Influences of Vitamin D and Iron Status on Skeletal Muscle Health: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
3.1. Vitamin D and Skeletal Muscle Health
3.1.1. Vitamin D and Skeletal Muscle Physiology
3.1.2. Vitamin D Status and Skeletal Muscle Health
3.1.3. Vitamin D Interventions and Skeletal Muscle Health
3.2. Iron and Skeletal Muscle Health
3.2.1. Iron and Skeletal Muscle Physiology
3.2.2. Iron and Skeletal Muscle Health
3.2.3. Iron Interventions and Skeletal Muscle Health
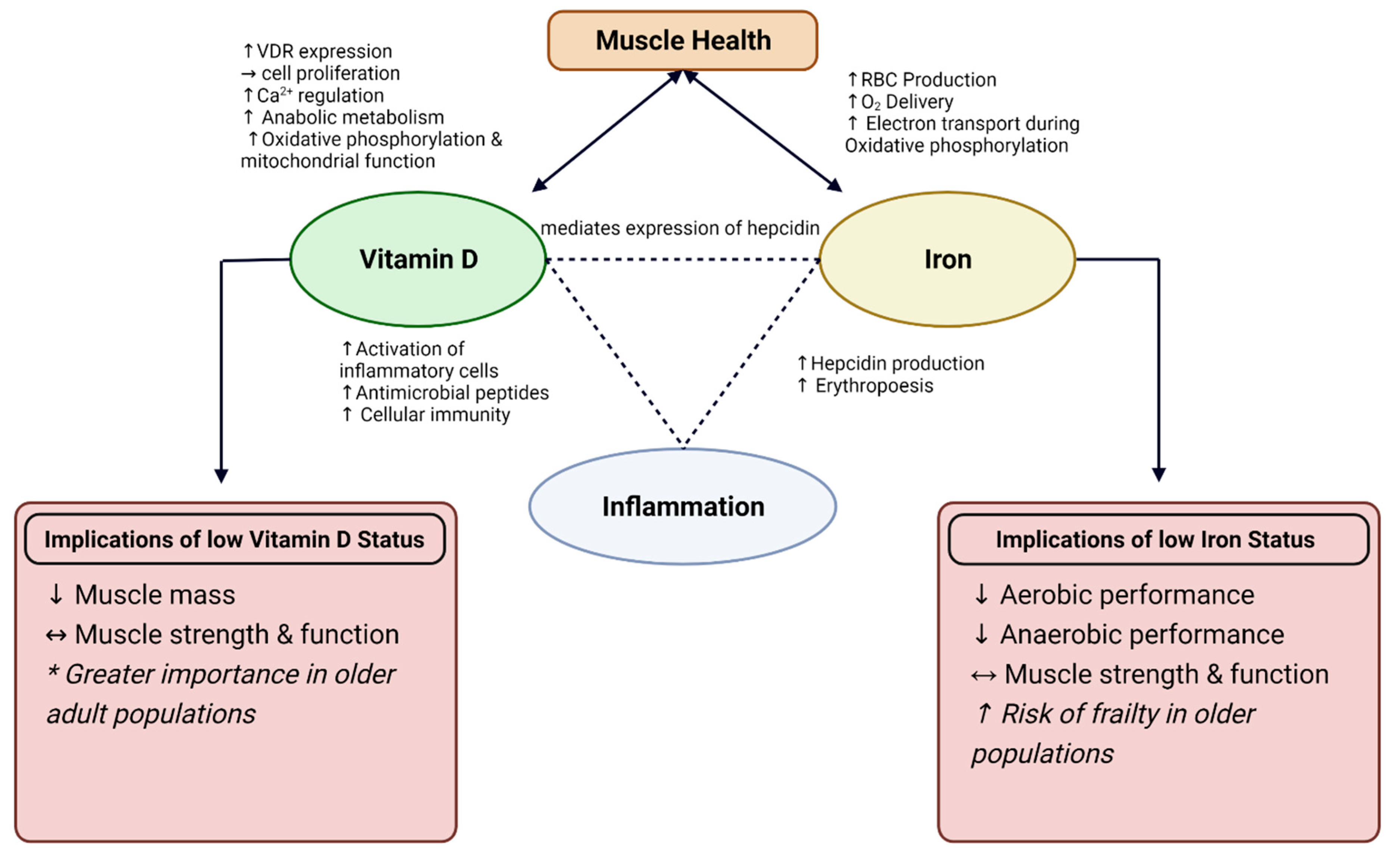
3.3. Interrelationship between Vitamin D, Iron, and Chronic Inflammation
3.3.1. Anti-Inflammatory Role of Vitamin D
3.3.2. Connection between Vitamin D and Iron Status
3.4. Animal Food Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gi, Y.-M.; Jung, B.; Kim, K.-W.; Cho, J.-H.; Ha, I.-H. Low Handgrip Strength Is Closely Associated with Anemia among Adults: A Cross-Sectional Study Using Korea National Health and Nutrition Examination Survey (KNHANES). PLoS ONE 2020, 15, e0218058. [Google Scholar] [CrossRef] [PubMed]
- Thein, M.; Ershler, W.B.; Artz, A.S.; Tecson, J.; Robinson, B.E.; Rothstein, G.; Liede, A.; Gylys-Colwell, I.; Lu, Z.J.; Robbins, S. Diminished Quality of Life and Physical Function in Community-Dwelling Elderly with Anemia. Medicine 2009, 88, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Penninx, B.W.J.H.; Lauretani, F.; Russo, C.R.; Carter, C.; Bandinelli, S.; Atkinson, H.; Onder, G.; Pahor, M.; Ferrucci, L. Hemoglobin Levels and Skeletal Muscle: Results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mastaglia, S.R.; Seijo, M.; Muzio, D.; Somoza, J.; Nuñez, M.; Oliveri, B. Effect of Vitamin D Nutritional Status on Muscle Function and Strength in Healthy Women Aged over Sixty-Five Years. J. Nutr. Health Aging 2011, 15, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey Protein, Amino Acids, and Vitamin D Supplementation with Physical Activity Increases Fat-Free Mass and Strength, Functionality, and Quality of Life and Decreases Inflammation in Sarcopenic Elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional Status, Body Composition, and Quality of Life in Community-Dwelling Sarcopenic and Non-Sarcopenic Older Adults: A Case-Control Study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen Weeks of Supplementation of Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement Attenuates Chronic Low-Grade Inflammation in Sarcopenic Older Adults: The PROVIDE Study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-Dose Vitamin D3 Reduces Circulating Hepcidin Concentrations: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Adults. Clin. Nutr. 2017, 36, 980–985. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and Anemia: Insights into an Emerging Association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The Role of Vitamin D in Regulating the Iron-Hepcidin-Ferroportin Axis in Monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D Deficiency Promotes Epithelial Barrier Dysfunction and Intestinal Inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The Vitamin D-Antimicrobial Peptide Pathway and Its Role in Protection against Infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.-R.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Khalaji, N.; Gharavi, A. Improvement of Vitamin D Status Resulted in Amelioration of Biomarkers of Systemic Inflammation in the Subjects with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2012, 28, 424–430. [Google Scholar] [CrossRef]
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. [Google Scholar] [CrossRef]
- De Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M.; et al. Relationship between Vitamin D and Inflammatory Markers in Older Individuals. Age 2014, 36, 9694. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Henriksen, V.T.; Dixon, B.M.; Schneider, E.D.; Dern, A.; Weaver, L.K. Different Doses of Supplemental Vitamin D Maintain Interleukin-5 without Altering Skeletal Muscle Strength: A Randomized, Double-Blind, Placebo-Controlled Study in Vitamin D Sufficient Adults. Nutr. Metab. 2012, 9, 16. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011.
- Toffanello, E.D.; Perissinotto, E.; Sergi, G.; Zambon, S.; Musacchio, E.; Maggi, S.; Coin, A.; Sartori, L.; Corti, M.-C.; Baggio, G.; et al. Vitamin D and Physical Performance in Elderly Subjects: The Pro.V.A Study. PLoS ONE 2012, 7, e34950. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Orces, C.; Lorenzo, C.; Guarneros, J.E. The Prevalence and Determinants of Vitamin D Inadequacy among U.S. Older Adults: National Health and Nutrition Examination Survey 2007–2014. Cureus 2019, 11, e5300. [Google Scholar] [CrossRef] [PubMed]
- Alagöl, F.; Shihadeh, Y.; Boztepe, H.; Tanakol, R.; Yarman, S.; Azizlerli, H.; Sandalci, O. Sunlight Exposure and Vitamin D Deficiency in Turkish Women. J. Endocrinol. Investig. 2000, 23, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Batieha, A.; Khader, Y.; Jaddou, H.; Hyassat, D.; Batieha, Z.; Khateeb, M.; Belbisi, A.; Ajlouni, K. Vitamin D Status in Jordan: Dress Style and Gender Discrepancies. Ann. Nutr. Metab. 2011, 58, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.F. Skin-Pigment Regulation of Vitamin-D Biosynthesis in Man. Science 1967, 157, 501–506. [Google Scholar] [CrossRef]
- Weishaar, T.; Rajan, S.; Keller, B. Probability of Vitamin D Deficiency by Body Weight and Race/Ethnicity. J. Am. Board Fam. Med. 2016, 29, 226–232. [Google Scholar] [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Law, A.D.; Dutta, U.; Kochhar, R.; Vaishnavi, C.; Kumar, S.; Noor, T.; Bhadada, S.; Singh, K. Vitamin D Deficiency in Adult Patients with Ulcerative Colitis: Prevalence and Relationship with Disease Severity, Extent, and Duration. Indian J. Gastroenterol. 2019, 38, 6–14. [Google Scholar] [CrossRef]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D Deficiency and Its Treatment in Cystic Fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. 2), S66–S73. [Google Scholar] [CrossRef]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D Deficiency in Patients with Inflammatory Bowel Disease: Association with Disease Activity and Quality of Life. JPEN J. Parenter. Enteral Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef]
- LaClair, R.E.; Hellman, R.N.; Karp, S.L.; Kraus, M.; Ofner, S.; Li, Q.; Graves, K.L.; Moe, S.M. Prevalence of Calcidiol Deficiency in CKD: A Cross-Sectional Study across Latitudes in the United States. Am. J. Kidney Dis. 2005, 45, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, J.; Holick, M.F. Aging Decreases the Capacity of Human Skin to Produce Vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. No Effects without Causes: The Iron Dysregulation and Dormant Microbes Hypothesis for Chronic, Inflammatory Diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1518–1557. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Anker, S.D.; von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; Marzetti, E. Biomarkers for Physical Frailty and Sarcopenia: State of the Science and Future Developments. J. Cachexia Sarcopenia Muscle 2015, 6, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Davies, K.; Martin-Ruiz, C.; Jagger, C.; Kirkwood, T.B.L.; von Zglinicki, T.; Aihie Sayer, A. Grip Strength and Inflammatory Biomarker Profiles in Very Old Adults. Age Ageing 2017, 46, 976–982. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory Markers and Loss of Muscle Mass (Sarcopenia) and Strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin Resistance and Inflammation as Precursors of Frailty: The Cardiovascular Health Study. Arch. Intern Med. 2007, 167, 635–641. [Google Scholar] [CrossRef]
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and Predictors of Vitamin D-Deficiency in Frail Older Hospitalized Patients. BMC Geriatr. 2018, 18, 219. [Google Scholar] [CrossRef]
- Seo, E.H.; Kim, H.; Kwon, O. Association between Total Sugar Intake and Metabolic Syndrome in Middle-Aged Korean Men and Women. Nutrients 2019, 11, 2042. [Google Scholar] [CrossRef]
- Villacis, D.; Yi, A.; Jahn, R.; Kephart, C.J.; Charlton, T.; Gamradt, S.C.; Romano, R.; Tibone, J.E.; Hatch, G.F.R. Prevalence of Abnormal Vitamin D Levels Among Division I NCAA Athletes. Sports Health 2014, 6, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Turer, C.B.; Lin, H.; Flores, G. Prevalence of Vitamin D Deficiency among Overweight and Obese US Children. Pediatrics 2013, 131, e152–e161. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of Anemia in Persons 65 Years and Older in the United States: Evidence for a High Rate of Unexplained Anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Robalo Nunes, A.; Fonseca, C.; Marques, F.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of Anemia and Iron Deficiency in Older Portuguese Adults: An EMPIRE Substudy. Geriatr. Gerontol. Int. 2017, 17, 1814–1822. [Google Scholar] [CrossRef]
- Dellavalle, D.M.; Haas, J.D. Iron Status Is Associated with Endurance Performance and Training in Female Rowers. Med. Sci. Sports Exerc. 2012, 44, 1552–1559. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Gillen, Z.M.; McKay, B.D.; Koehler, K.; Cramer, J.T. High Prevalence of Poor Iron Status Among 8- to 16-Year-Old Youth Athletes: Interactions Among Biomarkers of Iron, Dietary Intakes, and Biological Maturity. J. Am. Coll. Nutr. 2019, 39, 155–162. [Google Scholar] [CrossRef]
- Andriastuti, M.; Ilmana, G.; Nawangwulan, S.A.; Kosasih, K.A. Prevalence of Anemia and Iron Profile among Children and Adolescent with Low Socio-Economic Status. Int. J. Pediatrics Adolesc. Med. 2020, 7, 88–92. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Melamed, M.L.; Kumar, J.; Roy, C.N.; Miller, E.R.; Furth, S.L.; Fadrowski, J.J. Vitamin D, Race, and Risk for Anemia in Children. J. Pediatr. 2014, 164, 153–158.e1. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Pande, R.; Berliner, N.; Vanasse, G.J. Prevalence of 25-Hydroxyvitamin D Deficiency in Subgroups of Elderly Persons with Anemia: Association with Anemia of Inflammation. Blood 2011, 117, 2800–2806. [Google Scholar] [CrossRef]
