Effects of Developmental Failure of Swallowing Threshold on Obesity and Eating Behaviors in Children Aged 5–15 Years
Abstract
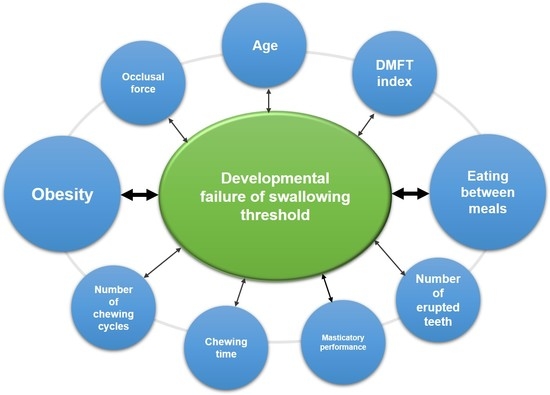
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire
2.3. Anthropometry and Dental Examination
2.4. Hand Grip Strength
2.5. Maximum Occlusal Force
2.6. Masticatory Performance
2.7. Swallowing Threshold
2.8. Reliability of Measurements
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Albala, C.; Vio, F.; Kain, J.; Uauy, R. Nutrition transition in Chile: Determinants and consequences. Public Health Nutr. 2002, 5, 123–128. [Google Scholar] [CrossRef]
- Carnell, S.; Wardle, J. Appetitive traits and child obesity: Measurement, origins and implications for intervention. Proc. Nutr. Soc. 2008, 67, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkila, V.; Rasanen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent dietary patterns identified from childhood to adulthood: The cardiovascular risk in Young Finns Study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P., Jr.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, L.; Flores-Barrantes, P.; Moreno, L.A.; Manios, Y.; Gonzalez-Gil, E.M. The Influence of Parental Dietary Behaviors and Practices on Children’s Eating Habits. Nutrients 2021, 13, 1138. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.; Lim, S. Overweight or obesity in children aged 0 to 6 and the risk of adult metabolic syndrome: A systematic review and meta-analysis. J. Clin. Nurs. 2017, 26, 3869–3880. [Google Scholar] [CrossRef]
- Velazquez-Bautista, M.; Lopez-Sandoval, J.J.; Gonzalez-Hita, M.; Vazquez-Valls, E.; Cabrera-Valencia, I.Z.; Torres-Mendoza, B.M. Association of metabolic syndrome with low birth weight, intake of high-calorie diets and acanthosis nigricans in children and adolescents with overweight and obesity. Endocrinol. Diabetes Nutr. 2017, 64, 11–17. [Google Scholar] [CrossRef]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Bozbulut, R.; Ertas-Ozturk, Y.; Doger, E.; Bideci, A.; Koksal, E. Increased Obesity Awareness and Adherence to Healthy Lifestyle-Diet Reduce Metabolic Syndrome Risk in Overweight Children. J. Am. Coll. Nutr. 2020, 39, 432–437. [Google Scholar] [CrossRef]
- Dejavitte, R.A.S.; Enes, C.C.; Nucci, L.B. Prevalence of metabolic syndrome and its associated factors in overweight and obese adolescents. J. Pediatric Endocrinol. Metab. 2020, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Haruyama, Y.; Muto, T.; Yamazaki, T. Association between eating speed and metabolic syndrome in a three-year population-based cohort study. J. Epidemiol. 2015, 25, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Yang, K.; Huang, F.; Liu, X.; Li, X.; Luo, Y.; Wu, L.; Guo, X. Association between self-reported eating speed and metabolic syndrome in a Beijing adult population: A cross-sectional study. BMC Public Health 2018, 18, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekuni, D.; Furuta, M.; Takeuchi, N.; Tomofuji, T.; Morita, M. Self-reports of eating quickly are related to a decreased number of chews until first swallow, total number of chews, and total duration of chewing in young people. Arch. Oral Biol. 2012, 57, 981–986. [Google Scholar] [CrossRef]
