Abstract
Dietary cholesterol has been suggested to increase the risk of cardiovascular disease (CVD). Phytosterols, present in food or phytosterol-enriched products, can reduce cholesterol available for absorption. The present study aimed to investigate the association between habitual intake of total and individual plant sterols (β-sitosterol, campesterol, and stigmasterol) or a diet combined with phytosterol-enriched products and CVD in a cross-section of Polish adults, participants of the Multicenter National Health Survey II (WOBASZ II). Among men (n = 2554), median intakes of plant sterols in terciles ranged between 183–456 mg/d and among women (n = 3136), 146–350 mg/d in terciles. The intake of phytosterols, when consumed with food containing phytosterols, including margarine, ranged between 184–459 mg/d for men and 147–352 mg/d for women. Among both men and women, beta-sitosterol intake predominated. Plant sterol intake was lower among both men and women with CVD (p = 0.016) compared to those without CVD. Diet quality, as measured by the Healthy Diet Index (HDI), was significantly higher in the third tercile of plant sterol intake for both men and women and the entire study group (p < 0.0001). This study suggests that habitual dietary intake of plant sterols may be associated with a lower chance of developing CVD, particularly in men.
1. Introduction
Cardiovascular disease (CVD) is a global health problem and a leading cause of death [1]. CVD risk factors are associated with poor lifestyle, including smoking, physical inactivity, obesity, unhealthy diet, and excessive alcohol consumption, leading to hypertension, hyperglycemia, and high LDL cholesterol [2,3]. Studies indicate a link between CVD and diabetes [3,4].
Type 2 diabetes mellitus (T2DM) predisposes patients to cardiovascular disease and cardiovascular mortality [5]. The development and progression of T2DM are strongly influenced by diet, physical inactivity, and increased body weight; therefore, intensive lifestyle modification is recommended for T2DM [6]. In patients with diabetes, the addition of soluble dietary fiber and phytosterols is recommended as a primary measure to prevent CVD before considering non-statin therapy [7].
Phytosterols (plant sterols and plant stanols) are natural bioactive plant substances with a structure similar to cholesterol. In the intestine, phytosterols and cholesterol compete for the same absorption mechanisms [8]. As a result, phytosterols can affect blood cholesterol concentrations by reducing the amount of cholesterol available for absorption. Studies have shown that consumption of 0.6–3.3 g of plant sterols per day reduces serum LDL-C concentrations by approximately 6–12%, and this effect was dose-dependent [9].
The diet typically provides 150–400 mg of plant sterols [10,11,12,13,14,15]. The phytosterols found in the highest amounts in plant-based foods, and, thus, in the human diet, are β-sitosterol, campesterol, and stigmasterol [16]. Food sources with the highest plant sterol content are vegetable oils, mainly corn oil, and sesame seeds [17]. Phytosterols isolated mainly from vegetable oils and their commercially produced esters can be ingredients of fortified foods and supplements as a non-pharmacological therapy of hypercholesterolemia. In European Union countries, products enriched in plant sterols are mainly milk and yogurt, margarine, and spreadable fats [18]. Plant sterol-enriched foods that provide 2 mg of phytosterols daily, combined with a healthy lifestyle, in patients with mild to moderate hypercholesterolemia have been found to reduce LDL-C levels by 10% [19,20]. However, the effect of long-term use of phytosterol-enriched foods on cardiovascular risk factors is unknown [21].
A few population-based studies, but not in the Polish population, have analyzed the effects of dietary phytosterol intake on CVD [10,11,14], but none included phytosterol-enriched products. Therefore, the present study aimed to investigate whether there is an association between habitual intake of total phytosterols and individual phytosterols (β-sitosterol, campesterol, and stigmasterol), or a diet combined with phytosterol-enriched products, and CVD in a cross-section of Polish adults.
2. Materials and Methods
2.1. Study Group
The study group consisted of 2554 men and 3136 women, of the National Multicenter Health Survey II (in Polish—WOBASZ II). WOBASZ II is a cross-sectional study representative of the Polish population of adults aged 20 years and older, which was conducted by the Institute of Cardiology (at present National Institute of Cardiology), Warsaw, Poland, in 2013–2014, in collaboration with five national medical universities. The design and methods of the WOBASZ II study have been described in detail elsewhere [22]. Approval for the WOBASZ II study was obtained from the Bioethics Committee at the National Institute of Cardiology (No. 1344), and was approved for the current study (No. 1837). Written informed consent was obtained from all participants.