- Ferrari, M.; Mistura, L.; Patterson, E.; Sjöström, M.; Díaz, L.E.; Stehle, P.; Gonzalez-Gross, M.; Kersting, M.; Widhalm, K.; Molnár, D.; et al. Evaluation of Iron Status in European Adolescents through Biochemical Iron Indicators: The HELENA Study. Eur. J. Clin. Nutr. 2011, 65, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Horton-French, K.; Dunlop, E.; Lucas, R.M.; Pereira, G.; Black, L.J. Prevalence and Predictors of Vitamin D Deficiency in a Nationally Representative Sample of Australian Adolescents and Young Adults. Eur. J. Clin. Nutr. 2021, 75, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Ritterhouse, L.L.; Lu, R.; Shah, H.B.; Robertson, J.M.; Fife, D.A.; Maecker, H.T.; Du, H.; Fathman, C.G.; Chakravarty, E.F.; Scofield, R.H.; et al. Vitamin D Deficiency in a Multiethnic Healthy Control Cohort and Altered Immune Response in Vitamin D Deficient European-American Healthy Controls. PLoS ONE 2014, 9, e94500. [Google Scholar] [CrossRef]
- van der Merwe, L.F.; Eussen, S.R. Iron Status of Young Children in Europe. Am. J. Clin. Nutr. 2017, 106, 1663S–1671S. [Google Scholar] [CrossRef]
- Constantini, N.W.; Eliakim, A.; Zigel, L.; Yaaron, M.; Falk, B. Iron Status of Highly Active Adolescents: Evidence of Depleted Iron Stores in Gymnasts. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, J.; Raczynski, G.; Stupnicki, R. Iron Status in Female Endurance Athletes and in Non-Athletes. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Asp, M.L.; Richardson, J.R.; Collene, A.L.; Droll, K.R.; Belury, M.A. Dietary Protein and Beef Consumption Predict for Markers of Muscle Mass and Nutrition Status in Older Adults. J. Nutr. Health Aging 2012, 16, 784–790. [Google Scholar] [CrossRef]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-Enriched Diet, with the Use of Lean Red Meat, Combined with Progressive Resistance Training Enhances Lean Tissue Mass and Muscle Strength and Reduces Circulating IL-6 Concentrations in Elderly Women: A Cluster Randomized Controlled Trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-Analysis123. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, O. The Relationship between Ultraviolet Radiation Exposure and Vitamin D Status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Clinical Review: The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef]
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of Vitamin D by the Liver. J. Clin. Investig. 1969, 48, 2032–2037. [Google Scholar] [CrossRef]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Vitamin D Treatment Modulates Immune Activation in Cystic Fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371. [Google Scholar] [CrossRef]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and Cardiovascular Disease Prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Briganti, S.; Vianello, E.; Dogliotti, G.; Barassi, A.; Malavazos, A.E.; Ermetici, F.; Morricone, L.; Sigruener, A.; Schmitz, G.; et al. Epicardial Adipose Tissue Inflammation Is Related to Vitamin D Deficiency in Patients Affected by Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 267–273. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D Deficiency Contributes Directly to the Acute Respiratory Distress Syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Treiber, G.; Prietl, B.; Fröhlich-Reiterer, E.; Lechner, E.; Ribitsch, A.; Fritsch, M.; Rami-Merhar, B.; Steigleder-Schweiger, C.; Graninger, W.; Borkenstein, M.; et al. Cholecalciferol Supplementation Improves Suppressive Capacity of Regulatory T-Cells in Young Patients with New-Onset Type 1 Diabetes Mellitus—A Randomized Clinical Trial. Clin. Immunol. 2015, 161, 217–224. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In Situ Detection of 1,25-Dihydroxyvitamin D3 Receptor in Human Skeletal Muscle Tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L.; da Silva Morais, M.; Park, L.K.; Morris, E.; Harris, S.S.; Bischoff-Ferrari, H.A.; Fielding, R.A.; Dawson-Hughes, B. Multi-Step Immunofluorescent Analysis of Vitamin D Receptor Loci and Myosin Heavy Chain Isoforms in Human Skeletal Muscle. J. Mol. Histol. 2010, 41, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Blau, H.M.; Feldman, D. 1,25-Dihydroxyvitamin D3 Receptors and Hormonal Responses in Cloned Human Skeletal Muscle Cells. Endocrinology 1986, 119, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Rybchyn, M.S.; Ning, Y.J.; Brennan-Speranza, T.C.; Girgis, C.M.; Gunton, J.E.; Fraser, D.R.; Mason, R.S. 1,25-Dihydroxycholecalciferol (Calcitriol) Modifies Uptake and Release of 25-Hydroxycholecalciferol in Skeletal Muscle Cells in Culture. J. Steroid Biochem. Mol. Biol. 2018, 177, 109–115. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-Hydroxyvitamin D Concentrations Are Associated with Better Lower-Extremity Function in Both Active and Inactive Persons Aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of Vitamin D Receptor Gene in Mice Results in Abnormal Skeletal Muscle Development with Deregulated Expression of Myoregulatory Transcription Factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.-K.; Esser, K.A. VDR and CYP27B1 Are Expressed in C2C12 Cells and Regenerating Skeletal Muscle: Potential Role in Suppression of Myoblast Proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef]
- Suzuki, T.; Kwon, J.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Yoshida, H. Low Serum 25-Hydroxyvitamin D Levels Associated with Falls among Japanese Community-Dwelling Elderly. J. Bone Miner. Res. 2008, 23, 1309–1317. [Google Scholar] [CrossRef]
- Sørensen, O.H.; Lund, B.; Saltin, B.; Lund, B.; Andersen, R.B.; Hjorth, L.; Melsen, F.; Mosekilde, L. Myopathy in Bone Loss of Ageing: Improvement by Treatment with 1 Alpha-Hydroxycholecalciferol and Calcium. Clin. Sci. 1979, 56, 157–161. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A Randomized Study on the Effect of Vitamin D₃ Supplementation on Skeletal Muscle Morphology and Vitamin D Receptor Concentration in Older Women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L. The Emerging Biomolecular Role of Vitamin D in Skeletal Muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D Concentration in 342 Professional Football Players and Association with Lower Limb Isokinetic Function. J. Sci. Med. Sport 2014, 17, 139–143. [Google Scholar] [CrossRef]
- Książek, A.; Zagrodna, A.; Dziubek, W.; Pietraszewski, B.; Ochmann, B.; Słowińska-Lisowska, M. 25(OH)D3 Levels Relative to Muscle Strength and Maximum Oxygen Uptake in Athletes. J. Hum. Kinet. 2016, 50, 71–77. [Google Scholar] [CrossRef]
- Zeitler, C.; Fritz, R.; Smekal, G.; Ekmekcioglu, C. Association between the 25-Hydroxyvitamin D Status and Physical Performance in Healthy Recreational Athletes. Int. J. Environ. Res. Public Health 2018, 15, 2724. [Google Scholar] [CrossRef]
- Most, A.; Dörr, O.; Nef, H.; Hamm, C.; Bauer, T.; Bauer, P. Influence of 25-Hydroxy-Vitamin D Insufficiency on Maximal Aerobic Power in Elite Indoor Athletes: A Cross-Sectional Study. Sports Med. Open 2021, 7, 74. [Google Scholar] [CrossRef]
- Fitzgerald, J.S.; Peterson, B.J.; Warpeha, J.M.; Wilson, P.B.; Rhodes, G.S.; Ingraham, S.J. Vitamin D Status and V[Combining Dot above]O2peak during a Skate Treadmill Graded Exercise Test in Competitive Ice Hockey Players. J. Strength Cond. Res. 2014, 28, 3200–3205. [Google Scholar] [CrossRef]
- Forney, L.A.; Earnest, C.P.; Henagan, T.M.; Johnson, L.E.; Castleberry, T.J.; Stewart, L.K. Vitamin D Status, Body Composition, and Fitness Measures in College-Aged Students. J. Strength Cond. Res. 2014, 28, 814–824. [Google Scholar] [CrossRef]
- Marantes, I.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J.; Amin, S. Is Vitamin D a Determinant of Muscle Mass and Strength? J. Bone Miner. Res. 2011, 26, 2860–2871. [Google Scholar] [CrossRef]
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.; van Loon, L.J.C.; De Groot, L.C.P.G.M. Low Vitamin D Status Is Associated with Reduced Muscle Mass and Impaired Physical Performance in Frail Elderly People. Eur. J. Clin. Nutr. 2013, 67, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Gumieiro, D.N.; Murino Rafacho, B.P.; Buzati Pereira, B.L.; Cavallari, K.A.; Tanni, S.E.; Azevedo, P.S.; Polegato, B.F.; Mamede Zornoff, L.A.; Dinhane, D.I.; Innocenti Dinhane, K.G.; et al. Vitamin D Serum Levels Are Associated with Handgrip Strength but Not with Muscle Mass or Length of Hospital Stay after Hip Fracture. Nutrition 2015, 31, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; de Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, R.; Gimigliano, F. Hypovitaminosis D Is Associated with a Reduction in Upper and Lower Limb Muscle Strength and Physical Performance in Post-Menopausal Women: A Retrospective Study. Aging Clin. Exp. Res. 2015, 27 (Suppl. 1), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Laird, E.; Healy, M.; Lawlor, B.; O’Sullivan, M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength and Physical Performance in Community-Dwelling Older Adults: Findings from the English Longitudinal Study of Ageing. Clin. Interv. Aging 2019, 14, 1751–1761. [Google Scholar] [CrossRef]
- Conzade, R.; Grill, E.; Bischoff-Ferrari, H.A.; Ferrari, U.; Horsch, A.; Koenig, W.; Peters, A.; Thorand, B. Vitamin D in Relation to Incident Sarcopenia and Changes in Muscle Parameters among Older Adults: The KORA-Age Study. Calcif. Tissue Int. 2019, 105, 173–182. [Google Scholar] [CrossRef]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. The Association between 25-Hydroxyvitamin D Concentration, Physical Performance and Frailty Status in Older Adults. Eur. J. Nutr. 2019, 58, 1173–1181. [Google Scholar] [CrossRef]
