) | 2 (12%) | |
| Body mass index (kg/m2) | 32.4 ± 7.4 | 35.4 ± 5.2 | 0.18 |
| Ever smokers, n (%) | 10 (59%) | 11 (65%) | 0.72 |
| Current alcohol use, n (%) | 8 (47%) | 7 (41%) | 0.46 |
| Hypertension, yes (%) | 11 (65%) | 14 (82%) | 0.24 |
| Type 2 diabetes, yes (%) | 7 (41) | 10 (59) | 0.30 |
| Healthy Eating Index | 64.1 ± 8.7 | 57.8 ± 8.3 | 0.04 |
| Saturated fat (grams/1000 kcal/day) | 10.5 ± 0.98 | 15.7 ± 2.16 | <0.0001 |
| Total carbohydrates (grams/1000 kcal/day) | 123 ± 21 | 103 ±14 | 0.004 |
| Protein (grams/1000 kcal/day) | 35.7 ± 8.1 | 40.5 ± 5.7 | 0.05 |
| Type of Fatty Acid | Phylum | Relative Abundance (%) | q Value a | |
|---|---|---|---|---|
| Lower Intake | Higher Intake | |||
| TFAs | Desulfobacterota | 1.05 | 1.84 | 0.005 |
| MUFAs | Desulfobacterota | 1.10 | 1.82 | 0.029 |
| Fusobacteria | 0.84 | 1.99 | 0.008 | |
| PUFAs | Desulfobacterota | 1.04 | 1.89 | 0.013 |
| n6-FA | Desulfobacterota | 1.04 | 1.89 | 0.013 |
| SFAs | Fusobacteria | 0.70 | 2.34 | 0.008 |
| Type Of Fatty Acid | Genus | Relative Abundance (%) | q Value a | |
|---|---|---|---|---|
| Lower Intake | Higher Intake | |||
| TFAs | Sutterella | 0.57 | 2.41 | <0.0001 |
| Acidaminococcus | 0.03 | 0.24 | 0.04 | |
| MUFAs | Lachnoclostridium | 0.87 | 1.57 | 0.002 |
| Sutterella | 0.80 | 2.29 | 0.002 | |
| Founierella | 0.02 | 0.13 | 0.035 | |
| Fusobacterium | 0.82 | 1.97 | 0.036 | |
| Intestinibacter | 0.15 | 0.17 | 0.041 | |
| PUFAs | Anaerostipes | 0.91 | 0.12 | 0.0006 |
| Sutterella | 0.66 | 2.44 | 0.0006 | |
| Acidaminococcus | 0.03 | 0.26 | 0.01 | |
| Bilophila | 0.50 | 0.99 | 0.02 | |
| Colidextribactor | 0.14 | 0.33 | 0.02 | |
| Prevotella | 1.50 | 3.39 | 0.02 | |
| n3-FAs | Alloprevotella | 0.60 | 0 | 0.004 |
| Faecalibacterium | 4.34 | 9.92 | 0.004 | |
| Subdoligranulum | 0.21 | 0.73 | 0.004 | |
| Acidaminococcus | 0.03 | 0.26 | 0.007 | |
| Sutterella | 0.85 | 2.30 | 0.026 | |
| Phascolarctobacterium | 0.44 | 0.78 | 0.03 | |
| Tyzzerella | 0.45 | 0.14 | 0.03 | |
| n6-FAs | Anaerostipes | 0.91 | 0.12 | <0.001 |
| Sutterella | 0.66 | 2.44 | <0.001 | |
| Acidaminococcus | 0.03 | 0.26 | 0.007 | |
| Bilophila | 0.50 | 0.99 | 0.007 | |
| Colidextribacter | 0.14 | 0.33 | 0.014 | |
| Prevotella | 1.51 | 3.39 | 0.014 | |
| SFAs | Tyzzerella | 0.11 | 0.43 | 0.0004 |
| Negativibacilus | 0.18 | 0.04 | 0.003 | |
| Butyricimonas | 0.15 | 0.20 | 0.032 | |
| Fusobacterium | 0.66 | 2.37 | 0.030 | |
| Bifidobacterium | 0.14 | 0.41 | 0.031 | |
| Intestinibacter | 0.26 | 0.06 | 0.031 | |
| Veillonella | 0.14 | 0.42 | 0.031 | |
| TrFAs | Bifidobacterium | 0.19 | 0.36 | 0.008 |
| Tyzzerella | 0.10 | 0.34 | 0.008 | |
| Butyricicoccus | 0.13 | 0.34 | 0.028 | |
| Genus | Type of Fatty Acid | Count | IRR (95% CI) b | IRR (95% CI) c | IRR (95% CI) d | |
|---|---|---|---|---|---|---|
| Lower Higher Intake a Intake | ||||||
| Median count | ||||||
| Sutterella | TFAs | 0 | 18 | 1.25 (1.15–1.37) | 1.24 (1.12–1.37) | 1.24 (1.12–1.37) |
| Sutterella | MUFAs | 0 | 17 | 1.67 (1.35–2.12) | 1.65 (1.32–2.08) | 1.65 (1.31–2.07) |
| Sutterella | PUFAs | 0 | 17 | 1.24 (1.04–1.47) | 1.19 (0.95–1.48) | 1.48 (1.12–1.94) |
| Sutterella | n6-FAs | 0 | 17 | 1.30 (1.05–1.60) | 1.23 (0.94–1.61) | 1.55 (1.11–2.16) |
| Tyzzerella | SFAs | 0 | 14.5 | 1.18 (0.76–1.83) | 2.04 (1.19–3.48) | 1.66 (1.00–2.76) |
| Fusobacterium | SFAs | 0 | 5 | 1.21 (1.02–1.43) | 1.38 (1.10–1.71) | 1.11 (0.83–1.48) |
| Tyzzerella | TrFAs | 0 | 0 | 1.52 (0.17–13.3) | 6.62 (1.01–43.0) | 6.61(1.02–43.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.A.; Kennedy, L.K.; Hoffman, K.; White, D.L.; Kanwal, F.; El-Serag, H.B.; Petrosino, J.F.; Jiao, L. Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients 2022, 14, 2722. https://doi.org/10.3390/nu14132722
Xu AA, Kennedy LK, Hoffman K, White DL, Kanwal F, El-Serag HB, Petrosino JF, Jiao L. Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients. 2022; 14(13):2722. https://doi.org/10.3390/nu14132722
Chicago/Turabian StyleXu, Anthony A., Luke K. Kennedy, Kristi Hoffman, Donna L. White, Fasiha Kanwal, Hashem B. El-Serag, Joseph F. Petrosino, and Li Jiao. 2022. "Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans" Nutrients 14, no. 13: 2722. https://doi.org/10.3390/nu14132722
APA StyleXu, A. A., Kennedy, L. K., Hoffman, K., White, D. L., Kanwal, F., El-Serag, H. B., Petrosino, J. F., & Jiao, L. (2022). Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients, 14(13), 2722. https://doi.org/10.3390/nu14132722