Data on participants’ demographics, diseases, leisure-time physical activities, tobacco use, and alcohol intake, were collected using a standardized questionnaire developed for the WOBASZ II study. The classification of cardiovascular disease (CVD) was adopted according to World Health Organization guidelines [23]. Respondents were defined as having CVD if they had a reported history of any of the following: coronary heart disease, myocardial infarction, stroke, atrial fibrillation and/or other cardiac arrhythmias, peripheral vascular disease of the lower limbs, heart failure, coronary angioplasty or coronary artery bypass grafting, and implanted pacemaker or cardioverter-defibrillator. The criterion for diabetes, according to the American Diabetes Association [24], was a glucose level ≥ 7.0 mmol/L and/or use of glucose-lowering medication. Blood pressure (BP) was measured three times on the right arm after 5 min of rest in a sitting position at 1 min intervals, and the final BP was reported as the mean of the second and third measurements. Hypertension was diagnosed when systolic blood pressure was ≥140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or when antihypertensive drugs were used. Height and weight measurements were taken by personnel trained in standard procedures. Body mass index (BMI) was calculated from body weight in kilograms divided by the square of height in meters. Biochemical analyses were performed at the Central Laboratory “Diagnostyka” at the National Institute of Cardiology in Warsaw.
2.2. Food Intake and Nutritional Assessment
Data on daily food intake were collected by trained interviewers using the single 24-h dietary recall method. To reduce the possibility of bias, individuals who described their diet as atypical were excluded. Based on the different types of food consumed, energy and dietary fiber of each patient’s diet were calculated using Polish food composition tables [25]. Polyphenols and antioxidants were calculated using previous studies [26,27,28,29].
2.3. Assessment of Healthy Diet Index (HDI) Score
Diet quality was determined by scoring the Healthy Diet Indicator (HDI), which was in accordance with the World Health Organization (WHO) dietary guidelines [18] and described in Fransen et al. [30]. HDI is based on six components—intake of saturated fatty acids (% total energy, %TE), intake of polyunsaturated fatty acids (%TE), dietary cholesterol (mg/d), dietary protein (%TE), fiber (g/d), and free sugars (%TE)—and fruits and vegetables (g/d), within the recommended range [31]. The final HDI score was the sum of all components, ranging from zero (minimal compliance with recommendations) to seven (maximum compliance with recommendations).
2.4. Assessment of Dietary Phytosterol Intake and the Intake of Plant Sterols from Enriched Margarine
Phytosterol intake was calculated as previously described using a developed database [12]. Total and individual phytosterol intakes were determined by multiplying the daily intake of each food by the total and individual phytosterol content of that food, respectively. Dietary recalls were reviewed by checking for consumption of phytosterol-enriched products. Based on the dietary history it was found that among the products enriched with phytosterols, only phytosterol-enriched margarine was consumed by 1.96% of men and 1.85% of women [12]. Manufacturers were identified and asked to report the plant sterol content of their products.
2.5. Statistical Analysis
The study population was divided into three groups according to the tercile distributions of plant sterol intakes (separately for total and individual phytosterols). All analyses were performed according to gender and overall. Quantitative variables were presented as mean (standard deviation) and/or median (interquartile range), while qualitative variables were presented as percentages. Mean values of plant sterol intake with a 95% confidence interval (95% CI), adjusted for age, were calculated using the general linear model and the Tukey-Kramer test was chosen for multiple comparisons, if appropriate. The odds ratios (ORs) with 95% CI for CVD were evaluated using logistic regression analysis in relation to total and particular phytosterol intake. Two models were applied: model 1, unadjusted in men and women but adjusted for sex, and combined, and model 2, adjusted for age, consumption of lipid-lowering drugs, HDI, BMI, alcohol intake, and, additionally, for sex, for the entire population. The first tercile (T1) in each model was adopted as a reference. Statistical analyses were carried out using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value less than 0.05 was considered statistically significant.
