Effects of Probiotics Supplementation on Gastrointestinal Symptoms in Athletes: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. PRISMA Guidelines and the PICO Principle
- (1)
- studies involving healthy adult athletes of both sexes who did physical activity;
- (2)
- interventions with probiotics, prebiotics, and synbiotics (the PRO group) with detailed information about the dose of supplementation, strain, and strain designation;
- (3)
- inclusion of a control/placebo group (the PLA group);
- (4)
- outcomes not previously defined (as an open question; all outcomes evaluated by the included studies were reported);
- (5)
- randomised clinical trials (crossover or parallel), with no language or date restrictions.
2.2. Literature Sources, Search Strategy, and Selection Criteria
2.3. Data Extraction and Quality Assessment
3. Results
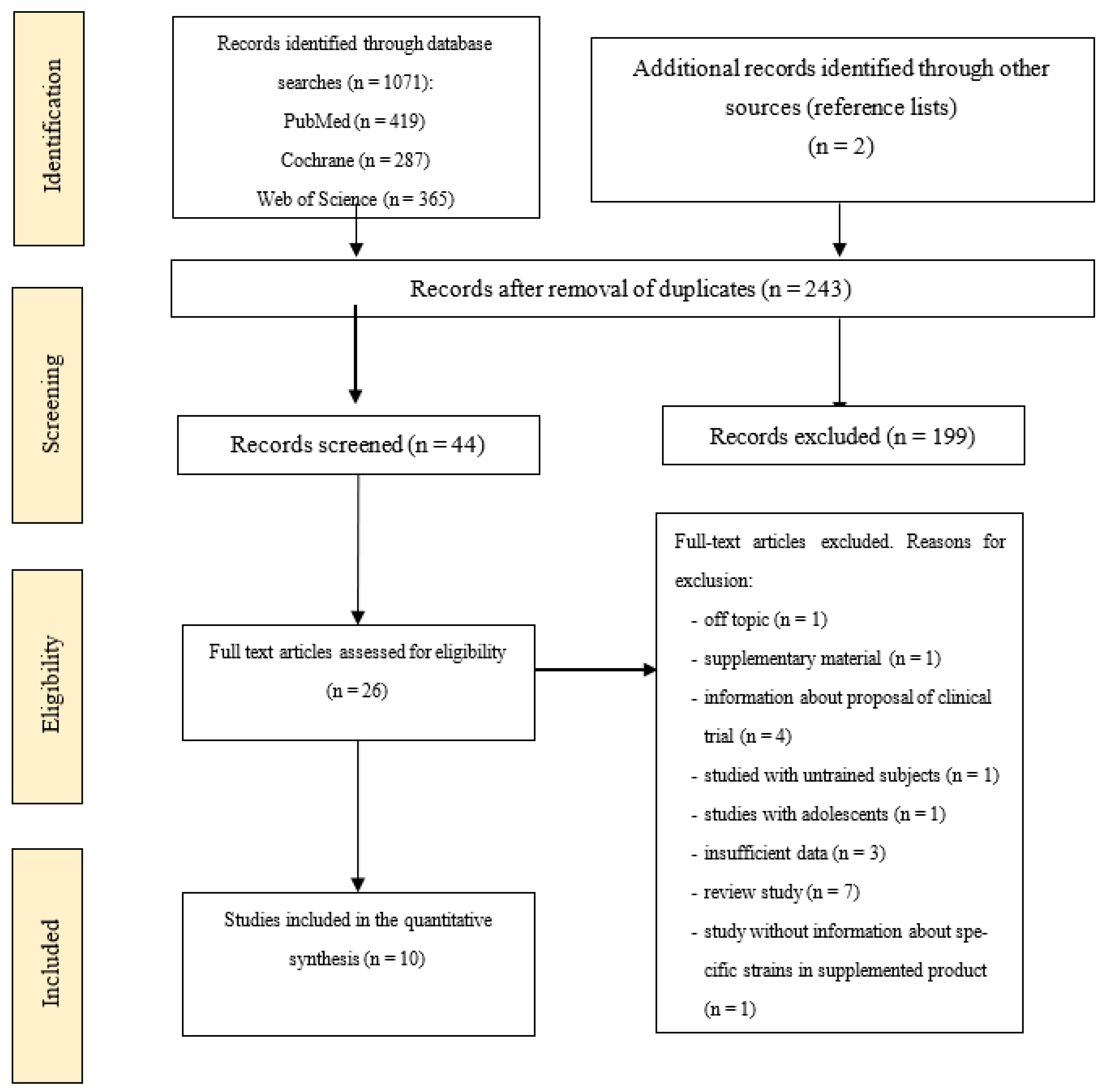
3.1. Study Selection
3.2. Population and Study Characteristics
3.3. Number of GI Symptoms
3.4. The Proportion of Subjects with GI Symptoms (%)
3.5. Total Symptom Severity of GI
3.6. Duration of GI Symptoms
3.7. Influence of Probiotic Supplementation on Inflammatory Markers and Gut Barrier Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pugh, J.N.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Prevalence, Severity and Potential Nutritional Causes of Gastrointestinal Symptoms during a Marathon in Recreational Runners. Nutrients 2018, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic Review: Exercise-Induced Gastrointestinal Syndrome-Implications for Health and Intestinal Disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between Gastro-Intestinal Complaints and Endotoxaemia, Cytokine Release and the Acute-Phase Reaction during and after a Long-Distance Triathlon in Highly Trained Men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Ter Steege, R.W.F.; Van Der Palen, J.; Kolkman, J.J. Prevalence of Gastrointestinal Complaints in Runners Competing in a Long-Distance Run: An Internet-Based Observational Study in 1281 Subjects. Scand. J. Gastroenterol. 2008, 43, 1477–1482. [Google Scholar] [CrossRef]
- Peters, H.P.F.; Bos, M.; Seebregts, L.; Akkermans, L.M.A.; van Berge Henegouwen, G.P.; Bol, E.; Mosterd, W.L.; de Vries, W.R. Gastrointestinal Symptoms in Long-Distance Runners, Cyclists, and Triathletes: Prevalence, Medication, and Etiology. Am. J. Gastroenterol. 1999, 94, 1570–1581. [Google Scholar] [CrossRef]
- Lamprecht, M.; Frauwallner, A. Exercise, Intestinal Barrier Dysfunction and Probiotic Supplementation. In Medicine and Sport Science; Lamprecht, M., Ed.; S. KARGER AG: Basel, Switzerland, 2012; Volume 59, pp. 47–56. ISBN 978-3-8055-9992-4. [Google Scholar]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Roberts, J.; Suckling, C.; Peedle, G.; Murphy, J.; Dawkins, T.; Roberts, M. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients 2016, 8, 733. [Google Scholar] [CrossRef] [Green Version]
- Leite, G.S.F.; Ayane, S.; Resende Master Student; West, N.P.; Lancha, A.H. Probiotics and Sports: A New Magic Bullet? Nutrients 2019, 60, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomised, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports. Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [Green Version]