- Llewellyn, C.H.; van Jaarsveld, C.H.; Boniface, D.; Carnell, S.; Wardle, J. Eating rate is a heritable phenotype related to weight in children. Am. J. Clin. Nutr. 2008, 88, 1560–1566. [Google Scholar] [CrossRef]
- Lin, M.; Pan, L.; Tang, L.; Jiang, J.; Wang, Y.; Jin, R. Association of eating speed and energy intake of main meals with overweight in Chinese pre-school children. Public Health Nutr. 2014, 17, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.E.; Ramos-Jorge, M.L.; de Alencar, B.M.; Oliveira, S.G.; Pereira, L.J.; Ramos-Jorge, J. Influence of masticatory function, dental caries and socioeconomic status on the body mass index of preschool children. Arch. Oral Biol. 2017, 81, 69–73. [Google Scholar] [CrossRef]
- Okubo, H.; Murakami, K.; Masayasu, S.; Sasaki, S. The Relationship of Eating Rate and Degree of Chewing to Body Weight Status among Preschool Children in Japan: A Nationwide Cross-Sectional Study. Nutrients 2018, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hollis, J.H. Increasing the number of chews before swallowing reduces meal size in normal-weight, overweight, and obese adults. J. Acad. Nutr. Diet. 2014, 114, 926–931. [Google Scholar] [CrossRef]
- Takeshima, T.; Fujita, Y.; Maki, K. Factors associated with masticatory performance and swallowing threshold according to dental formula development. Arch. Oral Biol. 2019, 99, 51–57. [Google Scholar] [CrossRef]
- Ohno, K.; Fujita, Y.; Ohno, Y.; Takeshima, T.; Maki, K. The factors related to decreases in masticatory performance and masticatory function until swallowing using gummy jelly in subjects aged 20–79 years. J. Oral Rehabil. 2020, 47, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Fujita, Y.; Hamaguchi, A.; Chaweewannakorn, W.; Maki, K. Association of tongue pressure with masticatory performance and dental conditions in Japanese children. Pediatric Dent. J. 2016, 26, 51–59. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization, Ed.; WHO Press: Geneva, Switzerland, 2013; pp. 29–56. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Domholdt, E. Physical Therapy Research: Principles and Applications; W.B Saunders Co.: Philadelphia, PA, USA, 1993. [Google Scholar]
- Itateyama, E.; Chiba, S.; Sakata, T.; Yoshimatsu, H. Hypothalamic neuronal histamine in genetically obese animals: Its implication of leptin action in the brain. Exp. Biol. Med. 2003, 228, 1132–1137. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Oishi, R.; Saeki, K. Feeding-induced increase in the extracellular concentration of histamine in rat hypothalamus as measured by in vivo microdialysis. Neurosci. Lett. 1991, 125, 235–237. [Google Scholar] [CrossRef]
- Ookuma, K.; Sakata, T.; Fukagawa, K.; Yoshimatsu, H.; Kurokawa, M.; Machidori, H.; Fujimoto, K. Neuronal histamine in the hypothalamus suppresses food intake in rats. Neurosci. Res. 1993, 628, 235–242. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Pries, A.M.; Huffman, S.L.; Adhikary, I.; Upreti, S.R.; Dhungel, S.; Champeny, M.; Zehner, E. High consumption of commercial food products among children less than 24 months of age and product promotion in Kathmandu Valley, Nepal. Matern. Child Nutr. 2016, 12 (Suppl. S2), 22–37. [Google Scholar] [CrossRef]
- Huffman, S.L.; Piwoz, E.G.; Vosti, S.A.; Dewey, K.G. Babies, soft drinks and snacks: A concern in low- and middle-income countries? Matern Child Nutr. 2014, 10, 562–574. [Google Scholar] [CrossRef]