3. Results
The general characteristics of the study participants are shown in Table 1. The mean age of the entire study group was 49.58 years. The highest percentage of the study participants had hypercholesterolemia 67.3% and hypertension 45.22%. CVD was diagnosed in 20% of the studied population, while diabetes in 10.82%.

Table 1.
General characteristics of the studied population.
Table 2 shows the phytosterol intake according to age, presence of diabetes, and CVD. The results are presented for men, women, and the entire study group. Dietary phytosterol content was found to be age-dependent and generally highest among the youngest age group and lowest among those aged 65 years and older. Among men and in the entire study group, sterol intake was significantly lower among people with diabetes (results were adjusted for age). No significant differences were found for women. With respect to CVD, plant sterol intake was lower among both men and women with CVD (p = 0.0016) and for both genders (p < 0.0001). With regard to diabetes, such a relationship was observed for men and the entire group, but not for women. With respect to individual plant sterols, we found that dietary intake of phytosterols was lower among both men with CVD and women and among men with diabetes (except campesterol in men with diabetes). No differences were found between women with diabetes and healthy women. For the whole group, only campesterol was not statistically significant. The intake of individual plant sterols with the fortified margarine was not considered, because manufacturers only reported the total phytosterol content. Thus, it was not possible to determine what the individual plant sterol content of the margarine was.

Table 2.
Phytosterol intakes depending on age, diabetes, and CVD.
Table 3 shows terciles of plant sterol intake with food, and with food including phytosterol-enriched margarine. Terciles of individual plant sterol intake for the entire study group and by gender were used as means (crude, adjusted), medians, and ranges for particular phytosterols intake. Among men, the median plant sterol intake in the first tercile was 183, in the second tercile 292, and in the third tercile 456 mg/d. For food intake, including margarine with phytosterols, the values were 184; 294, and 459 mg/d, respectively. Among women, the median intakes of plant sterols with diet were: 146 in the first tercile, 231 in the second tercile, and 350 mg/d in the third tercile. For food intake, including margarine with phytosterols, these values for women were, respectively: 147; 232, and 352 mg/d. For individual plant sterols, they are ranked in Table 3 by the volume of intake. Among both men and women, beta-sitosterol intake predominated, with a median range of 112–280 mg/d per tercile among men and 91–222 mg/d among women. For campesterol, the median range was 31–107 mg/d among men and 24–78 mg/d among women, and for stigmasterol, 12–39 mg/d among men and 12–34 mg/d for women.

Table 3.
Intake of phytosterols in terciles.
The odds ratio of developing CVD was related to phytosterol intake with diet (Figure 1). In the crude model, it was found that in both men and women, and in the entire study group (adjusted for gender), OR of CVD were significantly lower in the second and third terciles compared to the first terciles, with the lowest incidence of CVD in the third tercile. After adjusting for confounding factors, among men statistical significance was maintained, except for the second tercile of beta-sitosterol intake. Among women, only the intake of total plant sterols from the diet and their total intake together with margarine in the third tercile, and the intake of beta-sitosterol in the third tercile remained statistically significant. In the entire study group, significant values were observed in the third tercile of total plant sterol intake (without and with phytosterol-enriched products), and for all individual plant sterols.
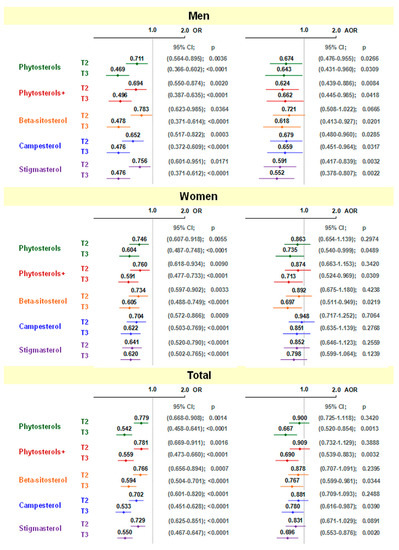
Figure 1.
Odds ratio (95% confidence interval) for CVD in relation to total and individual phytosterol intake (relative to 1st tercile). T2—2nd tercile; T3—3rd tercile; OR—odds ratio; AOR—adjusted odds ratio; ORs were unadjusted in men and women but adjusted for sex combined; AORs—adjusted for age, lipid-lowering medication, HDI, BMI, alcohol, and additionally for sex in total.