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-Hydroxyvitamin D Levels in Adolescents: Race, Season, Adiposity, Physical Activity, and Fitness. Pediatrics 2010, 125, 1104–1111. [Google Scholar] [CrossRef]
- Gracia-Marco, L.; Valtueña, J.; Ortega, F.B.; Pérez-López, F.R.; Vicente-Rodríguez, G.; Breidenassel, C.; Ferrari, M.; Molnar, D.; Widhalm, K.; de Henauw, S.; et al. Iron and Vitamin Status Biomarkers and Its Association with Physical Fitness in Adolescents: The HELENA Study. J. Appl. Physiol. 2012, 113, 566–573. [Google Scholar] [CrossRef]
- Valtueña, J.; Gracia-Marco, L.; Huybrechts, I.; Breidenassel, C.; Ferrari, M.; Gottrand, F.; Dallongeville, J.; Sioen, I.; Gutierrez, A.; Kersting, M.; et al. Cardiorespiratory Fitness in Males, and Upper Limbs Muscular Strength in Females, Are Positively Related with 25-Hydroxyvitamin D Plasma Concentrations in European Adolescents: The HELENA Study. QJM Int. J. Med. 2013, 106, 809–821. [Google Scholar] [CrossRef]
- Carson, E.L.; Pourshahidi, L.K.; Hill, T.R.; Cashman, K.D.; Strain, J.J.; Boreham, C.A.; Mulhern, M.S. Vitamin D, Muscle Function, and Cardiorespiratory Fitness in Adolescents From the Young Hearts Study. J. Clin. Endocrinol. Metab. 2015, 100, 4621–4628. [Google Scholar] [CrossRef]
- Bezrati, I.; Hammami, R.; Ben Fradj, M.K.; Martone, D.; Padulo, J.; Feki, M.; Chaouachi, A.; Kaabachi, N. Association of Plasma 25-Hydroxyvitamin D with Physical Performance in Physically Active Children. Appl. Physiol. Nutr. Metab. 2016, 41, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S.; Sacheck, J.M. Relationship between Muscle Strength and Dyslipidemia, Serum 25(OH)D, and Weight Status among Diverse Schoolchildren: A Cross-Sectional Analysis. BMC Pediatr. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Wakayo, T.; Belachew, T.; Whiting, S.J. Serum Vitamin D Level Associates With Handgrip Muscle Strength among Ethiopian Schoolchildren: A Cross-Sectional Study. Food Nutr. Bull 2018, 39, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270. [Google Scholar] [CrossRef]
- Bislev, L.S.; Grove-Laugesen, D.; Rejnmark, L. Vitamin D and Muscle Health: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Bone Miner. Res. 2021, 36, 1651–1660. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of Vitamin D Supplementation on Upper and Lower Body Muscle Strength Levels in Healthy Individuals. A Systematic Review with Meta-Analysis. J. Sci. Med. Sport 2015, 18, 575–580. [Google Scholar] [CrossRef]
- Wyon, M.A.; Wolman, R.; Nevill, A.M.; Cloak, R.; Metsios, G.S.; Gould, D.; Ingham, A.; Koutedakis, Y. Acute Effects of Vitamin D3 Supplementation on Muscle Strength in Judoka Athletes: A Randomized Placebo-Controlled, Double-Blind Trial. Clin. J. Sport Med. 2016, 26, 279–284. [Google Scholar] [CrossRef]
- Fairbairn, K.A.; Ceelen, I.J.M.; Skeaff, C.M.; Cameron, C.M.; Perry, T.L. Vitamin D3 Supplementation Does Not Improve Sprint Performance in Professional Rugby Players: A Randomized, Placebo-Controlled, Double-Blind Intervention Study. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Knab, A.M.; Gillitt, N.D.; Meaney, M.P.; Jin, F.; Sha, W.; Cialdella-Kam, L. Influence of Vitamin D Mushroom Powder Supplementation on Exercise-Induced Muscle Damage in Vitamin D Insufficient High School Athletes. J. Sports Sci. 2014, 32, 670–679. [Google Scholar] [CrossRef]
- Close, G.L.; Russell, J.; Cobley, J.N.; Owens, D.J.; Wilson, G.; Gregson, W.; Fraser, W.D.; Morton, J.P. Assessment of Vitamin D Concentration in Non-Supplemented Professional Athletes and Healthy Adults during the Winter Months in the UK: Implications for Skeletal Muscle Function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The Effects of Vitamin D(3) Supplementation on Serum Total 25[OH]D Concentration and Physical Performance: A Randomised Dose-Response Study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; McSorley, E.M.; Pourshahidi, L.K.; Madigan, S.M.; Laird, E.; Healy, M.; Magee, P.J. Vitamin D3 Supplementation Using an Oral Spray Solution Resolves Deficiency but Has No Effect on VO2 Max in Gaelic Footballers: Results from a Randomised, Double-Blind, Placebo-Controlled Trial. Eur. J. Nutr. 2017, 56, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-Weekly Dose of 8400 IU Vitamin D(3) Compared with Placebo: Effects on Neuromuscular Function and Tolerability in Older Adults with Vitamin D Insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991. [Google Scholar] [CrossRef]
- Lagari, V.; Gómez-Marín, O.; Levis, S. The Role of Vitamin D in Improving Physical Performance in the Elderly. J. Bone Miner. Res. 2013, 28, 2194–2201. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Cangussu, L.M.; Nahas-Neto, J.; Orsatti, C.L.; Bueloni-Dias, F.N.; Nahas, E.a.P. Effect of Vitamin D Supplementation Alone on Muscle Function in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Osteoporos Int. 2015, 26, 2413–2421. [Google Scholar] [CrossRef]
- Apaydin, M.; Can, A.G.; Kizilgul, M.; Beysel, S.; Kan, S.; Caliskan, M.; Demirci, T.; Ozcelik, O.; Ozbek, M.; Cakal, E. The Effects of Single High-Dose or Daily Low-Dosage Oral Colecalciferol Treatment on Vitamin D Levels and Muscle Strength in Postmenopausal Women. BMC Endocr. Disord. 2018, 18, 48. [Google Scholar] [CrossRef]
- Vaes, A.M.M.; Tieland, M.; Toussaint, N.; Nilwik, R.; Verdijk, L.B.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Cholecalciferol or 25-Hydroxycholecalciferol Supplementation Does Not Affect Muscle Strength and Physical Performance in Prefrail and Frail Older Adults. J. Nutr. 2018, 148, 712–720. [Google Scholar] [CrossRef]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D Supplementation and Muscle Strength in Pre-Sarcopenic Elderly Lebanese People: A Randomized Controlled Trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Mølmen, K.S.; Hammarström, D.; Pedersen, K.; Lian Lie, A.C.; Steile, R.B.; Nygaard, H.; Khan, Y.; Hamarsland, H.; Koll, L.; Hanestadhaugen, M.; et al. Vitamin D3 Supplementation Does Not Enhance the Effects of Resistance Training in Older Adults. J. Cachexia Sarcopenia Muscle 2021, 12, 599–628. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.A.; Das, G.; Roberts, S.A.; Berry, J.L.; Adams, J.E.; Rawer, R.; Mughal, M.Z. A Randomized, Controlled Trial of Vitamin D Supplementation upon Musculoskeletal Health in Postmenarchal Females. J. Clin. Endocrinol. Metab. 2010, 95, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Laing, E.M.; Pollock, N.K.; Hausman, D.B.; Weaver, C.M.; Martin, B.R.; McCabe, G.P.; Peacock, M.; Warden, S.J.; Hill Gallant, K.M.; et al. Serum 25-Hydroxyvitamin D and Intact Parathyroid Hormone Influence Muscle Outcomes in Children and Adolescents. J. Bone Miner. Res. 2018, 33, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, C.; Mølgaard, C.; Hauger, H.; Kristensen, M.; Damsgaard, C.T. Winter Vitamin D3 Supplementation Does Not Increase Muscle Strength, but Modulates the IGF-Axis in Young Children. Eur. J. Nutr. 2019, 58, 1183–1192. [Google Scholar] [CrossRef]
- Janssen, H.C.J.P.; Samson, M.M.; Verhaar, H.J.J. Muscle Strength and Mobility in Vitamin D-Insufficient Female Geriatric Patients: A Randomized Controlled Trial on Vitamin D and Calcium Supplementation. Aging Clin. Exp. Res. 2010, 22, 78–84. [Google Scholar] [CrossRef]
- van der Wielen, R.P.J.; de Groot, L.C.P.G.M.; van Staveren, W.A.; Löwik, M.R.H.; van den Berg, H.; Haller, J.; Moreiras, O. Serum Vitamin D Concentrations among Elderly People in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Sakuma, K. Vitamin D Signaling in Myogenesis: Potential for Treatment of Sarcopenia. BioMed Res. Int. 2014, 2014, 121254. [Google Scholar] [CrossRef]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Vitamin D and Muscle Sarcopenia in Aging. Methods Mol. Biol. 2020, 2138, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef]
- Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Shih, M.-H.; Chen, C.-D.; Yeh, S.-L. Effects of Adequate Dietary Protein with Whey Protein, Leucine, and Vitamin D Supplementation on Sarcopenia in Older Adults: An Open-Label, Parallel-Group Study. Clin. Nutr. 2021, 40, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Molecular Control of Iron Transport. J. Am. Soc. Nephrol. 2007, 18, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-Dependent Changes in Cellular Energy Metabolism: Influence on Citric Acid Cycle and Oxidative Phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- Xu, W.; Barrientos, T.; Andrews, N.C. Iron and Copper in Mitochondrial Diseases. Cell Metab. 2013, 17, 319–328. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron Status in the Elderly. Mech. Ageing Dev. 2014, 136–137, 22–28. [Google Scholar] [CrossRef]
- Buratti, P.; Gammella, E.; Rybinska, I.; Cairo, G.; Recalcati, S. Recent Advances in Iron Metabolism: Relevance for Health, Exercise, and Performance. Med. Sci. Sports Exerc. 2015, 47, 1596–1604. [Google Scholar] [CrossRef]
- Telford, R.D.; Sly, G.J.; Hahn, A.G.; Cunningham, R.B.; Bryant, C.; Smith, J.A. Footstrike Is the Major Cause of Hemolysis during Running. J. Appl. Physiol. 2003, 94, 38–42. [Google Scholar] [CrossRef]
- Monsen, E.R. Iron Nutrition and Absorption: Dietary Factors Which Impact Iron Bioavailability. J. Am. Diet Assoc. 1988, 88, 786–790. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers/[United Nations Children’s Fund, United Nations University, World Health Organization].—Version Details. Available online: https://trove.nla.gov.au/version/31276429 (accessed on 5 March 2018).
