Abstract
Gastric cancer is a malignant neoplasm of the gastrointestinal tract, with one of the standard treatment methods remaining gastrectomy. The authors conducted a systemic review of the Medline and Embase databases concerning the serum vitamin D level in post-gastrectomy gastric cancer patients, regarding all articles published until 22 May 2022 according to the PRISMA guidelines. 18 studies with a total number of 908 gastric cancer survivors were included in the analysis. The initial rate of vitamin D deficiency in gastric cancer patients undergoing gastrectomy appears to be similar to the global population deficiency. In post-gastrectomy survivors, the level of 25(OH)D may remain stable or decrease, while the level of 1, 25(OH)2D remains normal. Supplementation with vitamin D results in an improvement in its serum concentration and positively affects bone mineral density, which is gradually reduced in post-gastrectomy survivors. Combining vitamin D supplementation with calcium and bisphosphonates enables us to obtain better results than vitamin D and calcium only. The type of surgery influences the level of serum vitamin D and its metabolites, with total or partial gastrectomy and maintenance of the duodenal food passage remaining the most important factors. There is a strong need for randomized, controlled trials that would investigate this matter in the future.
1. Introduction
Gastric cancer. Gastric cancer is a malignant neoplasm of the gastrointestinal tract that continues to be a significant global clinical and epidemiological problem. It is estimated to be the fifth most common and third highest mortality cancer in the world, with the most dominant type of adenocarcinoma. According to gender, men are 2.2 times more likely to suffer from it. A relationship is also observed between the incidence and the region of the world with the highest prevalence and mortality in eastern and central Asia, as well as Latin America [1,2,3].
Gastrectomy. Gastric cancer is often diagnosed at a late stage, leading to unfavorable treatment results. In the case of early gastric cancer, 5-year survival can exceed 80%, while survival in more advanced stages decreases significantly. However, in recent decades, the incidence of gastric cancer has been declining due to the treatment of Helicobacter pylori infection and treatment results being better due to earlier detection and new treatment options. However, gastric resection remains a method necessary for the treatment of gastric cancer. The standard is a partial or total gastric resection with D2 lymphadenectomy (D2-LND). There are several techniques, such as endoscopic removal in the very early stages of gastric cancer, laparoscopic, robotic, and open surgery. Several gastrectomy techniques are available. Billroth I gastrectomy, called gastroduodenostomy, is a procedure in which the pylorus is removed and the distal stomach is anastomosed directly to the duodenum. In Billroth II, called a partial gastrectomy and gastrojejunostomy, a partial gastrectomy is performed and the cut end of the stomach is closed with the greater curvature of the stomach connected to the first part of the jejunum in an end-to-side anastomosis. In Roux-en-Y anastomosis (Roux-en-Y), a total gastrectomy is performed, with the small intestine connected end to end with the oesophagus, and the far end of the duodenum connected end to side with the small intestine. Systemic treatments also play an important role in the treatment of gastric cancer, including adjuvant and neoadjuvant chemotherapy, intraperitoneal chemotherapy, and chemoradiotherapy. One cannot fail to mention the latest scientific achievements: immunotherapy and targeted therapy [2,3,4,5,6,7].
Nutritional status after gastrectomy. Gastrectomy significantly affects nutritional status and exerts a change in metabolism. The main parameters of nutritional status, body weight and Body Mass Index (BMI), hemoglobin, cholesterol level, and lymphocyte count decrease significantly after surgery. The decrease in some parameters, such as body weight, glucose level, protein level, calcium level, and parameters assessing liver function remains even after a 5-year follow-up of patients undergoing gastrectomy. Research showed significant alterations in the nutritional status of post-gastrectomy gastric cancer patients. The importance of nutritional status was demonstrated in a study in which 1415 patients were examined preoperatively and postoperatively 3, 6, and 12 months after the operation. The level of albumin and the prognostic nutritional index score (PNI) decreased in the follow-up. Moreover, various parameters of the nutritional status examined perioperatively were independent prognostic factors, which illustrates the importance of research into the nutritional status of patients after gastrectomy due to cancer. Similar conclusions were drawn in a study published in Annals of Surgical Oncology in 2018, malnutrition 12 months after gastrectomy significantly and negatively affected the overall survival of patients with gastric cancer. A study focused on the long-term 5-year follow-up of hematological parameters and nutritional status in patients after gastric resection for cancer, published in 2017 in the Journal of Gastrointestinal Surgery, showed that ferritin and triglycerides levels gradually decreased after gastrectomy, while other parameters of nutritional status declined slightly or remained at a constant level [8,9,10,11].
Vitamin D. One of the indicators that should be considered in the context of nutrition for gastric cancer patients is the serum vitamin D level. Vitamin D is a fat-soluble vitamin obtainable from the diet, either in the form of vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol). Vitamin D3 is also synthesized from 7-dehydrocholesterol by ultraviolet B (UVB, 290–320 nm) radiation in the epidermis of the skin. Both D2 obtained from the diet and D3 from the skin or gastrointestinal tract bind in circulation to the vitamin D binding protein (VDBP) and are delivered to the liver, which is the first place in a two-step metabolism, where vitamin D 25-hydroxylase transforms the delivered metabolites into 25(OH)D (calcidiol). Calcidiol is the dominant vitamin D form in the serum. However, it is further metabolized by 25(OH)D 1α-hydroxylase in the proximal tubules of the kidneys into 1α,25-dihydroxyvitamin D (1α,25(OH)2D)—calcitriol, which is the most biologically active form of vitamin D that binds to the vitamin D receptor (VDR) in tissues, allowing it to perform its biological functions. 25(OH)D can also be transformed by 25-hydroxyvitamin D3-24-hydroxylase into 24,25-dihydroxyvitamin D3—24,25(OH)2D3, which is considered a metabolite of 25(OH)D showing weak activity. The level of vitamin D deficiency is ≤50 nmol/L 25(OH)D according to the guidelines of the Endocrine Society Task Force on Vitamin D [12,13].
