Abstract
Crassocephalum rabens (Asteraceae) is a common herb used in Taiwanese folk medicine to treat inflammation-related syndromes. Pharmacological studies have revealed that galactolipids exhibit anti-oxidative, anti-inflammatory, and anti-hyaluronidase activities and improve skin wrinkles, moisture, and elasticity in healthy subjects. However, the anti-aging effects of C. rabens and its primary active compound, 1,2-di-O-linolenoyl-3-O-β-galactopyranosyl-sn-glycerol (dLGG), remain elusive. Here, we investigated whether C. rabens can improve skin conditions in healthy individuals using a double-blind approach. Forty enrolled volunteers were randomly and equally assigned to the control or treatment group and were required to take either a placebo or a C. rabens extract capsule daily for one month. Skin parameters were measured before and after the study. The results showed significant differences in skin elasticity, wrinkles, collagen content, brightness, and hydration between the baseline and week 4 in the treatment group. Particularly, compared with those in the placebo group, skin wrinkles (p < 0.05), brightness (p < 0.001), collagen content (p < 0.01), and UV spots (p < 0.05) were notably improved after treatment with the C. rabens extract. Our study successfully demonstrated the application of C. rabens in preventing skin aging. Further investigations will be conducted to study the underlying anti-aging mechanism of dLGG.
1. Introduction
The skin is the largest organ in human body, accounting for approximately 15% of the total adult body weight [1]. The skin protects the human body from direct environmental impact and aids in thermoregulation and osmoregulation [2]. Skin health may represent the overall well-being of humans; therefore, the use of cosmetic or dietary supplements with functional ingredients has become a popular way to slow skin aging and maintain good skin conditions [3,4,5]. Skin aging is associated with increased oxidative stress in skin cells, elicited by internal (e.g., genes and cellular metabolism) and external (e.g., air pollutants, smoking, and ultraviolet (UV) radiation) factors [6]. In particular, 80% of premature aging (photoaging) results from chronic exposure to UV radiation (i.e., UVA and UVB) [7,8]. Aged skin is generally characterized by laxity, roughness, wrinkles, dullness, pigmentation, and a thickened epidermis [9]. Exposure to UV radiation leads to the production of reactive oxygen species (ROS) and increases the expression of transcription factor activator protein 1 (AP-1) via activation of the mitogen-activated protein kinase (MAPK) signaling pathway [10]. The upregulation of AP-1 increases the expression of matrix metalloproteinases (MMPs) and inhibits collagen expression in fibroblasts; the degradation of collagen leads to the formation of wrinkles [11]. Collagen is present in 70% of the dermis and aids in the stabilization of the extracellular matrix structure [12]. In addition, ROS cause DNA, lipid, and protein oxidation while driving melanogenesis in melanocytes [13].
Crassocephalum rabens (Asteraceae), also known as Zhaohe Cao, is a common herbal plant that is conventionally used as a folk remedy to treat inflammation-related syndromes in Taiwan [14]. Studies have revealed that C. rabens possesses anti-inflammatory and anti-cancer activities [14,15,16,17,18]. The prominent active compound of C. rabens is 1,2-di-O-linolenoyl-3-O-β-galactopyranosyl-sn-glycerol (dLGG), a phytogalactolipid that exhibits anti-oxidative, anti-tumor, anti-inflammatory, and hepatoprotective effects in cells and rodents [15,16,17,18,19,20]. A study showed that dLGG effectively attenuates the recurrence of triple-negative breast cancer and lung metastasis through the downregulation of fatty acid-binding proteins, peroxisome proliferator activated receptor γ, and epoxyeicosatrienoic acids [21]. Furthermore, Takahashi et al. demonstrated that dLGG scavenges free radicals in promyeloblasts [20]. Moreover, botanical extracts (e.g., Rosa canina) containing galactolipids (e.g., monogalactosyl diacylglycerol and digalactosyl monoacylglycerol) have been shown to delay skin aging and ameliorate skin conditions in in vitro and clinical studies [22,23,24,25,26,27,28].