- Harvey, L.J.; Armah, C.N.; Dainty, J.R.; Foxall, R.J.; John Lewis, D.; Langford, N.J.; Fairweather-Tait, S.J. Impact of Menstrual Blood Loss and Diet on Iron Deficiency among Women in the UK. Br. J. Nutr. 2005, 94, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, A.; Koschizke, J.W.; Leitzmann, C.; Hahn, A. Dietary Iron Intake and Iron Status of German Female Vegans: Results of the German Vegan Study. Ann. Nutr. Metab. 2004, 48, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Saliba, A.N. Iron Overload in Thalassemia: Different Organs at Different Rates. Hematology Am. Soc. Hematol. Educ. Program 2017, 2017, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef]
- Jacobs, P.; Dommisse, J. The Plasma Ferritin Level as a Reliable Index of Body Iron Stores Following Intravenous Iron Dextran. J. Med. 1982, 13, 309–321. [Google Scholar]
- Yang, Z.; Dewey, K.G.; Lönnerdal, B.; Hernell, O.; Chaparro, C.; Adu-Afarwuah, S.; McLean, E.D.; Cohen, R.J.; Domellöf, M.; Allen, L.H.; et al. Comparison of Plasma Ferritin Concentration with the Ratio of Plasma Transferrin Receptor to Ferritin in Estimating Body Iron Stores: Results of 4 Intervention Trials. Am. J. Clin. Nutr. 2008, 87, 1892–1898. [Google Scholar] [CrossRef][Green Version]
- Pfeiffer, C.M.; Looker, A.C. Laboratory Methodologies for Indicators of Iron Status: Strengths, Limitations, and Analytical Challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S. [Google Scholar] [CrossRef]
- Soppi, E.T. Iron Deficiency without Anemia—A Clinical Challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Feelders, R.A.; Vreugdenhil, G.; Eggermont, A.M.; Kuiper-Kramer, P.A.; van Eijk, H.G.; Swaak, A.J. Regulation of Iron Metabolism in the Acute-Phase Response: Interferon Gamma and Tumour Necrosis Factor Alpha Induce Hypoferraemia, Ferritin Production and a Decrease in Circulating Transferrin Receptors in Cancer Patients. Eur. J. Clin. Investig. 1998, 28, 520–527. [Google Scholar] [CrossRef]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting Ferritin Concentrations for Inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Northrop-Clewes, C.A.; Knowles, J. The Use of Adjustment Factors to Address the Impact of Inflammation on Vitamin A and Iron Status in Humans123. J. Nutr. 2015, 145, 1137S–1143S. [Google Scholar] [CrossRef] [PubMed]
- Bainton, D.F.; Finch, C.A. The diagnosis of iron deficiency anemia. Am. J. Med. 1964, 37, 62–70. [Google Scholar] [CrossRef]
- Skikne, B.S.; Punnonen, K.; Caldron, P.H.; Bennett, M.T.; Rehu, M.; Gasior, G.H.; Chamberlin, J.S.; Sullivan, L.A.; Bray, K.R.; Southwick, P.C. Improved Differential Diagnosis of Anemia of Chronic Disease and Iron Deficiency Anemia: A Prospective Multicenter Evaluation of Soluble Transferrin Receptor and the STfR/Log Ferritin Index. Am. J. Hematol. 2011, 86, 923–927. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron Deficiency or Anemia of Inflammation?: Differential Diagnosis and Mechanisms of Anemia of Inflammation. Wien Med. Wochenschr. 2016, 166, 411–423. [Google Scholar] [CrossRef]
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123. [Google Scholar] [CrossRef]
- WHO|Worldwide Prevalence of Anaemia 1993–2005. Available online: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596657/en/ (accessed on 6 March 2018).
- Beard, J.L. Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–579S, discussion 580S. [Google Scholar] [CrossRef]
- Dziegala, M.; Josiak, K.; Kasztura, M.; Kobak, K.; von Haehling, S.; Banasiak, W.; Anker, S.D.; Ponikowski, P.; Jankowska, E. Iron Deficiency as Energetic Insult to Skeletal Muscle in Chronic Diseases. J. Cachexia Sarcopenia Muscle 2018, 9, 802–815. [Google Scholar] [CrossRef]
- Galy, B.; Ferring-Appel, D.; Sauer, S.W.; Kaden, S.; Lyoumi, S.; Puy, H.; Kölker, S.; Gröne, H.-J.; Hentze, M.W. Iron Regulatory Proteins Secure Mitochondrial Iron Sufficiency and Function. Cell Metab. 2010, 12, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Robach, P.; Cairo, G.; Gelfi, C.; Bernuzzi, F.; Pilegaard, H.; Viganò, A.; Santambrogio, P.; Cerretelli, P.; Calbet, J.A.L.; Moutereau, S.; et al. Strong Iron Demand during Hypoxia-Induced Erythropoiesis Is Associated with down-Regulation of Iron-Related Proteins and Myoglobin in Human Skeletal Muscle. Blood 2007, 109, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Boulton, F.E. The Myoglobin Content of Human Skeletal Muscle. Br. J. Haematol. 1973, 25, 281. [Google Scholar] [PubMed]
- Wang, J.; Huo, J.-S.; Sun, J.; Ning, Z.-X. Physical Performance of Migrant Schoolchildren with Marginal and Severe Iron Deficiency in the Suburbs of Beijing. Biomed. Environ. Sci. 2009, 22, 333–339. [Google Scholar] [CrossRef]
- Arsenault, J.E.; Mora-Plazas, M.; Forero, Y.; Lopez-Arana, S.; Jáuregui, G.; Baylin, A.; Gordon, P.M.; Villamor, E. Micronutrient and Anthropometric Status Indicators Are Associated with Physical Fitness in Colombian Schoolchildren. Br. J. Nutr. 2011, 105, 1832–1842. [Google Scholar] [CrossRef]
- Tsai, K.-Z.; Lai, S.-W.; Hsieh, C.-J.; Lin, C.-S.; Lin, Y.-P.; Tsai, S.-C.; Chung, P.-S.; Lin, Y.-K.; Lin, T.-C.; Ho, C.-L.; et al. Association between Mild Anemia and Physical Fitness in a Military Male Cohort: The CHIEF Study. Sci. Rep. 2019, 9, 11165. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Gillen, Z.M.; Mckay, B.D.; Bohannon, N.A.; Gibson, S.M.; Koehler, K.; Cramer, J.T. Sex-Specific Relationships among Iron Status Biomarkers, Athletic Performance, Maturity, and Dietary Intakes in Pre-Adolescent and Adolescent Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 42. [Google Scholar] [CrossRef]
- Juárez-Cedillo, T.; Basurto-Acevedo, L.; Vega-García, S.; Manuel-Apolinar, L.; Cruz-Tesoro, E.; Rodríguez-Pérez, J.M.; García-Hernández, N.; Pérez-Hernández, N.; Fragoso, J.M. Prevalence of Anemia and Its Impact on the State of Frailty in Elderly People Living in the Community: SADEM Study. Ann. Hematol. 2014, 93, 2057–2062. [Google Scholar] [CrossRef]
- Kim, T.H.; Hwang, H.-J.; Kim, S.-H. Relationship between Serum Ferritin Levels and Sarcopenia in Korean Females Aged 60 Years and Older Using the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLoS ONE 2014, 9, e90105. [Google Scholar] [CrossRef]
- Pires Corona, L.; Drumond Andrade, F.C.; de Oliveira Duarte, Y.A.; Lebrao, M.L. The Relationship between Anemia, Hemoglobin Concentration and Frailty in Brazilian Older Adults. J. Nutr. Health Aging 2015, 19, 935–940. [Google Scholar] [CrossRef]
- Moon, J.-H.; Kong, M.-H.; Kim, H.-J. Relationship between Low Muscle Mass and Anemia in Korean Elderly Men: Using the Korea National Health and Nutrition Examination Survey (KNHANES IV–V). J. Clin. Gerontol. Geriatr. 2015, 6, 115–119. [Google Scholar] [CrossRef]
- Ruan, Y.; Guo, Y.; Kowal, P.; Lu, Y.; Liu, C.; Sun, S.; Huang, Z.; Zheng, Y.; Wang, W.; Li, G.; et al. Association between Anemia and Frailty in 13,175 Community-Dwelling Adults Aged 50 Years and Older in China. BMC Geriatr. 2019, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Neidlein, S.; Wirth, R.; Pourhassan, M. Iron Deficiency, Fatigue and Muscle Strength and Function in Older Hospitalized Patients. Eur. J. Clin. Nutr. 2020, 75, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.J.; Pollock, N.; Whyte, G.P.; Richards, T.; Moore, B.; Busbridge, M.; Srai, S.K.; Otto, J.; Pedlar, C.R. Effect of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med. Sci. Sports Exerc. 2015, 47, 1399–1407. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Haas, J.D. Iron Supplementation Improves Energetic Efficiency in Iron-Depleted Female Rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215. [Google Scholar] [CrossRef]
- Garvican, L.A.; Saunders, P.U.; Cardoso, T.; Macdougall, I.C.; Lobigs, L.M.; Fazakerley, R.; Fallon, K.E.; Anderson, B.; Anson, J.M.; Thompson, K.G.; et al. Intravenous Iron Supplementation in Distance Runners with Low or Suboptimal Ferritin. Med. Sci. Sports Exerc. 2014, 46, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S. Iron Supplementation Prevents a Decline in Iron Stores and Enhances Strength Performance in Elite Female Volleyball Players during the Competitive Season. Appl. Physiol. Nutr. Metab. 2015, 40, 615–622. [Google Scholar] [CrossRef]
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.I.; Caballero-García, A.; Roche, E.; Fernández-Lázaro, D. Effect of Iron Supplementation on the Modulation of Iron Metabolism, Muscle Damage Biomarkers and Cortisol in Professional Cyclists. Nutrients 2019, 11, 500. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, Z.; Jiang, Q.; Hu, N.; Yu, B.; Zhu, L.; Shen, L.; Zhang, S.; Chen, L.; Chen, H.; et al. Vitamin D Alleviates Rotavirus Infection through a Microrna-155-5p Mediated Regulation of the TBK1/IRF3 Signaling Pathway In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 3562. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; López-Vancell, M.R.; Fernández de Córdova-Aguirre, J.C.; Rojas-Serrano, J.; Chavarría, A.; Velasco-Medina, A.; Velázquez-Sámano, G. Reduction of Respiratory Infections in Asthma Patients Supplemented with Vitamin D Is Related to Increased Serum IL-10 and IFNγ Levels and Cathelicidin Expression. Cytokine 2018, 108, 239–246. [Google Scholar] [CrossRef]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D Deficiency Is Associated with Inflammation in Older Irish Adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.S.; Ishimura, M.E.; de Oliveira Duarte, Y.A.; Bueno, V. Parameters of the Immune System and Vitamin D Levels in Old Individuals. Front. Immunol. 2018, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Alvarez, J.A.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Hao, L.; Brown, L.A.S.; Martin, G.S.; Ziegler, T.R. Impact of High-Dose Vitamin D3 on Plasma Free 25-Hydroxyvitamin D Concentrations and Antimicrobial Peptides in Critically Ill Mechanically Ventilated Adults. Nutrition 2017, 38, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Shoemaker, M.E.; Gawel, S.; Davis, G.J.; Luo, M.; Mustad, V.A.; Cramer, J.T. Biomarker Changes in Response to a 12-Week Supplementation of an Oral Nutritional Supplement Enriched with Protein, Vitamin D and HMB in Malnourished Community Dwelling Older Adults with Sarcopenia. Nutrients 2022, 14, 1196. [Google Scholar] [CrossRef]
- Song, S.-N.J.; Tomosugi, N.; Kawabata, H.; Ishikawa, T.; Nishikawa, T.; Yoshizaki, K. Down-Regulation of Hepcidin Resulting from Long-Term Treatment with an Anti-IL-6 Receptor Antibody (Tocilizumab) Improves Anemia of Inflammation in Multicentric Castleman Disease. Blood 2010, 116, 3627–3634. [Google Scholar] [CrossRef]
- Sim, J.J.; Lac, P.T.; Liu, I.L.A.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D Deficiency and Anemia: A Cross-Sectional Study. Ann. Hematol. 2010, 89, 447–452. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernandez-Lázaro, D. Effects of Vitamin D Supplementation on Haematological Values and Muscle Recovery in Elite Male Traditional Rowers. Nutrients 2018, 10, 1968. [Google Scholar] [CrossRef]
- Lee, J.A.; Hwang, J.S.; Hwang, I.T.; Kim, D.H.; Seo, J.-H.; Lim, J.S. Low Vitamin D Levels Are Associated with Both Iron Deficiency and Anemia in Children and Adolescents. Pediatr. Hematol. Oncol. 2015, 32, 99–108. [Google Scholar] [CrossRef]
- Syed, S.; Michalski, E.S.; Tangpricha, V.; Chesdachai, S.; Kumar, A.; Prince, J.; Ziegler, T.R.; Suchdev, P.S.; Kugathasan, S. Vitamin D Status Is Associated with Hepcidin and Hemoglobin Concentrations in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1650–1658. [Google Scholar] [CrossRef]
- USDA Ground Beef Calculator: USDA ARS. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/ground-beef-calculator/ (accessed on 29 August 2019).