Although many articles discuss vitamin D level in the context of risk of gastric cancer development or changes in bone density after gastrectomy leading to osteomalacia or osteoporosis, hardly any investigate changes in serum vitamin D and its concentration of metabolites in gastric cancer survivors after gastrectomy. The purpose of our paper was to provide a systematic review of the available studies on vitamin D levels in patients post-gastrectomy due to gastric cancer.
2. Materials and Methods
The systematic review was carried out according to the PRISMA guidelines (Figure 1) The authors conducted research in Medline and Embase databases, using MeSH and Emtree terms: “stomach cancer”, “gastric cancer”, “gastrectomy”, “vitamin D”, “25-hydroxyvitamin d”, “cholecalciferol derivative”, “cholecalciferol”, “calcitriol “, “24,25 dihydroxyvitamin”; including all types of articles published until 22 May 2022, including data on the type of study, population, intervention, and outcome. 55 records in Embase, 5 in MEDLINE, and 89 records in both databases were found. After removing duplicates, the articles were manually searched to fulfill the inclusion criteria. Only original studies, case reports, or case series that had at least an abstract published in English, on human subjects who underwent gastrectomy due to gastric cancer and had their serum vitamin D levels determined at least once after gastrectomy were included in the review.
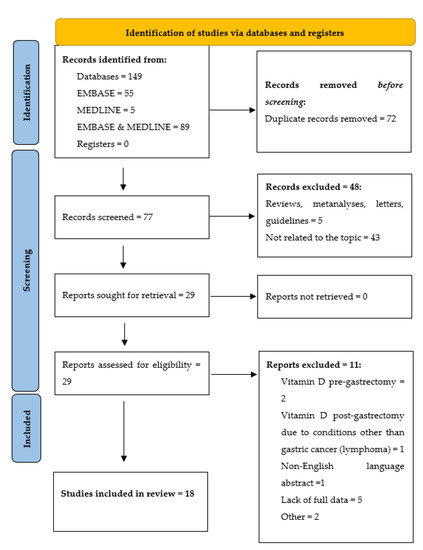
Figure 1.
Identification of studies via databases and registers according to the PRISMA guidelines.
3. Results
Eighteen original studies or case reports on serum vitamin D status were identified in post-gastrectomy patients due to gastric cancer, with a total number of 908 gastric cancer survivors included (Table 1).

Table 1.
Original studies and case reports on serum vitamin D concentration in patients with gastric cancer after gastrectomy.
The majority of the patients were men. The first report was made by Kobayashi et al. in 1994 [31], while the newest data were reported by Atsumi et al. in 2021 [14]. The investigated vitamin D metabolites were 25(OH)D (11 studies) [14,17,18,19,21,23,24,25,26,28,31], 1,25(OH)2D (6 studies) [14,17,24,25,27,31], and 24,25(OH)2D (2 studies) [24,31]. Six studies did not report the investigated metabolite [15,16,20,22,29,30], declaring that they analyzed “serum vitamin D”. The other concomitant and most frequently analyzed parameters were bone mineral density (nine studies) [14,16,17,19,20,21,23,26,30], serum estradiol level (three studies) [14,17,25] and other bone metabolism biomarkers, as part of the studies focused on bone density and risk of osteoporosis after gastric cancer surgery with vitamin D and its metabolites evaluated only as indicators. Five studies reported the type of surgery with the declared techniques Billroth I, Billroth II, and Roux-en-Y [18,24,25,28,31]. Seven out of 18 studies analyzed changes or serum vitamin D concentration 12 months after surgery [14,16,17,18,19,22,23]; the longest reported time from surgery to analysis was 18 years [29].
In seven out of 18 studies, vitamin D had been supplemented in patients with gastric cancer who underwent gastrectomy [15,16,19,21,22,26,27]. However, only two studies reported the doses administered, ranging from 1000 IU of cholecalciferol per day with calcium or 70 mg/week of alendronate [16] to 16,000 IU of vitamin D3 administered every 10 days [19]. Virik [21], Ribeiro [22], Cuerda [26], Veeralakshmanan [15], and Rino [27] only reported that patients were supplemented with vitamin D and/or calcium, multivitamin and mineral supplement, or 1α(OH)D3, with no report of daily doses, the regimen of administration, type of supplementation, or information whether it was advised by the physician or performed by the patient himself. The reported supplementing time ranged from 12 to 18 months [15,16,19,21,22,26,27].