We also investigated whether the results obtained could be biased by diet (Table 4). For this purpose, data from the extreme terciles of total and single plant sterol intake before and after energy adjustment were presented by sex and the entire study group. It was found that both before and after adjustment the results were significant for total and single dietary plant sterol. For intakes of phytosterol-enriched margarine, a significant difference was found between the first and third terciles before energy adjustment, which did not occur after adjustment. Intakes of polyphenols, antioxidants, dietary fiber, and HDI were also divided according to the tercile of total and individual plant sterol intake, with polyphenols, antioxidants, and dietary fiber adjusted for energy value. It was found that before adjustment, dietary polyphenol, antioxidant, and fiber contents were higher in the third tercile among both men and women and in the group as a whole (p < 0.0001). After adjustment for energy, differences were not observed. Diet quality, as measured by HDI, was significantly higher in the third tercile of plant sterol intake for both men and women and for the entire study group (p < 0.0001).

Table 4.
Dietary quality in relation to tercile of dietary total and individual phytosterol intake (1st tercile vs. 3rd tercile).
Intakes of atherogenic and antiatherogenic products were also examined in the first and third terciles of total and individual phytosterol intake (Table 5). For atherogenic products, butter and animal fat consumption was found to be higher in the third tercile of plant sterol intake, but after adjustment for energy there was an inverse difference, i.e., with higher plant sterol intake, animal fat and butter consumption was lower. For red meat and meat products before and after adjustment for energy, consumption was higher in the third tercile. All the above observations were true for both men and women and for the entire study group.

Table 5.
Consumption of selected products by tercile of phytosterol intake (1st tercile vs. 3rd tercile).
In the case of intake of antiatherogenic products, it was found that in both sexes and in the entire study group, both before and after adjustment for energy, the intake of vegetable oils, vegetable fats, fish, fruits, legumes, nuts, and seeds was higher in the third tercile of plant sterol intake. For soft margarine and vegetables, there were similar differences among men and the overall study group, but not among women. In women, after adjustment, differences were not observed. For whole grain bread, higher consumption by both sexes and in the entire study group was observed in the third tercile, but after adjusting for energy, differences were not significant.
4. Discussion
The prevalence of CVD and its risk factors among Poles is high [32]. CVD in this present population-based cross-sectional study was found in one fifth of the participants, which is concordant with the literature. This population requires interventions to reduce the incidence of CVD. One of the non-pharmacological treatment measures is a dietary modification to improve the quality of nutrition. Phytosterols, present in food and phytosterol-enriched food products, depending on the dose, can be effective in reducing LDL cholesterol, which is one of the risk factors for CVD [9].
Scientific evidence based on supplementation studies shows that the intake of 2 g of phytosterols is effective in lowering LDL cholesterol [20]. The relationship between dietary phytosterols and CVD is, however, controversial, as foods provide phytosterols in lower doses than dietary supplements do. The usual intake of phytosterols is generally less than 400 mg/day [10,11,12,13,14,15], and higher levels have been found only in vegans [33]. In this study, intakes higher than 400 mg/day were observed only in the highest tercile of phytosterol consumption, both in men and women. Previous evidence indicates, however, that phytosterols from natural foods may have an LDL cholesterol lowering effect [9]. In this study, both men and women with CVD were found to have lower intakes of total and individual plant sterols from diet and from diet and phytosterol-enriched margarine, than their healthy counterparts.
Diabetes predisposes one to CVD and people identified with diabetes are at a greater risk of developing cardiovascular diseases [5]. Scientific evidence shows that plant sterols can have beneficial effects on diabetes by reducing insulin resistance [34]. In this study, men with diabetes had significantly lower intakes of total and individual plant sterols, but no significant difference was observed in women.