- Valenzuela, C.; de Romaña, D.L.; Olivares, M.; Morales, M.S.; Pizarro, F. Total Iron and Heme Iron Content and Their Distribution in Beef Meat and Viscera. Biol. Trace Elem. Res. 2009, 132, 103–111. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma Concentrations of 25-Hydroxyvitamin D in Meat Eaters, Fish Eaters, Vegetarians and Vegans: Results from the EPIC-Oxford Study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US). Lifestyle Approaches to Promote Bone Health; Office of the Surgeon General: Bethesda, MD, USA, 2004.
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D Fortification in the United States and Canada: Current Status and Data Needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S. [Google Scholar] [CrossRef] [PubMed]
- Yetley, E.A. Assessing the Vitamin D Status of the US Population. Am. J. Clin. Nutr. 2008, 88, 558S–564S. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.; James, A.P.; Cunningham, J.; Strobel, N.; Lucas, R.M.; Kiely, M.; Nowson, C.A.; Rangan, A.; Adorno, P.; Atyeo, P.; et al. Vitamin D Composition of Australian Foods. Food Chem. 2021, 358, 129836. [Google Scholar] [CrossRef]
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of Vitamin D Fortified Foods on Bone Markers and Muscle Strength in Women of Pakistani and Danish Origin Living in Denmark: A Randomised Controlled Trial. Nutr. J. 2019, 18, 82. [Google Scholar] [CrossRef]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein after Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef]
- Hanach, N.I.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef]
- Radavelli-Bagatini, S.; Zhu, K.; Lewis, J.R.; Dhaliwal, S.S.; Prince, R.L. Association of Dairy Intake with Body Composition and Physical Function in Older Community-Dwelling Women. J. Acad Nutr. Diet 2013, 113, 1669–1674. [Google Scholar] [CrossRef]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between Dietary Nutrient Intake and Sarcopenia in the SarcoPhAge Study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025, 9th ed. Available online: https://www.dietaryguidelines.gov/ (accessed on 15 February 2022).
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Diet Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef]
- Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 88–94. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F. Total Protein, Animal Protein and Physical Activity in Relation to Muscle Mass in Middle-Aged and Older Americans. Br. J. Nutr. 2013, 109, 1294–1303. [Google Scholar] [CrossRef]
- Hawley, A.L.; Liang, X.; Børsheim, E.; Wolfe, R.R.; Salisbury, L.; Hendy, E.; Wu, H.; Walker, S.; Tacinelli, A.M.; Baum, J.I. The Potential Role of Beef and Nutrients Found in Beef on Outcomes of Wellbeing in Healthy Adults 50 Years of Age and Older: A Systematic Review of Randomized Controlled Trials. Meat Sci. 2022, 189, 108830. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein Intake Distribution Pattern Does Not Affect Anabolic Response, Lean Body Mass, Muscle Strength or Function over 8 Weeks in Older Adults: A Randomized-Controlled Trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef]
- Formica, M.B.; Gianoudis, J.; Nowson, C.A.; O’Connell, S.L.; Milte, C.; Ellis, K.A.; Daly, R.M. Effect of Lean Red Meat Combined with a Multicomponent Exercise Program on Muscle and Cognitive Function in Older Adults: A 6-Month Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 112, 113–128. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1429. [Google Scholar] [CrossRef]
- ten Haaf, D.S.M.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of Protein Supplementation on Lean Body Mass, Muscle Strength, and Physical Performance in Nonfrail Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef]
- Charlton, K.; Walton, K.; Batterham, M.; Brock, E.; Langford, K.; McMahon, A.; Roodenrys, S.; Koh, F.; Host, A.; Crowe, R.; et al. Pork and Chicken Meals Similarly Impact on Cognitive Function and Strength in Community-Living Older Adults: A Pilot Study. J. Nutr. Gerontol. Geriatr. 2016, 35, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Parker, B.; Dyer, K.A.; Davis, C.R.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. A Comparison of Regular Consumption of Fresh Lean Pork, Beef and Chicken on Body Composition: A Randomized Cross-over Trial. Nutrients 2014, 6, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hurst, C.; Dismore, L.; Aspray, T.; Stevenson, E.; Witham, M.D.; Sayer, A.A.; Robinson, S. Milk for Skeletal Muscle Health and Sarcopenia in Older Adults: A Narrative Review. Clin. Interv. Aging 2020, 15, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A.; et al. Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients 2021, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Huschtscha, Z.; Parr, A.; Porter, J.; Costa, R.J.S. The Effects of a High-Protein Dairy Milk Beverage With or Without Progressive Resistance Training on Fat-Free Mass, Skeletal Muscle Strength and Power, and Functional Performance in Healthy Active Older Adults: A 12-Week Randomized Controlled Trial. Front. Nutr. 2021, 8, 644865. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Study Participants (Mean ± SD) | Measurements | Conclusions |
|---|---|---|---|
| Athletes | |||
| Koundourakis et al., 2014, Greece [84] | Caucasian male soccer players, mean age: 25.6 ± 6.2 years n = 67 | Serum 25(OH)D concentrations and performance of the squat jump, countermovement jump, sprint performance, and VO2max, | Concentrations of serum 25(OH)D were positively associated with an increase in performance during the squat jump, counter movement jump, VO2max (r = 0.394–0.740), and negatively associated with sprint performance at 10 m and 20 m (r = −410–−0.649) before the soccer season (pre) and during the six-week off-season period (post) (p > 0.01) |
| Fitzgerald et al., 2014, United States [89] | Professional male ice hockey players, mean age: 20.1 ± 1.5 n = 52 | Serum 25(OH)D concentrations and performance during skate treadmill graded exercise testing | Concentration of serum 25(OH)D was not associated with VO2max, max heart rate, peak respiratory exchange ratio, final stage completed, and total exercise time completed during the graded exercise test (p = 0.22–0.71) |
| Hamilton et al., 2014, Qatar [85] | Male soccer players stratified based on 25(OH)D concentration (<10–>30 ng/mL) mean age: 24.4 ± 8.1 n = 342 | Serum 25(OH)D concentrations and lower limb isokinetic performance (peak torque) | Soccer players with serum 25(OH)D levels >30 ng·mL−1 displayed 17% greater concentric and 13% greater eccentric hamstring peak torque in the non-dominant leg compared to those with 25(OH)D levels of ≤10 ng·mL−1 (p = 0.015–0.021) |
| Forney et al., 2014, United States [90] | Recreationally active college students, mean age: 23.0 ± 0.7 years n = 39; n = 20 males, n = 19 females | Serum 25(OH)D concentrations and performance during aerobic testing (Bruce Protocol [VO2max]), anaerobic power (Wingate), strength (upright bench press, bicep curl, triceps pushdown, leg curl, leg extension, and upright row [8-repetition max]), and power (maximal vertical and horizontal jump). | Concentrations of serum 25(OH)D were associated with VO2max (r = 0.360, p = 0.018), however, there was no association of 25(OH)D with anaerobic power, muscular strength, and muscular power |
| Ksiazek et al., 2016, Poland [86] | Polish premier league soccer players, mean age: 22.7 ± 5.3 years n = 43 | Serum 25(OH)D concentrations and performance during hand grip strength, lower-limb isokinetic strength, and aerobic performance (VO2max) | Soccer players with serum 25(OH)D concentrations >20 ng·mL−1 displayed a 12% greaterpeak torque compared to those with 25(OH)D levels of ≤20 ng·mL−1 (p ≤ 0.05) A significant positive correlation between 25(OH)D levels and concentric leg extension peak torque (r = 0.410, p < 0.040). |
| Zeitler et al., 2018, Austria [87] | Healthy recreational athletes age: 18–65 years; 40.5 ± 9.2 (males), 38.7 ± 9.8 (females) n = 581; n = 287 males, n = 284 females | Serum 25(OH)D concentrations and performance during maximal and submaximal treadmill running | Males with serum 25(OH)D levels <20 ng·mL−1 had significantly lower submaximal performance on the treadmill compared with those with normal 25(OH)D levels (p = 0.045) Associations between 25(OH)D levels and maximal and submaximal treadmill performance in males and females displayed no significant differences |
| Most et al., 2021, Germany [88] | 88 male handball and 24 male ice hockey players stratified based on 25(OH)D concentration (<30 and >30 ng/mL) mean age: 26.1 ± 5.2 years n = 112 | Serum 25(OH)D concentrations and performance during a maximal cycle ergometer test (W/kg) | Athletes with serum 25(OH)D levels <30 ng·mL−1 achieved an 11% higher maximal aerobic power compared to those with insufficient levels (>30 ng·mL−1) (p = 0.030) |
| Older Adults | |||
| Marantes et al., 2011, United States [91] | Age-stratified, random sample of males and females ages 21–97 years old, mean age: 57.0 ± 18.0 years. n = 700; n = 325 males, n = 375 females | Concentrations of serum 25(OH)D and 1,25(OH)2D, fat mass and muscle mass, handgrip strength, isometric leg extension strength | Lower serum 25(OH)D levels were inversely associated with greater fat mass, while lower 1,25(OH)2D levels were positively associated with lower muscle mass and muscle strength in males and females |
| Mastaglia et al., 2011, Argentina [4] | Females over age 70 years attending bone health assessments at the Buenos Aires Hospital, mean age: 71.0 ± 4.0 years. n = 54 | Lower limb lean mass, muscle function (walking speed, chair stand, balance), muscle strength (hip flexors and abductors, leg extensors), levels of calcium, phosphorus, serum 25(OH)D concentrations, and urinary calcium and creatinine | Older adults with serum 25(OH)D levels ≥20 ng·mL−1 (n = 25) had 11% better scores on muscle function tests, 0.4 s faster walking speed, and were 14% and 13% stronger in tests of leg extension and hip abduction strength, respectively, than those with serum 25(OH)D levels <20 ng·mL−1 (n = 29). |