Several studies compared vitamin D concentration both at baseline and at specific time points after surgery. The initial deficiency was present in 36.67% of the participants in the study by Veeralakshmanan et al. [15]; Virik et al. reported that at baseline, 24/27 (89%) patients had vitamin D levels < 50 nmol/L (mean 35.3 nmol/L) and 26/27 (96%) had vitamin D levels < 75 nmol/L (mean 38.4 nmol/L) with a normal range from 50 nmol/L to 125 nmol/L [21]. In contrast, Rino Y. et al. found that the level of 1,25(OH)2D was normal in all patients; however, 25(OH)D was below the normal range in 7 of the 22 patients (31.8%) [24]. The findings on serum vitamin D concentration levels after gastrectomy are ambiguous. Atsumi et al., who performed research on the level of 25(OH)D after gastric resection due to cancer, reported in 2019 a significant decrease in serum concentration 24 months after surgery [17]. His findings were confirmed by Jeong et al., whose study found that the vitamin D level was lower in gastric cancer survivors (20.3 ± 0.5 IU vs. 17.5 ± 1.2 IU) than in healthy individuals [20]. Ribeiro reported that in his study on fat-soluble vitamins, vitamin D was the only deficient one in 82% of patients, and the deficiency remained high throughout the entire period of follow-up, which lasted up to 24 months after surgery [22]. Rino et al. investigated the topic in two studies: they reported that the crucial time for the change in serum vitamin D concentration is 12 months, as the mean serum level of 25(OH)D was significantly lower in patients at 1 year or more postoperatively than in those at less than 1 year postoperatively. The prolonged analysis, performed on the group of patients who underwent gastrectomy due to gastric cancer less than 10 years before the study, showed that 25(OH)D was reduced in 6 of 21 patients (29%) [24,25]. In the study by Cuerda et al., 25(OH)D deficiency was initially observed in 45% of the patients. However, 25(OH)D concentration depends on the type of surgery, with a higher level in patients belonging to the pouch group than Roux-en-Y reconstruction (47.3 nmol/L compared to 33.9 nmol/L, respectively). Another study concerning the level of 24,25(OH)2D, a weak activity metabolite of 25(OH)D, showed that after a year from gastrectomy, its level was below the normal range in 19 of the 22 patients (86%), and analyzing survivors with gastrectomy who underwent surgery less than 10 years prior to the study, 24,25(OH)2D was reduced in 17 patients (81%) [26]. Kobayashi also reported a decrease in serum 24,25(OH)2D concentration, as well as its dependence on the type of surgery, with patients who underwent Billroth II manifesting lower 24,25(OH)2D levels than those with Billroth I [31].
However, the findings of several authors suggest that gastric cancer gastrectomy does not affect serum vitamin D concentration levels, or at least some of its metabolites, as stated previously. A study by Atsumi et al. in 2021 showed that the concentration levels of 25(OH)D, 1,25(OH)2D remained stable 12 months after the surgery [14]. In 2019, the same author reported that despite surgery, 1,25(OH)2D levels were consistently in the normal range up to two years of follow-up [17]. The findings of Baek seem to confirm this trend, as the investigated level of 25(OH)D did not change significantly compared to the baseline at 12 months after surgery [23]. Two studies by Rino in 2007 showed that the level of 1,25(OH)2D was normal in all patients, as well as in those who had undergone gastrectomy for gastric cancer and had been followed for even 10 years [24,25]. Schmidel et al., who analyzed the serum vitamin D concentration level in patients 3–18 years after gastrectomy, stated in their study its serum level remained normal [29].
None of the studies reported an increase in serum vitamin D levels in all patients; the reported improvements were relative to another study group or related to supplementation. In patients undergoing Billroth II surgery, 24,25(OH)2D concentrations were reported to be reduced, while 25(OH)D and 1,25(OH)2D concentrations increased [31]. Toyomasu reported that serum vitamin D in the Billroth-I group was significantly higher than in Roux-en-Y patients [18]. This remains consistent with the findings of Iivonen, who observed that patients with Roux-en-Y tended to have lower serum vitamin D concentrations than patients in the pouch group [28]. The passage of food through the duodenum also affected the results obtained. Rino Y. found, that the 1,25(OH)2D/25(OH)D ratio was significantly higher in the patients without passage of food through the duodenum due to the reconstructive method, while the 25(OH)D/24,25(OH)2D ratio was significantly higher in the patients with remaining duodenal food passages [25]. In another study, he proved that the serum level of 25(OH)D was significantly lower in patients who had received total gastrectomy than in patients who underwent other gastrectomy procedures [24].
The issue of vitamin D supplementation after gastrectomy has not been fully elucidated in all studies; however, in every reported case, a positive influence on serum vitamin D level was demonstrated. In a study by Veeralakshmanan, supplementation for 18 months with multivitamin tablets did not only significantly improve vitamin D levels, but also ferritin, folate, and vitamin B12 deficiencies [15]. A study by Climent M. showed, that even shorter periods of supplementation may result in positive outcomes, as providing the patients with 16,000 IU of vitamin D3 every 10 days over 3 months made 35 out of 40 patients included in the study reach values of 25(OH)D over 30 ng/mL, while over 12 months of vitamin D supplementation caused 38 patients to achieve serum vitamin D levels within the normal range of 25(OH)D [19]. Supplementation with vitamin D and calcium provided by Virik in his study resulted in an evaluation of the mean serum vitamin D level of 81.1 nmol/L (range 34–147). Throughout the study period, vitamin D levels improved to >50 nmol/L in 26/27 (96%) patients and to >75 nmol/L in 13/27 (48% of patients) [21]. In a study by Ha et al., 1000 IU of cholecalciferol had been administered per day with calcium or 70 mg/week of alendronate. The results of the study, investigating the prevention of bone loss in post-gastrectomy gastric cancer survivors, showed that supplementation with both vitamin D and calcium was less effective than treatment of vitamin D and calcium combined with bisphosphonate [16]. However, the study by Rino in 2000 showed that the severity of metabolic bone disorder analyzed by the MD/MS method improved after only 1α(OH)D treatment in 56.3% of patients [27]. Wu in his case report also stated that in a patient with osteomalacia secondary to vitamin D deficiency after gastric cancer gastrectomy, supplementing the patient with vitamin D caused the appearance of repeated bone scintigram to normalize [30]. The question of the change in bone density after gastrectomy appears to bring important information on the change in bone metabolism [32], as in the study by Atsumi in 2019, where BMD decreased significantly by 0.04 ± 0.03 g/cm2 12 months after gastrectomy and by 0.05 ± 0.04 g/cm2 24 months after gastrectomy [17]. The same author in 2021 reported that BMD decreased by median degrees of 3.4% and 3.9% in male and female patients 12 months after surgery [14]. Jeong proved in his study that gastric cancer survivors who underwent gastrectomy have a significantly higher risk of osteopenia (RR = 2.90) and osteoporosis (adjusted RR = 4.63) than the healthy population [20].