- Pugh, J.N.; Sparks, A.S.; Doran, D.A.; Fleming, S.C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Four Weeks of Probiotic Supplementation Reduces GI Symptoms during a Marathon Race. Eur. J. Appl. Physiol. 2019, 119, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Kekkonen, R.A.; Vasankari, T.J.; Vuorimaa, T.; Haahtela, T.; Julkunen, I.; Korpela, R. The Effect of Probiotics on Respiratory Infections and Gastrointestinal Symptoms during Training in Marathon Runners. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 352–363. [Google Scholar] [CrossRef]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic Supplementation for Respiratory and Gastrointestinal Illness Symptoms in Healthy Physically Active Individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Savović, J.; Page, M.; Elbers, R.; Sterne, J. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021. [Google Scholar]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Lactobacillus Fermentum (PCC®) Supplementation and Gastrointestinal and Respiratory-Tract Illness Symptoms: A Randomised Control Trial in Athletes. Nutr. J. 2011, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Pumpa, K.L.; McKune, A.J.; Harnett, J. A Novel Role of Probiotics in Improving Host Defence of Elite Rugby Union Athlete: A Double-Blind Randomised Controlled Trial. J. Sci. Med. Sport 2019, 22, 876–881. [Google Scholar] [CrossRef]
- Schreiber, C.; Tamir, S.; Golan, R.; Weinstein, A.; Weinstein, Y. The Effect of Probiotic Supplementation on Performance, Inflammatory Markers and Gastro-intestinal Symptoms in Elite Road Cyclists. J. Int. Soc. Sports. Nutr. 2021, 18, 36. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.K.; Vitetta, L. Effects of Probiotics Supplementation on Gastrointestinal Permeability, Inflammation and Exercise Performance in the Heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Pugh, J.N.; Wagenmakers, A.J.M.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic Supplementation Increases Carbohydrate Metabolism in Trained Male Cyclists: A Randomised, Double-Blind, Placebo-Controlled Crossover Trial. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily Probiotic’s (Lactobacillus Casei Shirota) Reduction of Infection Incidence in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, Prebiotics and Synbiotics: Useful for Athletes and Active Individuals? A Systematic Review. Benef. Microbes 2020, 11, 135–149. [Google Scholar] [CrossRef]
- Miles, M.P. Probiotics and Gut Health in Athletes. Curr. Nutr. Rep. 2020, 9, 129–136. [Google Scholar] [CrossRef]
- Brock-Utne, J.G.; Gaffin, S.L.; Wells, M.T.; Gathiram, P.; Sohar, E.; James, M.F.; Morrell, D.F.; Norman, R.J. Endotoxaemia in Exhausted Runners after a Long-Distance Race. S. Afr. Med. J. 1988, 73, 533–536. [Google Scholar]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Vasile, A.; Bahrim, G.E.; Orpianesi, C.; Cresci, A. Effect of Buckwheat Flour and Oat Bran on Growth and Cell Viability of the Probiotic Strains Lactobacillus Rhamnosus IMC 501®, Lactobacillus Paracasei IMC 502® and Their Combination SYNBIO®, in Synbiotic Fermented Milk. Int. J. Food Microbiol. 2013, 167, 261–268. [Google Scholar] [CrossRef]