However, to the best of our knowledge, the anti-aging effects of C. rabens have not been evaluated in detail. The present study investigated the effects of C. rabens extract on healthy skin using a randomized, parallel, double-blind, and placebo-controlled approach.
2. Materials and Methods
2.1. Preparation of C. rabens Extract
The voucher specimen for C. rabens refers to the specimen no. 21152, 33768, 36303, 38867, 38959 in the Taiwan Wild Plant Database [29]. The aerial parts of C. rabens (harvested approximately 120 days after seeds were sown) were cleaned with running water and distilled water. The samples were dried using a food dehydrator at 40 °C, followed by crushing with a pulverizer. The powder was processed via ultrasonic extraction with 95% ethanol (1:10 w/v) at 40 °C for 3 h, and the extract was filtered using Whatman filter paper No. 1. The filtrate was freeze-dried and stored in a freezer. For this study, the C. rabens powder was filled in capsules.
2.2. dLGG Analysis
The levels of dLGG were analyzed using a high-performance liquid chromatography (HPLC) system (e2695; Waters Corporation, Milford, MA, USA) equipped with a photodiode array detector (2998; Waters Corporation) and an Inertsil ODS-HL analytical column (4.6 × 250 mm, 5 μm; GL Sciences Inc., Tokyo, Japan). The flow rate of the mobile phase (98% of methanol) was 1.0 mL/min, and the column temperature was set at 30 °C. The injection volume was 10 μL, and the detection wavelength was 210 nm.
2.3. Study Design
This clinical study was approved by the Ethics Committee of the Antai Medical Care Corporation, Antai Tian-Sheng Memorial Hospital (IRB No. 21-129-A), and the study protocol was registered with ClinicalTrials.gov (NCT05309161). This study was performed in accordance with the principles of the Declaration of Helsinki, and all subjects provided written informed consent before the study. Forty healthy subjects were enrolled in this study, and all subjects completed the study. Eligible participants were healthy adults over 20 years of age. The exclusion criteria were as follows: (i) non-volunteers; (ii) skin disorders; (iii) liver or kidney diseases; (iv) allergy to cosmetics, drugs, or food supplements; (v) pregnancy and lactation; (vi) acceptance of esthetic medicine treatments (e.g., intense pulse light, medical peelings, or laser therapy) before 4 weeks of the study; (vii) suffering from chronic or severe diseases; (viii) members of this research group.
The study had a randomized (1:1 ratio), double-blind, parallel, and placebo-controlled design. The subjects were instructed to take a maltodextrin capsule (placebo; 180 mg) or a C. rabens extract capsule (treatment; 180 mg) every day for 4 weeks. Skin parameters were recorded at weeks 0 and 4.
2.4. Efficacy Analysis
The number of skin pores, spots, UV spots, and brown spots and wrinkles, red areas, and the presence of texture on the full face (i.e., forehead, orbital rim, upper and lower cheeks, chin, and nose) were measured using the VISIA®-CR skin analysis system (Canfield, Parsippany-Troy Hills, NJ, USA). Skin lightness of the upper cheek was measured using Chroma Meter MM-500 (Minolta, Osaka, Japan). Skin hydration content of the upper cheek was measured using Corneometer® CM825 (Courage + Khazaka Electroni, Cologne, Germany), and the final value was the average of triplicate measurements. The skin elasticity of the upper cheek was measured using Soft Plus (Callegari 1930, Parma, Italy), and the final value was the average of triplicate measurements.
2.5. Statistical Analysis
Data were presented as mean values and standard deviations. The age comparison and measured results of skin parameters within groups and between groups were analyzed using a paired t-test and an independent t-test, respectively, with Excel 2021 (Microsoft, Redmond, WA, USA), and p < 0.05 was considered statistically significant.
3. Results
3.1. dLGG Analysis
C. rabens contains a variety of phytochemicals, but dLGG is its most well-known marker compound, as evidenced by several pharmacological studies [12,13,14,15,16,17,18]. In the HPLC analysis, dLGG was detected in the C. rabens extract at 13:58 min based on the running time of the standard sample, and its concentration was determined to be 218.05 mg/mL (Figure 1).