4. Discussion
Gastric cancer and vitamin D deficiency as a risk factor for its development. Gastric cancer is a malignant neoplasm of the gastrointestinal tract which, despite the constant efforts of doctors, scientists, and the health care system, continues to be a significant clinical and epidemiological problem in the world [1]. The risk factors for gastric cancer are: Helicobacter pylori infection; Ebstein–Barr viral infection; gastric ulcer disease; smoking and alcohol consumption; exposure to dust; high temperature; metals such as chromium; a diet rich in salt and N-nitroso compounds; obesity; pernicious anemia; blood group A; and even genetic syndromes such as familial adenomatous polyposis (FAP); Lynch syndrome; interleukin-17 (IL-17) and interleukin-10 (IL-10) polymorphisms of interleukin genes; hereditary diffuse gastric cancer (HDGC) and gastric adenocarcinoma; and proximal polyposis of the stomach (GAPPS) [2,3]. Epidemiologic evidence supports the role of vitamin D and vitamin D receptor (VDR) polymorphisms in the risk of several cancers, 1,25(OH)2D affecting cell differentiation and growth, as well as the appearance of invasion, angiogenesis, and metastasis in certain types of cancer [33]. At the same time, gastric cancer is a cancer that is extremely dependent on lifestyle; therefore, research on the potential role of nutrients, including vitamin D, was carried out, to investigate the matter of their influence on the prevalence of this malignancy. A systematic review published by Khayatzadeh et al. in 2015 on the relationship between vitamin D intake, serum vitamin D level, and risk of gastric cancer, found no evidence of a significant association between vitamin D status and risk of gastric cancer [34]. However, further studies by Tagliabue et al., which focused on the role of vitamin D and the VDR, found a borderline decrease in cancer risk for subjects with high levels of vitamin D binding protein compared those with low levels [33]. The latest reports show that vitamin D may be related to an anticancer mechanism of action in gastric cancer development by regulating epigenetic pathways, affecting the expression of microRNAs (miRNAs), accelerating the effect of cisplatin, and regulating intracellular signal transduction. Shah et al., who investigated this matter, found that serum vitamin D level is lower in patients suffering from Helicobacter pylori infection than those unaffected. Furthermore, patients suffering from vitamin D deficiency were found to have fewer chances to eliminate bacteria than those with normal vitamin D levels. UVB radiation, which promotes vitamin D synthesis in the skin, was found to reduce the incidence and mortality rate in patients with gastric cancer with an adequate serum vitamin D level related to a better survival rate. Also, the polymorphisms in the vitamin D receptor gene were found to be related to an increased risk of gastric cancer [35]. Vitamin D may modulate the expression of some miRNAs that are deregulated in gastric cancer cells. In this mechanism, vitamin D reduces chemoresistance at the cellular level [36]. Our review found that patients with gastric cancer have vitamin D deficiency, with reported rates ranging from the initial 36.67% of patients [15] up to 89% [21]. In patients with gastric cancer, it was also the vitamin D metabolite investigated that mattered, as demonstrated by Rino Y et al. al, who found that although 1,25(OH)2D may be normal in gastric patients, the level of the most active metabolite, 25(OH)D, may remain below the normal range in a significant number of them (31.8%) [24]. However, there is still no single study that would support with strong evidence that vitamin D supplementation could result in a decrease in gastric cancer incidence rate neither in the general population nor in relapsed patients [36], and the deficiency reported initially in gastric cancer patients remains consistent with the general global population deficiency, estimated at 37.3% (circulating concentrations of 25(OH)D below 20 ng/mL) [37].
Vitamin D level in post-gastrectomy gastric cancer survivors. The results of the provided studies remain ambiguous. The observational studies that reported serum vitamin D levels or its metabolites compared to baseline, without supplementation or relative concentration versus different types of surgery, etc., seem to indicate that in post-gastrectomy gastric cancer survivors the level of 25(OH)D may remain normal or decrease [14,17,18,19,21,23,24,25,26,28,31], while the level of 1,25(OH)2D remains normal [14,17,24,25,27,31].