Recent studies conducted in Poland support the belief that it is men who require special preventive measures to reduce cardiovascular risk factors, especially hypertension, dyslipidemia, diabetes, excessive body weight, and smoking [32,35]. Our cross-sectional study suggests that it is men who may benefit from habitual plant sterol intake. This is particularly evident after adjusting plant sterol intake for confounding variables, which were age, lipid-lowering medication, HDI, BMI, and alcohol. Among women, the findings are ambiguous because, after adjusting for confounders, most of the previously significant differences were not further observed for the second tercile of total and individual plant sterol intake. This might be due to the generally lower intake of plant sterols among women relative to that observed among men, and in the second quartile, it is low enough to observe beneficial effects. It is only in the third tercile of total and individual plant sterol intake that a lower incidence of CVD is observed among women.
The results of our study are in line with those of a Swedish study, which found that consumption of naturally occurring plant sterols was associated with a lower risk of a first heart attack in men, but not among women [10]. It is possible that women may benefit not from a single dietary component, but from a combination of foods and nutrients, which, for example, can be found in the Mediterranean diet or Dietary Portfolio [36]. Dietary recommendations to date regarding the consumption of a varied diet, and particularly emphasizing the consumption of plant-derived products, are reasonable in terms of providing various compounds of importance in the prevention of noncommunicable diseases. The contribution of phytosterols to the diet is highlighted by the Dietary Portfolio, which uses a combination of established nutritional approaches to lowering cholesterol, such as consumption of plant protein, nuts, soluble fiber, and monounsaturated fats and phytosterols [37,38]. It has been shown to improve LDL cholesterol fraction and other CVD risk factors [37,38,39]. In several other studies, lower levels of total cholesterol and LDL cholesterol were observed in relation to dietary phytosterols [40,41,42]. A recent study found that closer adherence to a plant-based diet was significantly associated with a lower risk of total CVD, coronary heart disease, and heart failure in postmenopausal women [36]. In contradiction to the Swedish study is the Danish study, which found no reduced CVD risk despite lower LDL-C concentrations in men [14]. However, the authors concluded that the study population had a narrow range of phytosterol intake.
Our study suggests that, in terms of intake of substances with beneficial effects on CVD, such as polyphenols, antioxidants, and dietary fiber, individuals with low and high intakes of plant sterols do not differ. However, they do differ in their intake of foods considered pro- and anti-atherogenic. It was found that study participants who had higher plant sterol intakes consumed more anti-atherogenic foods and fewer animal fats.
Phytosterol-enriched foods are recommended for people with hypercholesterolemia for the prevention of CVD [19]. However, in the WOBASZ II study, consumption of phytosterol-enriched foods was observed in a small proportion of the study group (less than 2% of participants). This translated into similar intakes of plant sterols and plant sterols along with phytosterols from fortified products.
Limitations
The main limitation of the study was its cross-sectional nature, as a result of which causal inferences cannot be drawn. Another limitation was the use of a single 24-h recall method, which may not reflect the usual pattern of food consumption. To reduce the possibility of bias, subjects who described their diet as atypical were excluded.
The strengths of this study are its representativeness to the Polish population and the assessment of diet quality, which may act synergistically with plant sterols, which has not been studied before. To minimize the synergistic effect of plant sterols on the association with CVD, the results were adjusted for the Healthy Diet Index (HDI) score. A strength of the study was its consideration of phytosterol-enriched foods.
5. Conclusions
This study suggests that habitual dietary intake of plant sterols may be associated with a lower chance of developing CVD, particularly in men. However, this finding should be treated with caution because of the difficulty in separating the effects of plant sterols from the effects of other dietary components that may have synergistic effects.