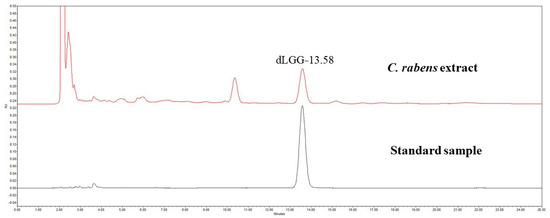
Figure 1.
HPLC analysis for dLGG in the C. rabens extract.
3.2. Baseline Characteristics
Forty subjects were recruited for this study and randomly assigned to the placebo or treatment group, with all participants completing the study (Table 1). The male-to-female ratio was identical for both groups, with mean ages of the placebo and treatment groups being 46.4 and 43.9 years, respectively, and no statistical difference in the mean age between the groups.

Table 1.
Baseline characteristics (mean value ± SD).
3.3. Improvement in Collagen-Associated Parameters
Table 2 displays the results of collagen-associated parameters before and after the study. No significant differences in skin pores were observed between baseline and week 4 for both the groups. The mean change (Δ) in skin pores in the treatment group was three times greater than that in the control group; however, the difference between the groups was not obvious. The mean changes in skin elasticity were similar for both groups, whereas both the placebo and C. rabens extract led to a significant increase in skin elasticity at week 4.

Table 2.
Measurement of collagen-related factors in the subjects (mean value ± SD).
The mean changes in skin texture in the placebo and treatment groups were +19.2 and −12.8, respectively, while there were no significant differences in skin texture within and between groups. In contrast, a noticeable reduction in wrinkles was observed in the treatment group. The mean changes in skin wrinkles in the placebo and treatment groups were +0.1 and −3.8, respectively. The improvement effect was weighted over placebo effects. Moreover, both groups exhibited significantly positive increments in collagen content after the study; nevertheless, the C. rabens extract showed a more prominent improvement in collagen synthesis than the placebo.
3.4. Improvement in Skin Pigmentation
Table 3 shows the number of measured skin spots, UV spots, and brown spots before and after the study. Skin spots refer to visible skin marks, UV spots represent epidermal melanin that can absorb UV light, whereas brown spots represent pigmentation on and beneath the skin. No significant changes in the number of skin spots or brown spots were observed within or between the groups. The difference in the number of UV spots between weeks 0 and 4 was –12.8 in the treatment group, but the mean value of the number of UV spots at week 4 increased by 7.5 relative to the baseline result. The comparison of UV spots between the groups was significant.

Table 3.
Measurement of skin pigmentation in the subjects (mean value ± SD).
3.5. Other Skin Parameters
Table 4 shows the measured results of skin brightness, hydration, and red areas before and after the study. C. rabens extract significantly improved the skin brightness of subjects over 4 weeks, and the improvement effect could be distinguished from the placebo effect. In addition, there was a significant improvement in skin hydration between the baseline and week 4 in the treatment group. The improvement in red areas after treatment with the C. rabens extract was not obvious.

Table 4.
Measurement of skin brightness, skin hydration, and red areas in the subjects (mean value ± SD).
4. Discussion
The safety of the C. rabens extract has been verified by acute and sub-acute toxicity studies in rats, and the no-observed-adverse-effect-level (NOAEL) was reported to be greater than 1666.7 mg/kg body weight in male and female rats [30]. Accordingly, the acceptable daily intake (ADI) of the extract for a 60 kg adult is approximately 1 g, based on a 100-fold safety factor [31]. Our intervention dose was 180 mg every day, which is 5.5 times lower than the ADI.