Vitamin D post-gastrectomy supplementation. Most available research on the influence of gastrectomy on serum vitamin D levels is focused on patients with bariatric surgery. As reported by Jamil et al., patients with obesity may suffer from vitamin D deficiency due to vitamin D sequestration in fat, decreased sun exposure, sedentary lifestyle, and the psychological component of covering more skin with vitamin D deficiency, the most common observed vitamin deficiency in patients prior to surgery treating obesity. Most studies indicate, however, that in obese patients undergoing bariatric surgery, vitamin D deficiencies do not develop de novo postoperatively, but rather are connected to the preoperative status and improve with supplementation [38]. Unfortunately, current Clinical Practice Guidelines on vitamin D supplementation in bariatric surgery differ between societies, with most recommending high doses of vitamin D supplementation following surgery, ranging from 3000 IU daily to 50,000 IU 1–3 times weekly, and increasing to 50,000 IU 1–3 times daily in case of severe malabsorption. However, it should be noted that they do not fulfill the criteria for optimal guideline development, in part possibly due to limited resources, and are based on expert opinions [39]. In our review, concerning post-gastrectomy gastric cancer patients, vitamin D supplementation has been implemented in seven of 18 studies [15,16,19,21,22,26,27], three of which reported the initial deficiency [15,21,24]. In all studies, vitamin D supplementation positively affected its serum level or bone mineral density; however, no strict recommendations on dose or regimen were made. Only a study by Ha et al. proved that in post-gastrectomy gastric cancer survivors with bone loss, it is more effective to combine vitamin D supplementation with calcium and bisphosphonates than calcium alone [16]. However, it should also be noted, that supplementing patients who suffer from a lack of calcium and vitamin D, resulting for example in osteoporosis, may also bring some dangers. Chiodini and Bollard provided a review of the risk-benefit profile of such supplementation with calcium, based on large randomized controlled trials, with a conclusion, that its benefit in preventing fractures (with or without vitamin D) is at most very small, if any. Also, the possible adverse events outweigh the possible benefits of such supplementation [40]. A study by Park et al. on a Korean population investigated the association between calcium supplementation and cardiovascular outcomes and showed that the cumulative incidence of acute myocardial infarction, ischemic stroke, and death was significantly higher in the calcium supplementation group than in the control group [41]. These findings were confirmed by the Women’s Health Initiative Calcium/Vitamin D Supplementation Study, which showed that calcium supplements with or without vitamin D modestly increased the risk of cardiovascular events, especially myocardial infarction in women [42]. The possible side effects of calcium supplementation may also include possible mild, but common gastrointestinal side effects [40], which may be especially important in the group of post-gastrectomy cancer patients.
As the composition of dietary supplements varied across the presented interventional studies, it should also be taken into consideration that it is not only calcium that is related to vitamin D metabolism. Vitamin K2 and magnesium both appear to be involved in bone metabolism with data suggesting that vitamin K2 supplementation might improve bone quality and reduce fracture risk in osteoporotic patients [43]. In the meta-analysis of eight randomized controlled trials including a total of 971 subjects, vitamin K combined with vitamin D significantly increased the total bone mineral density, with the most favorable effect expected for vitamin type K2 [44]. Continuous combination therapy with vitamin K2 and D3 was proved to be useful for increasing vertebral bone mass in postmenopausal women, demonstrating superiority over vitamin K2 alone [45]. Dai Qi et al. found in their original study, concerning the influence of magnesium supplementation on the vitamin D metabolism, that optimal magnesium status may be important for optimizing 25(OH)D status, as it increased the 25(OH)D concentration when baseline 25(OH)D concentrations were close to 30 ng/mL, but decreased it when baseline 25(OH)D was higher (from ∼30 to 50 ng/mL) [46]. All of this information outlines the complexity of vitamin D metabolism and indicates a strong need for further studies that could investigate whether post-gastrectomy gastric cancer patients should receive vitamin supplementation and what composition it should have, also in the context of diet. As it is known that food intake accounts for a smaller contribution to 25(OH)D concentration than does solar UVB exposure and supplements, it should be noted that meat is a good source of vitamin D as 25(OH)D. The EPIC-Oxford study, which investigated plasma 25(OH)D concentrations in meat eaters, fish eaters, vegetarians, and vegans found them lower in vegetarians and vegans than in meat and fish eaters [47]. A study by Liu et al. assessed the possible dietary daily intake of vitamin D in an Australian population, providing data that meats, chicken, fish, eggs, and dairy produce, may alone have contributed about 4.2 μg vitamin D equivalents per day to average Australian diets of adults > 18 years in 1995 and 4.3 μg in 2011–2013 [48]. The influence of undergoing gastrectomy, with its possible results concerning the recovery period and further impact on the type of used diet, should also be taken into consideration in further studies.
Bone mineral density loss in post-gastrectomy gastric cancer survivors. Oh et al. investigated the matter of bone mineral density and osteoporosis risk in post-gastrectomy patients in their meta-analysis, including 1204 patients. Factors leading to decreased bone mineral density in this population were calcium malabsorption, secondary hyperparathyroidism, and dominant bone resorption with a pooled incidence of decreased BMD estimated at 36%. It was also found that calcium and 25(OH)vitamin D levels were significantly decreased, while PTH and 1,25(OH)D levels increased significantly in the gastrectomy group compared to the control group [49]. Their findings remain consistent with those obtained in this review: in all studies investigating BMD after gastrectomy in gastric cancer survivors, it was decreased [14,16,17,19,20,21,23,26,30]. Vitamin D supplementation improved the observed BMD in studies including this intervention [15,16,19,21,22,26,27]. Bone mineral density loss may be only one of many changes related to vitamin D and calcium metabolism. In 2019, Choi et al. published a study based on a Korean population, which showed that gastric cancer patients who received a total gastrectomy had an increased incidence of Alzheimer’s disease, with vitamin B12 deficiency probably playing a crucial role [50]. However, there are significant associations between vitamin D deficiency and both dementia and Alzheimer’s disease [51,52]. The authors outlined a need for further studies in order to investigate if vitamin D deficiency may influence Alzheimer’s disease incidence rate in post-gastrectomy gastric patients, along with vitamin B12.