| Toffanello et al., 2012, Italy [20] | Older adults aged 65–98 years from a large cohort study in Italy (Pro.V.A), mean age: 75.6 ± 7.5. n = 2694 | Physical Performance (balance, chair stand, gait speed, 6 min walking test), handgrip strength, quadriceps strength, levels of PTH andserum 25(OH)D | Levels of serum 25(OH)D were positively associated with the chair stand, gait speed, 6 min walking test, and handgrip strength (p < 0.001). Concentrations of 100 nmol·L−1 was determined to be related to greater muscle function |
| Tieland et al., 2013, The Netherlands [92] | Older adults >age 65 years who were considered frail or pre-frail, mean age: 79.0 ± 7.8 years. n = 127 | Serum 25(OH)D, creatinine, glucose, and insulin concentrations, dietary intake, body composition, leg strength (leg press and leg extension), handgrip strength, and physical performance (SPPB) | Levels of serum 25(OH)D were associated with appendicular skeletal muscle mass (β = 0.012, p = 0.050). Levels of 25(OH)D and vitamin D intake were positively associated with higher SPPB scores (β = 0.020–0.180, p = 0.020–0.038). |
| Gumieriro et al., 2015, Brazil [93] | Older adults with a hip fracture and older than 65 years admitted to hospital, mean age: 80.0 ± 7.0 years. n = 100 | Serum 25(OH)D concentrations, handgrip strength, mid-upper arm muscle circumference, length of hospital stay, mortality | Participants with lower serum 25(OH)D concentrations had 40% lower handgrip strength and 52% higher mortality rate. Levels of serum 25(OH)D predicted handgrip strength when adjusted for age and sex (β = −1.945, p = 0.020). |
| Iolascon et al., 2015, Italy [94] | Post-menopausal females aged 50 years or older, mean age: 65.9 ± 7.7 years. n = 80; n = 46 with low vitamin D levels, n = 34 with normal vitamin D levels | Handgrip strength, isometric leg extension strength, SPPB, gait speed, serum 25(OH)D concentrations | Serum 25(OH)D concentrations were positively associated with handgrip strength (r = 0.234), leg extensor strength (r = 0.234), and inverselyl associated with time to complete physical performance tests such as walking speed (r = −0.457) and chair stand (r = −0.564). Those with serum 25(OH)D levels ≥30 ng·mL−1 had better results for handgrip strength, leg extension strength, and SPPB scores (p = 0.001–0.003) |
| Verlaan et al., 2017, The Netherlands [6] | Subsample of sarcopenic participants from the PROVIDE study, which were ≥65 years old, mean age: 71.0 ± 4.0 years. n = 132; sarcopenic participants (n = 66) and non-sarcopenic controls (n = 66) | Body composition (appendicular muscle mass and fat mass), muscle strength and function (handgrip strength, SPPB), ADLs, frailty status, nutritional status, and levels of serum 25(OH)D, vitamin B12, and folate | Serum 25(OH)D levels were not different between groups; however, there was a greater prevalence of vitamin B12 deficiency in sarcopenic individuals |
| Aspell et al., 2019, Ireland [95] | Older adults aged 60 years or older from the English Longitudinal Study of Aging, mean age: 69.8 ± 6.9 years. n = 4157 | Serum 25(OH)D concentrations, handgrip strength, SPPB | A greater number of older adults had low handgrip strength and SPPB score in the lowest serum 25(OH)D concentration quintile compared to the others quintiles (p < 0.0001–0.01). After adjusting for confounding factors, vitamin D deficiency was positively associated with low SPPB score [OR 1.65, p < 0.01) and positively predicted low handgrip strength (OR 1.44, p < 0.001). |
| Conzade et al., 2019, Germany [96] | Older adults aged 65 years or older, mean age: 75.7 ± 6.6 years. n = 702 | Muscle mass, handgrip strength, gait speed, TUG, Serum 25(OH)D levels | Low levels of serum 25(OH)D (<25 nmol·L−1) were had a 0.94% greater loss in muscle mass and 3.06% increase in time to complete TUG compared to higher levels (≥50 nmol·L−1) but was not related to change in handgrip strength or gait speed. |
| Vaes et al., 2019, The Netherlands [97] | Older adults 65 years or older that attended the screening visit of two clinical trials (D-DOSE and D-FIT), mean age: 74.0 ± 6.0 years. n = 756 | Serum 25(OH)D levels, handgrip strength, gait speed, TUG, isometric leg extension strength | Older adults with lower serum 25(OH)D levels (<50 nmol·L−1 and 50–75 nmol·L−1) had inverse relationships for time to complete TUG (β = 0.73–0.83, p = 0.01–0.05) and lower scores for gait speed (β = −0.04, p < 0.05), but there no relationships observed with handgrip or leg extension strength. Those with lower 25(OH)D levels were also more likely to be categorized as frail. |
| Youth | |||
| Dong et al., 2010, United States [98] | Adolescents aged 14–18 years old, mean age: 16.2 ± 1.2 years. n = 599 | Levels of serum 25(OH)D, time spent in physical activity, cardiovascular fitness determined from oxygen consumption during a treadmill test | Positive associations were found between serum 25(OH)D levels and unadjusted and adjusted vigorous physical activity (r = 0.132–0.139, p = 0.002–0.01) and maximal oxygen consumption (r = 0.100–0.212, p < 0.01–0.025). |
| Gracia-Marco et al., 2012, Europe (Sweden, Greece, Italy, Spain, Hungary, Belgium, France, Germany, Austria) [99] | Adolescents aged 12.5–17.5 years old across Europe that completed the blood sample analysis as part of the HELENA-CSS study, mean age: 15.0 ± 1.2 years. n = 1089; males, n = 509, females, n = 580 | Standing long jump, 20 m shuttle run to estimate VO2max, red blood cell parameters, biomarkers of iron status (sTfR and ferritin), other micronutrients (vitamins A, E, C, B6, and B12, folate, and serum 25(OH)D) concentrations | Concentrations of serum25(OH)D were positively correlated with estimated VO2max (from 20 m shuttle run) (β = 0.091, p = 0.030) and standing broad jump (β = 0.125, p = 0.010) in female adolescents. |
| Valtueña et al., 2013, Europe (Sweden, Greece, Italy, Spain, Hungary, Belgium, France, Germany, Austria) [100] | European adolescents ages 12.5–17.5 years, mean age: 14.9 ± 1.2 years. n = 3000, n = 1006 had samples for 25(OH)D and included in the analysis | Serum 25(OH)D concentrtions, BMI, fat mass, fat-free mass, fat mass index, fat-free mass index, 20 m shuttle run to estimate VO2max, handgrip strength, standing long jump | In males, VO2max had a positive correlation with serum 25(OH)D concentrations (r = 0.108, p = 0.022). Linear regression demonstrated a positive association between VO2max and serum 25(OH)D concentrations (β = 0.189, p = 0.002) and a negative associaton between BMI and serum 25(OH)D concentrations (β = −0.125, p = 0.023). In females, handgrip strength was positively associated with serum 25(OH)D concentrations (β = 0.168, p = 0.002). Greater long jump performance was a positively ssociated with higher serum 25(OH)D levels in males. |
| Carson et al., 2015, Ireland [101] | Males and females ages 12 and 15 years from Northern Ireland. n = 1015; (12-year-old males, n = 266; 12-year-old females, n = 260; 15-year-old males, n = 239; 15-year-old girls, n = 250) | Serum 25(OH)D concentrations, BMI, fat mass, fat-free mass, fat-free mass index, handgrip strength, jump height, jump power, 20 m shuttle run to estimate VO2max | Serum 25(OH)D concentrations in the highest tertile (>51 nmol·L−1) were positively associated with greater muscle strength in the 15-year-old males (β = 3.90, p < 0.001), but this relationship was not present in any other group categorized by age or sex. There were no associations between serum 25(OH)D concentrations and muscle mass, muscle power or VO2max |
| Bezrati et al., 2016, Tunisia [102] | Physically active males aged 7–15 years, mean age: 11.4 ± 2.0, 11.8 ± 2.2, and 11.0 ± 1.9 years for vitamin D deficient, insufficient, and sufficient, respectively. n = 125 | Serum 25(OH)D concentrations, body fat percentage, vertical jump, broad jump, triple hop, sprint agility, and trunk force | Serum 25(OH)D levels were positively associated with trunk force, vertical jump, and broad jump (β = 0.165–0.552, p < 0.001) and inversely related to 10 m sprint, 20 m sprint, and shuttle run (β = −4.330–06.436, p < 0.001). |
| Blakeley et al., 2018, United States [103] | Children in fourth through eighth grades in Boston area, mean age: 11.2 ± 1.3 years. n = 350 | Handgrip strength, levels of HDL cholesterol, triglycerides, and serum 25(OH)D concentrations, BMI | There were no associations between handgrip strength and serum 25(OH)D concentrations. |
| Wakayo et al., 2018, Ethiopia [104] | Ethiopian school-age children 11–18 years old, median age: 15 years. n = 174 | Serum 25(OH)D concentrations, handgrip strength | There was no association between handgrip strength and serum 25(OH)D levels |
| Author, Year, Country | Study Participants | Supplemental Treatment | Duration | Measurements | Conclusions |
|---|---|---|---|---|---|
| Athletes | |||||
| Shanely et al., 2014, USA [111] | Professional football, tennis, lacrosse, baseball players, and professional wrestlers. n = 33 mean age: 16.3 ± 0.25 years. | 600 IU·d−1 (Portobello mushroom powder) vs. Placebo | 6-weeks | Serum 25(OH)D concentrations, isometric deadlift strength and vertical jump performance | No associations between serum 25(OH)D concentrations with isometric muscle strength or vertical jump performance. Isometric strength and vertical jump performance was not different between supplement and placebo group. |
| Close et al., 2013, UK [112] | Professional rugby, soccer, flat jockeys and national hunt jockeys. n = 61 | 5000 IU·month−1 vs. placebo | 6-weeks | Serum 25(OH)D concentrations, isometric strength, 10 m sprint performance and vertical jump performance | There was approximately a 3-inch increase in vertical jump height (p = 0.008) and 0.04-s time improvement in 10 m sprint performance (p = 0.008) in the supplementation group with no change in the placebo. |
| Close et al., 2013, UK [113] | Professional rugby and soccer players. n = 30 | 20,000 or 40,000 IU·week−1 vs. placebo | 6 or 12-weeks | Serum 25(OH)D concentrations, dynamic strength (1-RM bench press, 1-RM leg press) and vertical jump performance | Serum 25(OH)D concentrations increased in both 6-week and 12 week periods (p < 0.0005) with concentrations higher after 6-weeks of 40,000 IUs compared to 20,000 IUs (p = 0.016). However, serum 25(OH)D concentrationswere not associated with improvements in 1-RM bench press, 1-RM leg press and vertical jump performance following 6 or 12-week of supplementation. |