While little is known regarding the mechanisms involved in the improvement of skin health, a few in vitro and clinical studies point towards the role of galactolipids in slowing down skin aging [22,23,24,25,26,27,28]. Rose (R. canina) hips, which contain abundant galactolipids (e.g., (2S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9-12-15-trienoyl]-3-O-β-d-galactopyranosyl glycerol), inhibit the expression of proinflammatory cytokines (e.g., tumor necrosis factor α and interleukins 1β and 6) and proinflammatory enzymes (e.g., MMPs and cyclooxygenase-2) while simultaneously reducing oxidative stress in cells [22,23]. The galactolipids isolated from Impatiens parviflora DC exhibit anti-hyaluronidase activity [24], while M. integrifolia leaf extract, which is rich in monogalactosyl diacylglycerol 36:4 and digalactosyl monoacylglycerol 18:2, exerts a strong tyrosinase inhibitory activity [25]. Furthermore, clinical studies have shown that R. canina hips can significantly improve skin wrinkles, hydration, and elasticity in healthy individuals [26,27]. A wheat polar lipid complex extract containing digalactosyl diglycerides notably increased skin hydration, elasticity, and smoothness while reducing trans-epidermal water loss, skin roughness, and skin wrinkles in healthy subjects after a month of treatment [28].
No adverse effects were observed throughout this study. After the 4 week intervention, the scores of skin elasticity, wrinkles, and collagen content in subjects were significantly improved in comparison with the baseline. The C. rabens extract reinforced skin elasticity and reduced skin wrinkles as compared with the placebo. Contrastingly, although the mean changes in skin pores and texture reached a certain degree of reduction, individual variations affected the outcomes. We propose that these results may be attributed to the anti-aging effects of galactolipids. Galactolipids are powerful anti-oxidants and anti-inflammatory agents that can effectively inhibit collagenase and hyaluronidase activities [22,23,24]. An excessive ROS production and the expression of pro-inflammatory cytokines exacerbate the degradation of collagen and elastin in the skin owing to the upregulation of MMPs and downregulation of collagen synthesis in fibroblasts, which undermines the skin structure and promotes the formation of skin wrinkles [32]. In addition, hyaluronic acid (HA), comprising D-glucuronic acid and N-acetyl-D-glucosamine, plays an essential role in the integration and retention of water in skin tissue [33]. ROS cause the degradation of HA and activation of hyaluronidase, consequently leading to dry and rough skin and increased skin wrinkles [34]. The anti-hyaluronidase activity of the C. rabens extract might partially be responsible for the significant improvement in skin hydration of the subjects (Table 4).
Regarding depigmentation, the C. rabens extract significantly reduced the number of UV spots (melanin in the basal and suprabasal layers of the epidermis) caused by sun damage, but its efficacy in reducing visible skin marks in the dermis was not remarkable [35]. We hypothesized that the positive changes in the appearance of spots and brown spots might be more pronounced if the extract was consumed for longer periods of time. This speculation was based on the anti-tyrosinase activity of galactolipids and the corresponding observation of a small improvement in spot appearance (Table 3) [25].
Skin brightness is related to skin tone, hydration, and surface roughness. The significant change in skin brightness was correlated with the complex effects of improvements in skin spots, texture, pores, and hydration. Skin hydration was remarkably improved in the treatment group, suggesting that dLGG could reduce oxidative stress and suppress hyaluronidase activity in skin cells [24]. Red areas result from sensitive skin, winter xerosis, alcohol ingestion, and UV radiation [36]. Since we did not recruit individuals with sensitive skin and conducted this study in spring, the study conditions might explain the insignificant change in red skin areas in the treatment group.
Our study suggests that C. rabens can be developed as a dietary supplement to improve skin health and delay skin aging; however, there are several limitations to this study. First, it is difficult to extrapolate the results to men, considering the disproportional female-to-male ratios in both groups. Second, the addition of a positive control, different concentrations of C. rabens extracts, more parameters (e.g., temperature, pH value, erythema, trans-epidermal water loss, stratum corneum hydration, and stiffness), weekly measurement, and follow up were not considered in this study, which might have slightly affected the accurate observation of the anti-aging activity of the C. rabens extract. Third, the underlying mechanism of dLGG in skin aging is unclear and has not been meticulously verified in this study; we speculate that the improvement in skin health by the extract is likely due to the synergistic effect of phytochemicals present in C. rabens. We will conduct further investigations to examine the anti-aging effects of dLGG in cells and rodents (including skin disease models). Finally, we will consider the shortcomings and further animal results and conduct another clinical study to strictly verify the anti-aging activity of C. rabens in terms of dietary supplements and cosmetics (in the form of gel or cream).