Type of surgery and serum vitamin D level. Although the techniques implemented in bariatric surgery and gastrectomy due to gastric cancer may differ, with sleeve gastrectomy performed in bariatric surgery as one of the possible options, some of them remain common, e.g., Roux-en-Y technique. A systematic review and meta-analysis performed by Salman et al., including 717 patients who underwent one of these procedures with 50.63% of the patients in the Roux-en-Y arm, investigating the influence of gastric bypass and sleeve gastrectomy on bone mineral density and bone turnover markers showed that there was no significant difference in bone mineral density between these types of surgery. Only bone alcaic phosphatase and parathormone levels were significantly higher after Roux-en-Y surgery [53]. Howevr, this interesting finding, pertaining to the influence of the type of surgery on postoperative serum vitamin D levels, does not fit our findings. In our review, in gastric cancer survivors, the type of gastrectomy affected the results obtained. By comparing the techniques reported, Billroth II resulted in statistically significant higher levels of 1,25(OH)2D and reduced levels of 24,25(OH)2D versus the Billroth I group [31]. However, the Billroth I technique resulted in better vitamin D serum levels compared to the Roux-en-Y technique up to 2 years after surgery [18], with Roux-en-Y technique also remaining inferior to the pouch group in maintaining serum vitamin D concentrations [28]. It is the passage of food through the duodenum that appears to influence the serum vitamin D level, as the ratio 1,25(OH)2D/25(OH)D remains significantly higher in patients without the passage of food through the duodenum [25]. Total gastrectomy results in a lower serum vitamin D level than other gastrectomy procedures [24]. The relationship between vitamin D level and surgery has been raised for many years, for example in the context of the association between perioperative vitamin D status and outcomes after surgery, as it is known that some factors affecting the operation outcome, e.g., the inflammatory state (assessed for example by CRP level), is differentially modulated by co-existing infections and vitamin deficiencies [54]. A systematic review by Iglar and Hogan provided that vitamin D hypovitaminosis is associated with adverse outcomes after diverse surgical procedures [55]. Barker et al. conducted a study, in which patients undergoing open-heart surgery were randomly assigned to one of two groups, receiving either cholecalciferol (50,000 IU/dose) or placebo on three separate occasions: orally the evening before surgery and either orally or per nasogastric tube on postoperative days 1 and 2. Supplemental vitamin D prevented the sudden decrease in 25(OH)D induced by open-heart surgery during postoperative care. What is more, plasma 25(OH)D gradually increased from baseline to day three and remained significantly increased thereafter but plateaued to discharge with supplemental vitamin D [56].
Limitations. The authors outline the heterogeneity of the studies included in the review, ranging from case reports to interventional studies with different groups, abundance, and types of patients. It was not for all of the studies that satisfactory data on the type, intervention, comparison, and outcome of the patients were provided. Laboratory results were not always provided with particular serum vitamin D metabolites. Also, data on the type and dosage of vitamin D supplementation implemented in interventional studies are lacking. The research also did not report exact pathological findings on the type of gastric cancer, its staging, and grading.
5. Conclusions
The initial rate of vitamin D deficiency in gastric cancer patients undergoing gastrectomy seems to be similar to the global population deficiency. In post-gastrectomy survivors, the level of 25(OH)D may remain stable or decrease, while the level of 1,25(OH)2D remains normal. Supplementation with vitamin D results in an improvement in its serum concentration and positively affects bone mineral density, which is gradually reduced in post-gastrectomy survivors. Combining vitamin D supplementation with calcium and bisphosphonates enables improving bone mineral density results; however, no specific strong recommendations have been made regarding dosage and supplementation regimen. The type of surgery influences the level of serum vitamin D and its metabolites, with total or partial gastrectomy and maintenance of the duodenal food passage remaining the most important factors. There is a strong need for more randomized, controlled trials that would investigate this matter in the future.
Author Contributions
Conceptualization, T.M., K.P., A.F. and B.M.; methodology T.M.; validation, T.M., K.P., A.F., B.M., A.S. and B.B.-C.; writing—original draft preparation, T.M., K.P. and A.F.; writing—review and editing, K.P., B.M. and B.B.-C.; supervision, A.S. and B.B.-C.; project administration, T.M.; funding acquisition, T.M., K.P. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 1α(OH)D | 1αhydroxyvitamin D |
| 1α,25(OH)2D | 1α,25-dihydroxyvitamin D, calcitriol |
| 24,25(OH)2D | 24,25-dihydroxyvitamin D |
| 25(OH)D | 25-hyroxyvitamin D, calcidiol |
| ALP | alkaline phosphatase |
| BMD | bone mineral density |
| BMI | Body Mass Index |
| D2-LND | D2 lymphadenectomy |
| DEXA | dual-energy X-ray absorptiometry |
| E2 | estradiol |
| FAP | familial adenomatous polyposis |
| GAPPS | gastric adenocarcinoma and proximal polyposis of the stomach |
| HDGC | hereditary diffuse gastric cancer |