Author Contributions
Conceptualization, A.W. and A.M.W.; methodology, A.M.W., A.W. and M.E.Z.; software, A.W., A.M.W. and A.C.-M.; validation, A.W., A.M.W. and A.C.-M.; formal analysis, A.C.-M. and A.W.; investigation, A.W. and A.M.W.; resources, A.M.W., A.W., M.E.Z., I.M.-C. and W.D.; data curation, A.W. and A.M.W.; writing—original draft preparation, A.M.W.; writing—review and editing, A.W., M.E.Z., I.M.-C., A.C.-M. and W.D.; visualization, A.W., A.C.-M. and A.M.W.; supervision, A.W., A.M.W. and W.D.; project administration, A.M.W. and A.W.; funding acquisition, A.W. and A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Cardiology (Grant no. 2.20/I/20) and Medical University of Bialystok (Grant no. SUB/1/DN/22/001/3317).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the National Institute of Cardiology (protocol code 1344, date of approval 5 November 2012, and protocol code 1837, date of approval 14 January 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data in this study are made available upon request to the authors at the following e-mail address: anna.witkowska@umb.edu.pl or awaskiewicz@ikard.pl.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention and Control of Noncommunicable Diseases: Guidelines for Primary Health Care in Low Resource Settings; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef]
- Newman, J.D.; Schwartzbard, A.Z.; Weintraub, H.S.; Goldberg, I.J.; Berger, J.S. Primary Prevention of Cardiovascular Disease in Diabetes Mellitus. J. Am. Coll. Cardiol. 2017, 70, 883–893. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Daly, D.D., Jr.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C., Jr. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2017, 70, 1785–1822. [Google Scholar] [CrossRef]
- Gylling, H.; Simonen, P. Phytosterols, Phytostanols, and Lipoprotein Metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [Green Version]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Klingberg, S.; Ellegård, L.; Johansson, I.; Jansson, J.H.; Hallmans, G.; Winkvist, A. Dietary intake of naturally occurring plant sterols is related to a lower risk of a first myocardial infarction in men but not in women in northern Sweden. J. Nutr. 2013, 143, 1630–1635. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.S.; Fonseca, F.A.H.; Fonseca, M.I.H.; Martins, C.M.; Fonseca, H.A.R.; Fonzar, W.T.; Goulart, A.C.; Bensenor, I.M.; Lotufo, P.A.; Izar, M.C. Phytosterol consumption and markers of subclinical atherosclerosis: Cross-sectional results from ELSA-Brasil. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1756–1766. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Mirończuk-Chodakowska, I.; Cicha-Mikołajczyk, A.; Drygas, W. Assessment of Plant Sterols in the Diet of Adult Polish Population with the Use of a Newly Developed Database. Nutrients 2021, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Sirirat, R.; Heskey, C.; Haddad, E.; Tantamango-Bartley, Y.; Fraser, G.; Mashchak, A.; Jaceldo-Siegl, K. Comparison of phytosterol intake from FFQ with repeated 24-h dietary recalls of the Adventist Health Study-2 calibration sub-study. Br. J. Nutr. 2019, 121, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; van der Schouw, Y.T.; Trautwein, E.A.; Sioen, I.; Dalmeijer, G.W.; Zock, P.L.; Beulens, J.W. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European Prospective Investigation into Cancer and Nutrition-the Netherlands (EPIC-NL) population. Eur. J. Prev. Cardiol. 2015, 22, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Huang, W.; Hu, Y.; Zhang, L.; Shao, Y.; Wang, M.; Zhang, F.; Zhao, Z.; Mei, X.; Li, T.; et al. Phytosterol Profiles of Common Foods and Estimated Natural Intake of Different Structures and Forms in China. J. Agric. Food Chem. 2018, 66, 2669–2676. [Google Scholar] [CrossRef]
- USDA. Composition of Foods, Raw, Processed, Prepared. National Nutrient Database for Standard Reference Release 28. Modified in 2019. 2015. Available online: https://data.nal.usda.gov/dataset/composition-foods-raw-processed-prepared-usda-national-nutrient-database-standard-reference-release-28-0 (accessed on 28 April 2022).