| Jastzebska et al., 2016, Poland [114] | Well trained soccer players. n = 36 | 5000 IU·d−1 vs. placebo | 8-weeks | Serum 25(OH)D concentrations, 30-s Wingate test for peak power, sprint tests for 5, 10, 20, and 30 m, squat jump, countermovement jump | Supplementation group displayed an increase in all power tests except for 30 m sprint time (p < 0.001); however, mean change scores were not different between supplementation and placebo groups. |
| Todd et al., 2016, Ireland [115] | Gaelic football players. n = 42 | 3000 IU·d−1 vs. placebo | 12-weeks | Serum 25(OH)D concentrations and VO2max | Serum 25(OH)D concentrations increased following supplementation, however, supplementation had no effect on VO2max. |
| Wyon et al., 2016, UK [109] | Judo athletes. n = 22 | 150,000 IU once vs. placebo | 8 days | Serum 25(OH)D concentrations, maximal isokinetic leg extension and leg curls | Supplement group displayed a 13% increase in muscle strength following 8 days of supplementation (p ≤ 0.001). |
| Fairbairn et al., 2017, New Zealand [110] | Professional rugby players. n = 57 | 50,000 IU once every 2 weeks | 11–12 weeks | Serum 25(OH)D concentrations, 30 m sprint performance and maximal dynamic strength (weighted chin-up 1-RM, bench pull 1-RM, and bench press 1-RM) | No difference in 30 m sprint performance; however, there was a 5.5 kg increase in dynamic strength (weighted chin-up 1-RM), (p = 0.002). |
| * Lips et al., 2010, USA, Mexico, The Netherlands, Germany, Canada [116] | Older adults aged 70 years or older who were ambulatory and had 25(OH)D levels between 6 and 20 ng·ml−1, mean age: 77.6 ± 6.6 and 78.5 ± 62.0 years in the placebo and experimental groups, respectively. n = 226 randomized, 202 completed the study. | 8400 IU vitamin D3 weekly (n = 105) or placebo (n = 97) | 16 weeks | Serum 25(OH)D concentrations, postural sway, SPPB, and levels PTH | 25(OH)D levels increased from approximately 14 to 26 ng·mL−1 (p < 0.001) in the supplementation group; however, there were no changes in postural sway or SPPB scores for either group |
| ** Ceglia et al., 2013, USA [82] | Older females aged 65 years or older who were ambulatory, community-dwelling, and postmenopausal, mean age: 78.0 ± 5.0 years. n = 24 randomized, 21 included in analysis | 4000 IU vitamin D3 (n = 11) or placebo (n = 13) | 4 months | Serum 25(OH)D concentrations, leg extension strength, muscle fiber type and intramyonuclear VDR from biopsies of the vastus lateralis | The supplementation group had a much greater increase in 25(OH)D levels after 4 months (36.4 vs. 4.2 nmol·L−1 increase, p < 0.001), as well as a 10.6% increase in total muscle fiber cross-sectional area (p = 0.048) and 29.7% increase in VDR concentration (p = 0.025) compared to changes of −7.4% and 7.8%, respectively, in the placebo group. |
| Lagari et al., 2013, USA [117] | Older adults aged 65–95 years who were ambulatory, and community dwelling, mean age: 73.4 ± 6.4 years. n = 105 randomized, n = 86 participated in sub-study | 400 IU or 2000 IU vitamin D3 daily | 6 months | Physical performance (gait speed, timed sit-to-stand, single leg balance, gallon jug test, handgrip), body composition, levels of serum 25(OH)D, calcium, creatinine, and spot urine calcium | There was no improvement in physical performance for either group; however, supplementation was more effective in those with low baseline 25(OH)D levels (<30 ng·dL−1. The relative change in serum 25(OH)D (%) was positively associated to change in chair stand test score (5.1%, p = 0.033) and inversely associated with an increase in fat mass (p = 0.027). |
| Bauer et al., 2015, The Netherlands [118] | Older adults aged 65 years or older with mild to moderate physical limitations and low skeletal muscle index, mean age: 77.3 ± 6.7 and 78.1 ± 7.0 years for the experimental and placebo group, respectively. n = 380 were randomized, 302 completed all three study visits | Active control product (20 g whey protein, 3 g leucine, 9 g carbohydrate, 3 g fat, 800 IU vitamin D) n = 184, or isocaloric control, n = 196 consumed twice daily | 13 weeks | Handgrip strength, SPPB, appendicular muscle mass, serum 25(OH)D concentrations | Handgrip strength increased by 0.79 kg in 13 weeks in the active group (p = 0.005), with no significant increase in the control. The SPPB scores increased in both groups (p < 0.001), with chair stand time improving more in the active group (p = 0.018). An estimated mean difference of 0.17 kg of appendicular muscle mass showed a significant increase over the placebo (p < 0.001). |
| Cangussu et al., 2015, Brazil [119] | Post-menopausal females aged 50–65 years, mean age: 55.6 ± 6.6 years. n = 160 randomized and included as intention to treat, n = 140 analyzed per protocol | 1000 IU Vitamin D3 (n = 80) or identical placebo (n = 80) | 9 months | Handgrip strength, Chair stand, lean body mass and fat mass assessed by DXA, serum 25(OH)D concentrations, creatinine, calcium, and parathyroid hormone levels | The supplementation group had a 45.4% increase in serum 25(OH)D levels at 9 months compared to the 18.5% decrease seen in the placebo group. Lean mass decreased 6.8% over 9 months only in the placebo group (p = 0.030). The supplementation group had a 25.3% increase in chair stand performance, whereas there were no changes in the placebo (p < 0.0001). Neither group had a change in handgrip strength. |
| * Apaydin et al., 2018, Turkey [120] | Postmenopausal females ages 50–68 years with vitamin D levels <20 ng·mL−1., mean age: 51.6 ± 5.8 and 51.58 ± 5.54 years in the daily and single dose groups, respectively. n = 60 | 800 IU daily (n = 32) or a single bolus of 300,000 IU (n = 28) of vitamin D3 | 3 months | Serum 25(OH)D concentrations, muscle strength of the quadriceps and hamstrings | There were no differences in muscle strength between groups; however, the daily dose group showed greater non-significant increases over time. Serum 25(OH)D levels increased in both groups but were higher in the single dose compared to the daily dose group. |
| ** Vaes et al., 2018, The Netherlands [121] | Older adults aged 65 years or older with 25(OH)D levels between 20 and 50 nmol·L−1 and were considered frail or prefrail, mean age: 74.0 ± 6.0 years. n = 78 | Daily supplements of 10 μg 25(OH)D3 (n = 26), 20 μg vitamin D3 (n = 24), or a placebo (n = 25) | 6 months | Muscle strength (leg extension and flexion, handgrip strength), Physical performance (TUG, SPPB, postural sway), serum 25(OH)D concentrations, muscle fiber type from biopsies of the vastus lateralis, Body composition (appendicular lean mass, BMI) | The two supplementation groups had an increase in serum 25(OH)D levels over time, with the 10 μg 25(OH)D3 showing the greatest increase (p < 0.01). There were no differences between groups for muscle strength or performance |
| * Hajj et al., 2019, Lebanon [122] | Older adults who were pre-sarcopenic and vitamin D deficient, mean age: 73.3 ± 2.1 years. n = 128 | vitamin D supplementation of 10,000 IU of vitamin D3 (n = 64) or a placebo (n = 64) | 6 months | Handgrip strength, appendicular skeletal muscle mass, serum 25(OH)D concentrations | The supplement group had a greater change in serum 25(OH)D levels (10.13 to 27.98 ng·mL−1, p < 0.001) compared to the placebo (10.56 to 15.71 ng·mL−1, p < 0.001). Handgrip strength increased approximately 0.85 kg (p = 0.007), and appendicular skeletal muscle mass increased 0.65 kg (p = 0.001) at 6 months, whereas the placebo group had no differences over time. |
| Molmen et al., 2021, Norway [123] | Older adults with healthy lung function or diagnosed with COPD, ages 65–77, mean age: 68.0 ± 5.0 years. n = 95 enrolled, 78 completed | Vitamin D3 supplementation of 10,000 IU per day for two weeks and 2000 IU per day for the remainder (n = 34) or the study or a placebo (n = 44) | 12 weeks of vitamin D supplementation only, followed by 13 weeks of supplementation + resistance training | One repetition maximum (1 RM) of leg extension and leg press, number of repetitions at 50% of 1 RM, isokinetic peak torque, VO2max, Wmax, sit-to-stand, 6 min step test, muscle thickness, leg lean mass, blood analysis for total testosterone, cortisol, growth hormone, IGF-1, SHBG, androstenedione, serum 25(OH)D concentrations, PTH, calcium, albumin, creatinine, creatine kinase AST, CRP, triglycerides, LDL, HDL, thyroid hormones, and iron status variables, and muscle fiber cross-sectional area and nuclei number from biopsies. | Overall, supplementation resulted in a 42 nmol·L−1 increase in serum 25(OH)D concentrations (p < 0.001) but did not lead to any additional improvement in training-related effects. |
| Youth | |||||
| ** Ward et al., 2010, UK [124] | Post-menarchal females ages 12–14 years, mean age: 13.8 ± 0.6 years. n = 73, n = 72 completed intervention | 4 doses per year of 150,000 IU vitamin D2 (n = 36) or placebo (n = 36) | 12 months | Muscle force, power velocity, and jumping height during countermovement vertical jumps, grip strength, serum 25(OH)D concentrations, PTH, bone mineral density | Serum 25(OH)D concentrations increased by 12.3 nmol·L−1 (p < 0.001) in the intervention group while decreasing by −0.8 nmol·L−1 in the placebo group. Mean PTH decreased in both groups. There were no significant changes between groups for muscle parameters or bone mineral density. |
| Wright et al., 2018, USA [125] | Children age 9–13 years in the United States, mean age: 11.3 ± 1.2 years. n = 324 | 0, 400, 1000, 2000, or 4000 IU vitamin D3 per day | 12 weeks | Serum 25(OH)D and 1,25(OH)2D concentrations, body composition (fat-free mass, fat mass, body fat percent, forearm and calf muscle cross-sectional area, muscle density, intramuscular adipose tissue, and handgrip strength. | Changes in muscle mass and strength over the 12 weeks were not related to changes in 25(OH)D, even with a 34.9% (p < 0.001) increase in serum 25(OH)D levels. However, increases in 25(OH)D were inversely associated with forearm intramuscular adipose tissue (r = −0.17, p = 0.029). |
| Mortensen et al., 2019, Denmark [126] | Children aged 4–8 years in Copenhagen, mean age: 6.6 ± 1.5 years. n = 117 | 10 μg·day−1 (n = 38), 20 μg·day−1 (n = 39), or a placebo (n = 40) | 20 weeks | Handgrip strength. BMI, fat mass index, fat-free mass index, and serum 25(OH)D, IGF, and IGF-binding protein concentrations | At baseline, serum 25(OH)D concentrations were positively associated with handgrip strength (non-adjusted: β = 0.73–0.74, p = 0.005–0.006, adjusted: β = 0.38–0.39, p = 0.022–0.025), fat-free mass index (non-adjusted: β = (0.26, p = 0.001, adjusted: β = 0.19, p = 0.006), and IGF binding protein (β = 0.19, p = 0.010) in females. Levels of serum 25(OH)D decreased by 24.2 μg·day−1 in the placebo group and increased by 4.9–17.4 μg·day−1 (p < 0.001) in both intervention groups. There were no differences between groups after the intervention for handgrip strength or fat-free mass; however, IGF-1 was higher in the 20 μg·day−1 group compared to the placebo |