| IL | interleukin |
| MD/MS | modified microdensitometry method |
| miRNAs | microRNAs |
| PERT | pancreatic enzyme replacement therapy |
| RR | relative risk |
| UVB | ultraviolet B |
| VDBP | vitamin D binding protein |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Marqués-Lespier, J.M.; González-Pons, M.; Cruz-Correa, M. Current Perspectives on Gastric Cancer. Gastroenterol. Clin. N. Am. 2016, 45, 413–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef]
- Charalampakis, N.; Economopoulou, P.; Kotsantis, I.; Tolia, M.; Schizas, D.; Liakakos, T.; Elimova, E.; Ajani, J.A.; Psyrri, A. Medical management of gastric cancer: A 2017 update. Cancer Med. 2018, 7, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Kurokawa, Y.; Mori, M.; Doki, Y. Update on the Treatment of Gastric Cancer. JMA J. 2018, 1, 40–49. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Oh, S.E.; Choi, M.G.; Seo, J.M.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin. Nutr. 2019, 38, 870–876. [Google Scholar] [CrossRef]
- Fujiya, K.; Kawamura, T.; Omae, K.; Makuuchi, R.; Irino, T.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Terashima, M. Impact of Malnutrition After Gastrectomy for Gastric Cancer on Long-Term Survival. Ann. Surg. Oncol. 2018, 25, 974–983. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, D.J.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Actual 5-Year Nutritional Outcomes of Patients with Gastric Cancer. J. Gastric Cancer 2017, 17, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K.; Ohira, M.; Tamura, T.; Toyokawa, T.; Amano, R.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Maeda, K.; et al. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Sola, D.Y.; Turin, T.C.; Ellis, L.; Ahmed, S.B. Vitamin D levels are associated with cardiac autonomic activity in healthy humans. Nutrients 2013, 5, 2114–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsumi, Y.; Rino, Y.; Aoyama, T.; Okuda, N.; Kawahara, S.; Kazama, K.; Numata, M.; Tamagawa, H.; Oshima, T.; Yukawa, N.; et al. A Gender Comparison of Bone Metabolic Changes After Gastric Cancer Surgery: A Prospective Observational Study. In Vivo 2021, 35, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Veeralakshmanan, P.; Tham, J.C.; Wright, A.; Bolter, M.; Wadhawan, H.; Humphreys, L.M.; Sanders, G.; Wheatley, T.; Berrisford, R.J.; Ariyarathenam, A. Nutritional deficiency post esophageal and gastric cancer surgery: A quality improvement study. Ann. Med. Surg. 2020, 56, 19–22. [Google Scholar] [CrossRef]
- Ha, J.; Lee, J.M.; Lim, Y.; Kim, M.K.; Kwon, H.S.; Song, K.H.; Jeon, H.M.; Kang, M.I.; Baek, K.H. Effect of bisphosphonate on the prevention of bone loss in patients with gastric cancer after gastrectomy: A randomized controlled trial. Bone 2020, 130, 115138. [Google Scholar] [CrossRef]
- Atsumi, Y.; Rino, Y.; Wada, H.; Kitani, Y.; Ozawa, Y.; Aoyama, T.; Oshima, T.; Yukawa, N.; Yoshikawa, T.; Masuda, M. Changes in bone metabolism after gastric cancer surgery in male patients: A prospective observational study. Gastric Cancer 2019, 22, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Toyomasu, Y.; Ogata, K.; Suzuki, M.; Yanoma, T.; Kimura, A.; Kogure, N.; Ohno, T.; Kamiyama, Y.; Mochiki, E.; Kuwano, H. Comparison of the Physiological Effect of Billroth-I and Roux-en-Y Reconstruction Following Laparoscopic Distal Gastrectomy. Surg. Laparosc Endosc. Percutan. Tech. 2018, 28, 328–333. [Google Scholar] [CrossRef]
- Climent, M.; Pera, M.; Aymar, I.; Ramón, J.M.; Grande, L.; Nogués, X. Bone health in long-term gastric cancer survivors: A prospective study of high-dose vitamin D supplementation using an easy administration scheme. J. Bone Miner. Metab. 2018, 36, 462–469. [Google Scholar] [CrossRef]
- Jeong, S.M.; Shin, D.W.; Lee, J.E.; Jin, S.M.; Kim, S. Increased Risk of Osteoporosis in Gastric Cancer Survivors Compared to General Population Control: A Study with Representative Korean Population. Cancer Res. Treat. 2019, 51, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Virik, K.; Wilson, R. Bone loss and vitamin D deficiency post gastrectomy for gastro-esophageal malignancy. J. Clin. Oncol. 2016, 34, 165. [Google Scholar] [CrossRef]
- Ribeiro, U., Jr.; Zaidan, E.P., Jr.; Tomitão, M.T.; Kubrusly, M.; Vaz Safatle-Ribeiro, A.; De Melo, E.S.; Da Rocha, R.M.; Sallum, R.; Cecconello, I. O101. 01: Cyclooxygenase-2 (Cox-2) and Methylenetetrahydrofolate Reductase (MTHFR) Gene Polymorphisms in Esophageal Cancer. Dis. Esophagus 2014, 27, 3A–168A. [Google Scholar] [CrossRef]
- Baek, K.H.; Jeon, H.M.; Lee, S.S.; Lim, D.J.; Oh, K.W.; Lee, W.Y.; Rhee, E.J.; Han, J.H.; Cha, B.Y.; Lee, K.W.; et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone 2008, 42, 61–67. [Google Scholar] [CrossRef]
- Rino, Y.; Yamamoto, Y.; Wada, N.; Yukawa, N.; Murakami, H.; Tamagawa, H.; Yamada, T.; Ohshima, T.; Masuda, M.; Imada, T. Changes in vitamin D after gastrectomy. Gastric Cancer 2007, 10, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rino, Y.; Takanashi, Y.; Yamamoto, Y.; Inagaki, D.; Kawamoto, M.; Harada, H.; Ashida, A.; Wada, H.; Yamada, R.; Ohshima, T.; et al. Bone disorder and vitamin D after gastric cancer surgery. Hepatogastroenterology 2007, 54, 1596–1600. [Google Scholar]
- Cuerda, C.; Camblor, M.; Bretón, I.; Velasco, C.; Parón, L.; Hervás, E.; Muñoz-Calero, A.; García-Peris, P. Cirugía gástrica como factor de riesgo nutricional [Gastric surgery as a nutritional risk factor]. Nutr. Hosp. 2007, 22, 330–336. [Google Scholar] [PubMed]