- Starkie, R.L.; Rolland, J.; Angus, D.J.; Anderson, M.J.; Febbraio, M.A. Circulating Monocytes Are Not the Source of Elevations in Plasma IL-6 and TNF-α Levels after Prolonged Running. Am. J. Physiol.-Cell Physiol. 2001, 280, C769–C774. [Google Scholar] [CrossRef]
- Łagowska, K.; Bajerska, J. Probiotic Supplementation and Respiratory Infection and Immune Function in Athletes: Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Athl. Train. 2021, 56, 1213–1223. [Google Scholar] [CrossRef]
- Vollmer-Conna, U.; Fazou, C.; Cameron, B.; Li, H.; Brennan, C.; Luck, L.; Davenport, T.; Wakefield, D.; Hickie, I.; Lloyd, A. Production of Pro-Inflammatory Cytokines Correlates with the Symptoms of Acute Sickness Behaviour in Humans. Psychol. Med. 2004, 34, 1289–1297. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Wosinska, L.; Cotter, P.D.; O’Sullivan, O.; Guinane, C. The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients 2019, 11, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PICOS Criteria | Definition of Criteria for Studies |
|---|---|
| Participants | Athletes (aged ≥ 18 years) |
| Intervention | Oral supplementation with probiotics, prebiotics, symbiotics |
| Comparator | Control/placebo |
| Outcomes | Primary outcome: gastrointestinal symptoms Secondary outcomes: IL-6, TNF-α, gastrointestinal permeability, |
| Study design | RCT randomised controlled trial |
| Study | Design | Population (n), Age | Supplementation Protocol | Control/ Placebo | Duration | Limitation of the Study | ||
|---|---|---|---|---|---|---|---|---|
| Gleeson et al. (2011) [24] | P | Runners, cyclists, swimmers, triathlons, racket sports, team gamers (male and females) PRO: n = 32; 32 (14) PLA: n = 26; 2 (9) All: 27.0 (11.6) | Lactobacillus casei Shirota (LcS) (6.5 × 109 CFU) per probiotic drink | 2 probiotic drinks per day | single strain | drink without Lactobacillus casei Shirota | 16 weeks | |
| Kekkonen et al. (2007) [15] | P | Marathon runners (male & female) PRO: n = 61; 40 (22–58) PLA: n = 58; 40 (23–69) | Lactobacillus rhamnosus LGG, ATCC 53,103 (3 × 108 CFU) in milk-based fruit drink or Lactobacillus rhamnosus LGG, ATCC 53,103 (5 × 109 CFU) per capsule | 2 milk-based fruit drinks per day (4 × 1010 CFU) or 2 capsules per day (1 × 1010 CFU) | single strain | milk-based fruit drink or capsule without probiotic bacteria | 12 weeks | |
| Pugh et al. (2019) [14] | P | Runners (male & female) PRO: n = 12; 34.8 (6.9) PLA: n = 12; 36.1 (7.5) | Lactobacillus acidophilus CUL60, L. acidophilus CUL21, Bifidobacterium bifidum CUL20, & Bifidobacterium animalis subsp. Lactis CUL34 (2.5 × 1010 CFU) per capsule | 1 capsule per day | multi-strain | cornstarch | 4 weeks | lack information about sweat rates and levels of dehydration and core temperature which can affect GI symptoms |
| Pugh et al. (2020) [23] | C | Cyclists (male) PRO: 7 PLA: 7 All: 23 (4) | Lactobacillus acidophilus CUL60, L. acidophilus CUL21, Bifidobacterium bifidum CUL20, & Bifidobacterium animalis subsp. lactis CUL34 (2.5 × 1010 CFU) per capsule | 1 capsule per day | multi-strain | cornstarch | 28 days | small sample size and lack of statistical power |