| Author, Year, Country | Study Participants | Measurements | Conclusions |
|---|---|---|---|
| Athletes | |||
| DellaValle & Hass, 2012, USA [46] | Female rower athletes n = 165, n = 44 of rowers were identified as iron depleted without anemia (IDNA) [serum ferritin < 20.0 μg·L−1]; n = 16, as anemic (hemoglobin < 12.0 g·dL−1). | Physical performance of 48 nonanemic rowers (n = 24 normal, n = 24 depleted) was assessed during a 4 km rowing event (VO2max, time and energetic efficiency), ferritin, sTfR, and Hb levels | Rowers with IDNA had 0.3 L·min−1 lower VO2max and VO2peak (p = 0.02–0.03) and higher lactate concentration during a 4 km row assessment (p = 0.02). Relationships between iron status and endurance performance (VO2max, VO2peak, time, and energetic efficiency), between groups was dependent on the rowers’ training load. A positive correlation was present between VO2peak and serum ferritin (r = 0.29, p = 0.05), but no other measures of iron status with performance. IDNA may influence the training load of athletes affecting performance. |
| Tsai et al., 2019, Taiwan [170] | Males Taiwanese Military n = 3666 | 3000 m run test, 2 min sit-ups and 2 min push-up test, levels of Hb and hematocrit | After adjusting for age, service occupation, BMI, waist size, and blood pressure, mild anemic males were more likely to be the worst 10% performers in the 3000 m run test (Odds Ratios 1.47, p = 0.043). However, mild anemic males had a higher possibility to be the best 10% performers in the 2 min push-ups test (Odds Ratio 1.68, p = 0.001). There was no associations between anemic status and 2 min sit-up test. |
| Shoemaker et al., 2019, USA [171] | Male (n = 179; mean age 12.0 ± 2.1 years) and female (n = 70; mean age 12.0 ± 2.2 years) youth athletes | Athletic performance (vertical jump, broad jump, agility drill times, 20-yard dash time, power push up force), dietary intakes, levels of ferritin, sTfR, and Hb | Athletic performance was consistently related to Hb in males (r = 0.237–0.375, p < 0.001–0.05) and with sTfR (r = 0.521–0.649, p < 0.001–0.004) and iron intake (r = 0.397–0.568, p = 0.001–0.027) in females. |
| Older Adults | |||
| Juárez-Cedillo et al., 2014, Mexico [172] | Older adults from the Study on Aging and Dementia, mean age by Hb quintiles: 71.7 ± 7.6, 70.8 ± 7.0, 71.6 ± 7.7, 69.7 ± 7.3, and 70.8 ± 7.4 years from lowest to highest Hb quintile, respectively. n = 1933 | Levels of Hb, frailty status (weight loss, exhaustion, grip strength, and walking speed) | Greater risk of frailty was present in those with lower Hb concentrations, with concentrations of Hb of 10.5 and 11.5 g·dL−1 having greater likelihood of frailty (Odds Ratio 6.3 and 2.3) compared to concentrations of 15.0 g·dL−1 (Odds Ratio 0.81). |
| Kim et al., 2014, Korea [173] | Older adults in South Korea from the KNHANES IV study that were 60 years or older, mean age: males, 69.0 ± 6.3; females, 69.3 ± 6.4 years. n = 2332 | Ferritin concentrations, HOMA-IR, sarcopenic status based on appendicular skeletal muscle mass | Ferritin concentrations were higher in the sarcopenic females compared to the non-sarcopenic females (70.7 vs. 85.4 ng·mL−1, p = 0.001). Appendicular skeletal muscle was negatively associated with ferritin concentrations (males; r = −0.111, p = 0.001, females; r = −0.104, p < 0.001). |
| Pires Corona et al., 2015, Brazil [174] | Older adults in Sao Paulo, Brazil, mean age 70.0 years. n = 1256 | Levels of Hb, frailty status (weight loss, exhaustion, grip strength, and walking speed) | Mean Hb concentrations were lower in frail older adults compared to non-frail older adults (13.3 g·dL−1 vs. 14.3 g·dL−1, p < 0.001). Frail individuals were more likely to have lower Hb than non-frail individuals, independent of confounding health conditions (Odds Ratio 3.27, p < 0.001). |
| Moon et al., 2015, Korea [175] | Korean males 65 years or older from the KNHANES and young males not meeting study criteria included as a reference group, mean ages: 71.6 ± 5.0 and 30.7 ± 5.9 years, respectively. n = 1464 | Levels of Hb, Skeletal Muscle Index | Low muscle mass was related to presence of anemia, independent of potential confounding factors (Odds Ratio 2.83). |
| Ruan et al., 2019, China, Ghana, India, Mexico, Russia, South Africa [176] | Older adults in China age 50 years or older that were part of the World Health Organization Study on Global Ageing and Adult Health, mean age 62.6 ± 0.2 # years. n = 13,175 | Levels of Hb, frailty status | Presence of anemia was associated with frailty, in which for each 1 g·dL−1 increase in Hb concentration, there was a 4% decrease in the odds of frailty (Odds Ratio 0.96, p < 0.001). |
| Neidlein et al., 2021, Germany [177] | Hospitalized older adults aged 65 years or older, mean age: 81.4 ± 6.2 years. n = 224 | Levels of ferritin, transferrin, iron, and Hb, CRP, handgrip strength, SPPB score, isometric leg extension strength. Iron supplementation protocol was recorded if provided during hospital stay | In those with iron deficiency, frailty scores were higher (4 vs. 3, p < 0.05), Hb was lower (males; 11.1 g·dL−1 vs. 12.4 g·dL−1, p < 0.01, females; 10.7 g·dL−1 vs. 11.7 g·dL−1, p < 0.001), and CRP was higher (3.2 mg·dL−1 vs. 1.9 g·dL−1, p < 0.01). There were no differences in muscle strength and function parameters between those with were iron deficient or non-iron deficient. However, a positive association was observed between Hb and handgrip strength at baseline and at hospital discharge in those with iron deficiency (β = 0.242–0.641, p = 0.020–0.039). |
| Youth | |||
| Arsenault et al., 2011, Colombia [169] | Youth aged 5–12 years in Colombia. n = 1945 | Standing long jump, 36 m shuttle run, and levels of Hb, ferritin, vitamin B12, complete blood count, CRP, erythrocyte folate | There were no differences in performance measurements between anemic and non-anemic youth. Females with low ferritin had 0.6 s slower performance on the shuttle run than females with normal ferritin levels (p = 0.020). Males with low ferritin had 7 cm lower jump scores than those with normal ferritin (p = 0.030). |
| Gracia-Marco et al., 2012, Sweden, Greece, Italy, Spain, Hungary, Belgium, France, Germany, Austria [99] | Adolescents aged 12.5–17.5 years old across Europe that completed the blood sample analysis as part of the HELENA-CSS study. n = 1089; males, n = 509, females, n = 580 | Standing long jump, 20 m shuttle run to estimate VO2max, red blood cell parameters, biomarkers of iron status (sTfR and ferritin), other micronutrients (vitamins A, E, C, B6, and B12, folate, and serum 25(OH)D) concentrations | Concentrations of Hb was positively associated with estimated VO2max (from 20 m shuttle run) in male adolescents (β = 0.192, p = 0.002). |
| Author, Year, Country | Study Participants | Supplemental Iron Treatment | Duration | Measurements | Conclusions |
|---|---|---|---|---|---|
| Athletes | |||||
| DellaValle & Hass, 2014, USA [179] | 31 Rowers | Treatment group: 100 mg·d−1 FeSO4 (n = 15) Placebo: (n = 16) | 6-weeks | Iron status (Hb, iron ferritin, transferrin receptor), body composition, performance (4 km time trial, VO2max, energetic efficiency, and blood lactate) | Treatment group showed a 0.3 g·dL−1 improvement in Hb (p = 0.04), slower lactate response during the 4 km time trial, and (12.2 nmol·L−1 vs. 11.4 nmol·L−1 at post-treatment compared to placebo, p < 0.001), and a 4.3 kcal higher energy expenditure (p = 0.03) post-treatment. |
| Garvican et al., 2014, Australia [180] | 27 distance runners with low or suboptimal iron status | Intravenous (IV) iron (550 ± 171 mg for low iron status, 375 ± 39 mg for suboptimal iron status) or oral supplementation of one (low) or two (suboptimal) tablets of 305 mg ferrous sulfate and 105 mg elemental iron) daily | 6-weeks | Iron status (Hb, sTfR, ferritin, erythropoietin, transferrin, Hb mass) and performance (VO2max, lactate threshold, running economy) | Both IV and oral supplementation showed a 83.7–417.5% increase ferritin at 6 and 8 weeks and a −5.6–−9.9% decrease in sTfR concentrations. VO2max increased, with a more profound increase in runners with low iron status (1.2–3.3 mL·kg−1·min−1). |
| Mielgo-Ayuso et al., 2015, Spain [181] | 22 Elite female volleyball players (27.0 ± 5.6 years) | Treatment group: 325 mg·d−1 ferrous sulphate daily (n = 11) Placebo: (n = 11) | 11-weeks | Iron status (serum iron, ferritin, transferrin saturation index, Hb), and strength (bench press, military press, half-squat, power clean, clean and jerk, and pull-over) | Treatment group showed significantly greater levels of serum iron, ferritin, transferrin saturation index, Hb compared to placebo group. Improvements in bench press (38.2 to 43.1 kg) clean and jerk (27.7 to 35.1 kg), power clean (33.3 to 35.1 kg), and total mean strength (35.2 to 41.9 kg occurred after the 11 weeks in the treatment group compared to placebo group (p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoemaker, M.E.; Salmon, O.F.; Smith, C.M.; Duarte-Gardea, M.O.; Cramer, J.T. Influences of Vitamin D and Iron Status on Skeletal Muscle Health: A Narrative Review. Nutrients 2022, 14, 2717. https://doi.org/10.3390/nu14132717
Shoemaker ME, Salmon OF, Smith CM, Duarte-Gardea MO, Cramer JT. Influences of Vitamin D and Iron Status on Skeletal Muscle Health: A Narrative Review. Nutrients. 2022; 14(13):2717. https://doi.org/10.3390/nu14132717
Chicago/Turabian StyleShoemaker, Marni E., Owen F. Salmon, Cory M. Smith, Maria O. Duarte-Gardea, and Joel T. Cramer. 2022. "Influences of Vitamin D and Iron Status on Skeletal Muscle Health: A Narrative Review" Nutrients 14, no. 13: 2717. https://doi.org/10.3390/nu14132717
APA StyleShoemaker, M. E., Salmon, O. F., Smith, C. M., Duarte-Gardea, M. O., & Cramer, J. T. (2022). Influences of Vitamin D and Iron Status on Skeletal Muscle Health: A Narrative Review. Nutrients, 14(13), 2717. https://doi.org/10.3390/nu14132717