- Rino, Y.; Imada, T.; Yamamoto, Y.; Takahashi, M.; Amano, T.; Takanashi, Y. The efficacy of 1 alpha hydroxy vitamin D3 treatment of the metabolic bone disorder in patients who underwent gastrectomy for gastric cancer. Hepatogastroenterology 2000, 47, 1498–1500. [Google Scholar]
- Iivonen, M.K.; Mattila, J.J.; Nordback, I.H.; Matikainen, M.J. Long-term follow-up of patients with jejunal pouch reconstruction after total gastrectomy. A randomized prospective study. Scand. J. Gastroenterol. 2000, 35, 679–685. [Google Scholar] [CrossRef]
- Schmiedl, A.; Schwille, P.O.; Stühler, C.; Göhl, J.; Rümenapf, G. Low bone mineral density after total gastrectomy in males: A preliminary report emphasizing the possible significance of urinary net acid excretion, serum gastrin and phosphorus. Clin. Chem. Lab. Med. 1999, 37, 739–744. [Google Scholar] [CrossRef]
- Wu, Y.W.; Seto, H.; Shimizu, M.; Kageyama, M.; Watanabe, N.; Kakishita, M. Postgastrectomy osteomalacia with pseudofractures assessed by repeated bone scintigraphy. Ann. Nucl. Med. 1995, 9, 29–32. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takahashi, C.; Kuroda, T.; Sugenoya, A.; Iida, F.; Katoh, K. Calcium regulating hormones and bone mineral content in patients after subtotal gastrectomy. Surg. Today 1999, 24, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rino, Y.; Aoyama, T.; Atsumi, Y.; Yamada, T.; Yukawa, N. Metabolic bone disorders after gastrectomy: Inevitable or preventable? Surg. Today 2022, 52, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1758–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayatzadeh, S.; Feizi, A.; Saneei, P.; Esmaillzadeh, A. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: A systematic review and meta-analysis. J. Res. Med. Sci. 2015, 20, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Iqbal, Z.; Alharbi, M.G.; Kalra, H.S.; Suri, M.; Soni, N.; Okpaleke, N.; Yadav, S.; Hamid, P. Vitamin D and Gastric Cancer: A Ray of Sunshine? Cureus 2021, 13, e18275. [Google Scholar] [CrossRef]
- Gallardo Martin, E.; Cousillas Castiñeiras, A. Vitamin D modulation and microRNAs in gastric cancer: Prognostic and therapeutic role. Transl. Cancer Res. 2021, 10, 3111–3127. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Jamil, O.; Gonzalez-Heredia, R.; Quadri, P.; Hassan, C.; Masrur, M.; Berger, R.; Bernstein, K.; Sanchez-Johnsen, L. Micronutrient Deficiencies in Laparoscopic Sleeve Gastrectomy. Nutrients 2020, 12, 2896. [Google Scholar] [CrossRef]
- Chakhtoura, M.T.; Nakhoul, N.; Akl, E.A.; Mantzoros, C.S.; El Hajj Fuleihan, G.A. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism 2016, 65, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Chiodini, I.; Bolland, M.J. Calcium supplementation in osteoporosis: Useful or harmful? Eur. J. Endocrinol. 2018, 178, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-M.; Lee, B.; Kim, Y.-S.; Hong, K.-W.; Park, Y.C.; Shin, D.H.; Kim, Y.; Han, K.; Kim, K.; Shin, J.; et al. Calcium Supplementation, Risk of Cardiovascular Diseases, and Mortality: A Real-World Study of the Korean National Health Insurance Service Data. Nutrients 2022, 14, 2538. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.; Grey, A.; Avenell, A.; Gamble, G.; Reid, I. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011, 342, d2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Liu, C.; Guo, X.; Li, K.; Deng, Q.; Li, D. The combination effect of vitamin K and vitamin D on human bone quality: A meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 3280–3297. [Google Scholar] [CrossRef] [PubMed]
- Ushiroyama, T.; Ikeda, A.; Ueki, M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas 2002, 41, 211–221. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Arcot, J.; Cunningham, J.; Greenfield, H.; Hsu, J.; Padula, D.; Strobel, N.; Fraser, D.R. New data for vitamin D in Australian foods of animal origin: Impact on estimates of national adult vitamin D intakes in 1995 and 2011–2013. Asia Pac. J. Clin. Nutr. 2015, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Yoon, B.H.; Ha, Y.C.; Suh, D.C.; Lee, S.M.; Koo, K.H.; Lee, Y.K. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: A meta-analysis. Osteoporos. Int. 2020, 31, 267–275. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, D.W.; Jang, W.; Lee, D.H.; Jeong, S.M.; Park, S.; Han, K.D.; Park, Y.G. Risk of Dementia in Gastric Cancer Survivors Who Underwent Gastrectomy: A Nationwide Study in Korea. Ann. Surg. Oncol. 2019, 26, 4229–4237. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.A.; Aradaib, M.; Salman, A.; Elewa, A.; Tourky, M.; Shaaban, H.E. Effects of Gastric Bypass and Sleeve Gastrectomy on Bone Mineral Density and Bone Turnover Markers: A Systematic Review and Meta-Analysis. World J. Surg. 2022, 46, 865–875. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, D.; del Carmen Pons, E.; Rueda, D.; Teresa Sininterra, O.; Murillo, E.; Scott, M.; Koski, K. C-reactive protein is differentially modulated by co-existing infections, vitamin deficiencies and maternal factors in pregnant and lactating indigenous Panamanian women. Infect. Dis. Poverty 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Iglar, P.J.; Hogan, K.J. Vitamin D status and surgical outcomes: A systematic review. Patient Saf. Surg. 2015, 30, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, T.; May, H.T.; Doty, J.R.; Lappe, D.L.; Knowlton, K.U.; Carlquist, J.; Konery, K.; Inglet, S.; Chisum, B.; Galenko, O.; et al. Vitamin D supplementation protects against reductions in plasma 25-hydroxyvitamin D induced by open-heart surgery: Assess-d trial. Physiol. Rep. 2021, 9, e14747. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).