5. Conclusions
In summary, we successfully demonstrated the application of C. rabens in preventing skin aging. However, considering the limitations of the study design, further investigations should be carried out to verify the anti-aging activities of C. rabens and dLGG in rodents and humans.
Author Contributions
C.-M.K., project administration, data analysis and curation, and manuscript preparation; C.-H.L., study implementation, data analysis, and manuscript preparation; W.-H.C., project administration, data curation, and manuscript preparation; T.-Y.L., study implementation and data analysis; P.-K.H., project supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Antai Medical Care Corporation, Antai Tian-Sheng Memorial Hospital (IRB No. 21-129-A); the study protocol was registered at ClinicalTrials.gov (NCT05309161). All participants provided informed consent before the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the research group of Chia-Hua Liang for providing support for the entire study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses. Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Hogan, M.B.; Peele, K.; Wilson, N.W. Skin barrier function and its importance at the start of the atopic march. J. Allergy 2012, 2012, 901940. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.H.; Kwon, S.B.; An, I.S.; An, S.; Ahn, K.J. Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp. Ther. Med. 2016, 12, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Cho, S. The role of functional foods in cutaneous anti-aging. J. Lifestyle Med. 2014, 4, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Puizina-Ivić, N. Skin aging. Acta Dermatovenerol. Alp. Panon. Adriat. 2008, 17, 47–54. [Google Scholar]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Alexander, R.A.; Liang, C.H.; Liu, C.; Lin, Y.H.; Lin, Y.H.; Chan, L.P.; Kuan, C.M. Collagen formula with Djulis for improvement of skin hydration, brightness, texture, crow’s feet, and collagen content: A double-blind, randomized, placebo-controlled trial. J. Cosmet. Dermatol. 2021, 20, 188–194. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Skin penetration of nanoparticles. In Emerging Nanotechnologies Immunology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–88. [Google Scholar]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.-C.; Chen, Y.-P.; Wu, J.-H.; Huang, C.-C.; Wang, S.-Y.; Yang, N.-S.; Shyur, L.-F. A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Res. 2007, 67, 6907–6915. [Google Scholar] [CrossRef] [Green Version]
- Apaya, M.K.; Chang, M.-T.; Shyur, L.-F. Phytomedicine polypharmacology: Cancer therapy through modulating the tumor microenvironment and oxylipin dynamics. Pharmacol. Ther. 2016, 162, 58–68. [Google Scholar] [CrossRef]
- Apaya, M.K.; Hsiao, P.-W.; Yang, Y.-C.; Shyur, L.-F. Deregulating the CYP2C19/epoxy-eicosatrienoic acid-associated FABP4/FABP5 signaling network as a therapeutic approach for metastatic triple-negative breast cancer. Cancers 2020, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Chang, C.-K.; Chang, M.-T.; Shyur, L.-F. Plant galactolipid dLGG suppresses lung metastasis of melanoma through deregulating TNF-α-mediated pulmonary vascular permeability and circulating oxylipin dynamics in mice. Int. J. Cancer 2018, 143, 3248–3261. [Google Scholar] [CrossRef] [Green Version]
- Hsan, K.-M.; Chen, C.-C.; Shyur, L.-F. Current research and development of chemotherapeutic agents for melanoma. Cancers 2010, 2, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Larsen, E.; Christensen, L.P. Common vegetables and fruits as a source of 1,2-di-O-b-linolenoyl-3-O-b-D-galactopyranosyl-snglycerol, a potential anti-inflammatory and antitumor agent. J. Food Lipids 2007, 14, 272–279. [Google Scholar] [CrossRef]