| Pumpa et al. (2019) [20] | P | Elite rugby union athletes PRO: n = 9; 27.0 (3.2) PLA: n = 10; 26.6 (2.9) | Lactobacillus rhamnosus CUL63 (1.555 × 1010 CFU), Lactobacillus casei CUL06 (9.45 × 109 CFU), Lactobacillus acidophilus CUL21 + CUL60 (2 × 1010 CFU), Lactobacillus plantarum CUL66 (3.15 × 109 CFU), Lactobacillus fermentum CUL67 (1.35 × 109 CFU), Bifidobacterium animalis subsp. lactis CUL 34 (6.55 × 109 CFU), Bifidobacterium bifidum CUL20 (3.45 × 108 CFU), Streptococcus thermophilus CUL68 (2.25 × 109 CFU) and Saccharomyces boulardi (S. cerevisiae) (250 mg) per capsule | 1 capsule per day | multi-strain | microcrystalline, iron oxide yellow, iron oxide red, gelatin capsule; and SB Floractiv: microcrystalline cellulose, lactose, calcium hydrogen phosphate dihydrate, povodine, silica colloidal anhydrous, magnesium stearate, gelatin capsule | 17 weeks | |
| Roberts (2016) [9] | P | Male and female triatheletes PRO: n = 10; 35 (2) PLA: n =10, 35 (3) | Lactobacillus acidophilus CUL60, NCIMB 30157 (1 × 1010 CFU) Lactobacillus acidophillus CUL21, NCIMB 30156 (1 × 1010 CFU), Bifidobacterium bifidum CUL20, NCIMB 30172 (9.5 × 109 CFU) Bifidobacterium animalis subsp. lactis CUL34, NCIMB 30153 (5 × 108 CFU) and fructooligosaccharides (55.8 mg) per capsule | 1 capsule per day | multi-strain + prebiotic | 200 mg cornflour | 12 weeks before and 6 days after a triathlon | - |
| Schreiber et al. (2021) [21] | P | Male cyclists PLA: n = 11 PRO: n = 16 (19-40) | Lactobacillus helveticus Lafti L10 (4.3 × 109 CFU), Bifidobacterium animalis subsp. lactis Lafti B94 (4.3 × 109 CFU), Enterococcus faecium R0026 (3.9 × 109 CFU), Bifidobacterium longum R0175 (2.1 × 109 CFU) & Bacillus subtilis R0179 (0.4 × 109 CFU) per capsule | 1 capsule per day | multi-strain | capsules contained the excipients only (potato starch, magnesium stearate, ascorbic acid, and white vegetable powder) without the bacteria | 90 days/~13 weeks | small sample size the cyclists were at various phases of their training/completion season thus, some were at their peak competition level while others were training for their upcoming competitions season |
| Shing et al. (2014) [22] | C | Male runners PRO: n = 5 PLA: n = 5 All: 27 (2) | Lactobacillus acidophilus CUL21 + CUL60 (7.45 × 109 CFU), Lactobacillus rhamnosus CUL66 (1.555 × 1010 CFU), Lactobacillus casei CUL07 (9.45 × 109 CFU), Lactobacillus plantarum CUL66 (3.15 × 109 CFU), Lactobacillus fermentum CUL67 (1.35 × 109 CFU), Bifidobacterium animalis subsp. lactis CUL34 (4.05 × 109 CFU), Bifidobacterium breve CUL69 (1.35 × 109 CFU), Bifidobacterium bifidum CUL20 (4.5 × 108 CFU), & Streptococcus thermophilus CUL68 (2.25 × 109 CFU) per capsule | 1 capsule per day | multi-strain | skim milk powder | 4 weeks | small sample size and only included males, short study duration of 4 weeks |
| West et al. [19] | P | Male and female cyclists and triathletes (not elite) PRO: n = 29; 35.2 (10.3) PLA: n = 33; 36.4 (8.9) 35 (9) | Lactobacillus fermentum VRI–003 PCC® (1 × 109 CFU) per capsule | 1 capsule per day | single strain | Microcrystalline cellulose | 11 weeks | |