- European Food Safety Authority (EFSA). Consumption of Food and Beverages with Added Plant Sterols. EFSA J. 2008, 6, 133. [Google Scholar]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; ESC Scientific Document Group; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Turini, E.; Sarsale, M.; Petri, D.; Totaro, M.; Lucenteforte, E.; Tavoschi, L.; Baggiani, A. Efficacy of Plant Sterol-Enriched Food for Primary Prevention and Treatment of Hypercholesterolemia: A Systematic Literature Review. Foods 2022, 11, 839. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [Green Version]
- Drygas, W.; Niklas, A.A.; Piwońska, A.; Piotrowski, W.; Flotyńska, A.; Kwaśniewska, M.; Nadrowski, P.; Puch-Walczak, A.; Szafraniec, K.; Bielecki, W.; et al. Multi-center National Population Health Examination Survey (WOBASZ II study): Assumptions, methods and implementation. Kardiol. Pol. 2016, 74, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011; pp. 3–18. [Google Scholar]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunachowicz, H.; Nadolna, I.; Przygoda, B.; Iwanow, K. Food Composition Tables; PZWL Medical Publishing House: Warsaw, Poland, 2005. [Google Scholar]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts and seeds. Int. J. Food Prop. 2014, 17, 86–92. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Fransen, H.P.; Beulens, J.W.; May, A.M.; Struijk, E.A.; Boer, J.M.; de Wit, G.A.; Onland-Moret, N.C.; van der Schouw, Y.T.; Bueno-de-Mesquita, H.B.; Hoekstra, J.; et al. Dietary patterns in relation to quality-adjusted life years in the EPIC-NL kohort. Prev. Med. 2015, 77, 119–124. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases; WHO Technical Report Series No. 797, Report of a WHO Study Group; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Jóźwiak, J.J.; Studziński, K.; Tomasik, T.; Windak, A.; Mastej, M.; Catapano, A.L.; Ray, K.K.; Mikhailidis, D.P.; Toth, P.P.; LIPIDOGRAM2015 Investigators; et al. The prevalence of cardiovascular risk factors and cardiovascular disease among primary care patients in Poland: Results from the LIPIDOGRAM2015 study. Atheroscler. Suppl. 2020, 42, e15–e24. [Google Scholar] [CrossRef]
- Jaceldo-Siegl, K.; Lütjohann, D.; Sirirat, R.; Mashchak, A.; Fraser, G.E.; Haddad, E. Variations in dietary intake and plasma concentrations of plant sterols across plant-based diets among North American adults. Mol. Nutr. Food Res. 2017, 61, 1600828. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, H.M.; Li, Y.Y.; Xia, S.; Wei, Y.; Yang, L.; Wang, D.; Ye, J.J.; Li, H.X.; Yuan, J.; et al. A combination of omega-3 and plant sterols regulate glucose and lipid metabolism in individuals with impaired glucose regulation: A randomized and controlled clinical trial. Lipids Health Dis. 2019, 18, 106. [Google Scholar] [CrossRef] [Green Version]
- Liput-Sikora, A.; Cybulska, A.M.; Fabian, W.; Fabian-Danielewska, A.; Stanisławska, M.; Kamińska, M.S.; Grochans, E. Cardiovascular Risk Distribution in a Contemporary Polish Collective. Int. J. Environ. Res. Publ. Health 2020, 17, 3306. [Google Scholar] [CrossRef]
- Glenn, A.J.; Lo, K.; Jenkins, D.J.A.; Boucher, B.A.; Hanley, A.J.; Kendall, C.W.C.; Manson, J.E.; Vitolins, M.Z.; Snetselaar, L.G.; Liu, S.; et al. Relationship Between a Plant-Based Dietary Portfolio and Risk of Cardiovascular Disease: Findings from the Women’s Health Initiative Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e021515. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; Trautwein, E.A.; Parker, T.L.; Josse, R.G.; Leiter, L.A.; et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism 2003, 52, 1478–1483. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Chiavaroli, L.; Wong, J.M.; Kendall, C.; Lewis, G.F.; Vidgen, E.; Connelly, P.W.; Leiter, L.A.; Josse, R.G.; Lamarche, B. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. CMAJ 2010, 182, 1961–1967. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, L.; Nishi, S.K.; Khan, T.A.; Braunstein, C.R.; Glenn, A.J.; Mejia, S.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Jenkins, D.J.A.; et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018, 61, 43–53. [Google Scholar] [CrossRef]
- Andersson, S.W.; Skinner, J.; Ellegård, L.; Welch, A.A.; Bingham, S.; Mulligan, A.; Andersson, H.; Khaw, K.T. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. Eur. J. Clin. Nutr. 2004, 58, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, Y.M.; He, L.P.; Chen, C.G.; Zhang, B.; Xue, W.Q.; Su, Y.X. Association of natural intake of dietary plant sterols with carotid intima-media thickness and blood lipids in Chinese adults: A cross-section study. PLoS ONE 2012, 7, e32. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, S.; Ellegard, L.; Johansson, I.; Hallmans, G.; Weinehall, L.; Andersson, H.; Winkvist, A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am. J. Clin. Nutr. 2008, 87, 993–1001. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).