- Takahashi, M.; Sugiyama, Y.; Kawabata, K.; Takahashi, Y.; Irie, K.; Murakami, A.; Kubo, Y.; Kobayashi, K.; Ohigashi, H. 1,2-Di-O-α-linolenoyl-3-O-β-galactosyl-sn-glycerol as a superoxide generation inhibitor from Perilla frutescens var. crispa. Biosci. Biotechnol. Biochem. 2011, 75, 2240–2242. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-N.; Chen, H.-M.; Shyur, L.-F. Current advancements of plant-derived agents for triple-negative breast cancer therapy through deregulating cancer cell functions and reprogramming tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 13571. [Google Scholar] [CrossRef]
- Cheng, B.C.; Fu, X.Q.; Guo, H.; Li, T.; Wu, Z.Z.; Chan, K.; Yu, Z.L. The genus Rosa and arthritis: Overview on pharmacological perspectives. Pharmacol. Res. 2016, 114, 219–234. [Google Scholar] [CrossRef]
- Winther, K.; Vinther Hansen, A.S.; Campbell-Tofte, J. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Podolak, I.; Galanty, A.; Załuski, D.; Makowska-Wąs, J.; Sobolewska, D.; Janeczko, Z.; Żmudzki, P. In vitro anti-denaturation and anti-hyaluronidase activities of extracts and galactolipids from leaves of Impatiens parviflora DC. Nat. Prod. Res. 2016, 30, 1219–1223. [Google Scholar] [CrossRef]
- El Hawary, S.S.; Abubaker, M.; Abd El-Kader, E.M.; Mahrous, E.A. Phytochemical constituents and anti-tyrosinase activity of Macadamia integrifolia leaves extract. Nat. Prod. Res. 2022, 36, 1089–1094. [Google Scholar] [CrossRef]
- Phetcharat, L.; Wongsuphasawat, K.; Winther, K. The effectiveness of a standardized rose hip powder, containing seeds and shells of Rosa canina, on cell longevity, skin wrinkles, moisture, and elasticity. Clin. Interv. Aging 2015, 10, 1849–1856. [Google Scholar]
- Winther, K.; Petcharat, L.; Wongsuphasawat, K. Rose-HIP including seeds and shells reported to reduce symptoms of osteoarthritis, improves quality of the skin by mechanisms which may involve collagen and longevity of cell membranes. Osteoarthr. Cartil. 2015, 23, A170. [Google Scholar] [CrossRef] [Green Version]
- Bizot, V.; Cestone, E.; Michelotti, A.; Nobile, V. Improving skin hydration and age-related symptoms by oral administration of wheat glucosylceramides and digalactosyl diglycerides: A human clinical study. Cosmetics 2017, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Taiwan Wild Plant Database. Available online: https://plant.tesri.gov.tw/plant106/WebPlantDetail.aspx?tno=539034010 (accessed on 15 June 2022).
- Hsu, P.-K.; Tsai, Y.-T.; Lin, Y.-C.; Kuan, C.-M. Assessment of the acute and sub-acute toxicity of the ethanolic extract of the aerial parts of Crassocephalum rabens (Asteraceae) in rats. Toxicol. Rep. 2021, 9, 58–63. [Google Scholar] [CrossRef]
- Dorne, J.L.; Renwick, A.G. The refinement of uncertainty/safety factors in risk assessment by the incorporation of data on toxicokinetic variability in humans. Toxicol. Sci. 2005, 86, 20–26. [Google Scholar] [CrossRef]
- Kim, M.; Park, H. Molecular mechanisms of skin aging and rejuvenation. In Molecular Mechanism of the Aging Process and Rejuvenation; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Moon, S.H.; Hong, Y.; Ahn, D.U.; Paik, H.D. Anti-elastase and anti-hyaluronidase activity of phosvitin isolated from hen egg yolk. Br. Poult. Sci. 2020, 61, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.L.; Matos, L.F.; Brunstein, F.; Ferreira, L.M.; Silva, A.; Costa, D., Jr. A clinical, prospective, randomized, double-blind trial comparing skin whitening complex with hydroquinone vs. placebo in the treatment of melasma. Int. J. Dermatol. 2003, 42, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Yamada, W.; Miyasaka, K.; Shimoda, H. Ameliorating effects of Delphinol®, anthocyanin standardized maqui berry extract, on skin brightness and redness in Japanese females: A randomized double-blind placebo-controlled pilot study. J. Cosmet. Dermatol. Sci. Appl. 2020, 10, 149–162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).