| West et al. [16] | P | Athletes (male and female) PRO: n = 161; 36 (12) PRO1: n = 155; 36 (11) PLA: n= 149; 37 (11) | PRO: Bifidobacterium animalis subsp. lactis Bi-04 (2.0 × 109 CFU) per sachet PRO1: Lactobacillus acidophilus NCFM (5.0 × 109 CFU) and Bifidobacterium animalis subsp. lactis Bi-07 (5.0 × 109 CFU) per sachet | PRO: 1 sachet per day PRO1: 1 sachet per day | PRO: single strain PRO1: multi-strain | sucrose base without the probiotic bacteria | 11 weeks | |
| Authors | Number of GI Symptoms | The Proportion of Subjects with GI Symptoms (%) | Total Symptom Severity Score of GI | Duration of Symptoms (Days) | Gut Barrier Function | TNF-α | IL-6 |
|---|---|---|---|---|---|---|---|
| Impact of probiotics on GI symptoms during training | |||||||
| Gleeson et al. 2011 [24] | - |  |  |  | - | ↓ (after 8 weeks of intervention) (after 16 weeks of intervention) (after 16 weeks of intervention) | ↓ (after 8 weeks of intervention) (after 16 weeks of intervention) (after 16 weeks of intervention) |
| Kekkonen et al. 2007 [15] |  |  | - |  | - | - | - |
| Pugh et al. 2019 [14] | ↓ | - | - | - |  | - |  |
| Roberts et al. 2016 [9] | ↓ | - | ↓ | - |  | - | - |
| Schreiber et al. 2021 [21] | ↓ | - | - | - | - |  |  |
| West et al. 2011 [19] | ↑ | - | ↓ | ↑ | - | ↓ | ↓ |
| West et al. 2014 [16] |  | - | - | - | - | - | - |
 | - | - | - | - | - | - | |
| Impact of probiotics on GI symptoms during competition or single event | |||||||
| Kekkonen et al. 2007 [15] | - | - | - | - | - | - | - |
| Pugh et al. 2019 [14] | - | - | ↓ (during final 1/3 of marathon race) | - |  | - |  |
| Pugh et al. 2020 [23] | - | - |  | - |  | - | ↓ |
| Pumpa et al. 2019 [20] |  | - | - | - | - | - |  |
| Roberts et al. 2016 [9] | - | - | - | - | - | - | - |
| Schreiber et al. 2021 [21] | - | - | - | - | - |  |  |
| Shing et al. 2014 [22] |  | - | - | - |  |  |  |
| Impact of probiotics on GI symptoms after the competition | |||||||
| Kekkonen et al. 2007 [15] |  |  | - | ↓ | - | - | - |
| Pugh et al. 2019 [14] | - | - | - | - |  | - |  |
| Schreiber et al. 2021 [21] | - | - |  | - | - |  |  |
 there was no (significant) effect. ↑ a significant increase in effect.
there was no (significant) effect. ↑ a significant increase in effect.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łagowska, K.; Bajerska, J.; Kamiński, S.; Del Bo’, C. Effects of Probiotics Supplementation on Gastrointestinal Symptoms in Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 2645. https://doi.org/10.3390/nu14132645
Łagowska K, Bajerska J, Kamiński S, Del Bo’ C. Effects of Probiotics Supplementation on Gastrointestinal Symptoms in Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients. 2022; 14(13):2645. https://doi.org/10.3390/nu14132645
Chicago/Turabian StyleŁagowska, Karolina, Joanna Bajerska, Szymon Kamiński, and Cristian Del Bo’. 2022. "Effects of Probiotics Supplementation on Gastrointestinal Symptoms in Athletes: A Systematic Review of Randomized Controlled Trials" Nutrients 14, no. 13: 2645. https://doi.org/10.3390/nu14132645
APA StyleŁagowska, K., Bajerska, J., Kamiński, S., & Del Bo’, C. (2022). Effects of Probiotics Supplementation on Gastrointestinal Symptoms in Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients, 14(13), 2645. https://doi.org/10.3390/nu14132645