- Tenjin, K.; Sekine, M.; Yamada, M.; Tatsuse, T. Relationship Between Parental Lifestyle and Dietary Habits of Children: A Cross-Sectional Study. J. Epidemiol. 2020, 30, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piernas, C.; Popkin, B.M. Food portion patterns and trends among U.S. children and the relationship to total eating occasion size, 1977–2006. J. Nutr. 2011, 141, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.; Slavin, J. Snacking for a cause: Nutritional insufficiencies and excesses of U.S. children, a critical review of food consumption patterns and macronutrient and micronutrient intake of U.S. children. Nutrients 2014, 6, 4750–4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hujoel, P.P.; Bollen, A.M.; Yuen, K.C.; Hujoel, I.A. Phenotypic characteristics of adolescents with concave and convex facial profiles—The National Health Examination Survey. Homo 2016, 67, 417–432. [Google Scholar] [CrossRef]
- Araujo, D.S.; Marquezin, M.C.; Barbosa Tde, S.; Gaviao, M.B.; Castelo, P.M. Evaluation of masticatory parameters in overweight and obese children. Eur. J. Orthod. 2016, 38, 393–397. [Google Scholar] [CrossRef] [Green Version]

| Age (Years) | Sex (n = 83) | Height (m) | Body Weight (kg) | Kaup Index/Rohrer Index | Number of Erupted Teeth | DMFT Index |
|---|---|---|---|---|---|---|
| 5 | M (n = 4) | 1.18 ± 0.08 | 17.75 ± 0.96 | 13.01 ± 2.09 | 20.25 ± 0.50 | 2.50 ± 2.38 |
| F (n = 4) | 1.17 ± 0.14 | 16.75 ± 1.26 | 12.86 ± 2.81 | 20.00 ± 0.00 | 7.75 ± 7.50 | |
| 6 | M (n = 5) | 1.13 ± 0.03 | 20.40 ± 1.67 | 143.81 ± 19.83 | 19.40 ± 1.34 | 7.00 ± 5.39 |
| F (n = 6) | 1.13 ± 0.04 | 20.67 ± 1.37 | 144.30 ± 12.77 | 20.00 ± 0.00 | 8.00 ± 5.02 | |
| 7 | M (n = 4) | 1.29 ± 0.09 | 26.25 ± 4.99 | 122.24 ± 8.08 | 21.25 ± 1.50 | 5.50 ± 2.52 |
| F (n = 6) | 1.22 ± 0.07 | 22.50 ± 2.07 | 126.02 ± 21.46 | 21.50 ± 1.76 | 6.00 ± 3.16 | |
| 8 | M (n = 3) | 1.21 ± 0.06 | 21.33 ± 3.06 | 120.54 ± 16.38 | 23.67 ± 0.58 | 2.67 ± 2.08 |
| F (n = 3) | 1.27 ± 0.07 | 24.67 ± 4.16 | 119.10 ± 13.05 | 23.67 ± 0.58 | 5.00 ± 4.36 | |
| 9 | M (n = 4) | 1.39 ± 0.05 | 32.88 ± 8.53 | 122.06 ± 20.64 | 24.00 ± 0.00 | 6.00 ± 0.82 |
| F (n = 4) | 1.32 ± 0.07 | 29.75 ± 6.90 | 127.73 ± 18.58 | 23.25 ± 1.50 | 3.25 ± 3.20 | |
| 10 | M (n = 3) | 1.38 ± 0.03 | 29.50 ± 0.50 | 111.56 ± 5.50 | 23.67 ± 0.58 | 6.67 ± 1.53 |
| F (n = 5) | 1.32 ± 0.07 | 26.60 ± 4.55 | 115.37 ± 5.97 | 23.20 ± 0.84 | 7.60 ± 3.91 | |
| 11 | M (n = 4) | 1.36 ± 0.09 | 29.25 ± 2.99 | 121.96 ± 16.85 | 24.00 ± 2.94 | 2.00 ± 2.71 |
| F (n = 3) | 1.41 ± 0.01 | 30.33 ± 1.15 | 108.18 ± 2.45 | 23.00 ± 1.00 | 10.33 ± 1.53 * | |
| 12 | M (n = 3) | 1.43 ± 0.04 | 50.00 ± 7.21 | 173.45 ± 34.21 | 25.00 ± 2.65 | 3.33 ± 4.04 |
| F (n = 3) | 1.37 ± 0.10 | 32.67 ± 8.39 | 123.82 ± 7.72 | 25.00 ± 1.00 | 1.33 ± 1.53 | |
| 13 | M (n = 3) | 1.64 ± 0.14 | 49.67 ± 12.70 | 110.73 ± 4.59 | 26.67 ± 2.31 | 4.33 ± 2.08 |
| F (n = 3) | 1.54 ± 0.02 | 45.67 ± 0.58 | 128.40 ± 4.49 | 28.00 ± 0.00 | 6.00 ± 4.36 | |
| 14 | M (n = 3) | 1.76 ± 0.02 | 72.33 ± 2.08 | 131.91 ± 1.75 | 28.00 ± 0.00 | 3.67 ± 3.51 |
| F (n = 4) | 1.55 ± 0.08 | 45.25 ± 1.71 * | 123.35 ± 17.04 | 27.00 ± 2.00 | 1.25 ± 1.50 | |
| 15 | M (n = 3) | 1.74 ± 0.05 | 72.00 ± 14.80 | 135.16 ± 20.15 | 28.00 ± 0.00 | 4.67 ± 2.31 |
| F (n = 3) | 1.51 ± 0.08 * | 47.33 ± 6.81 | 125.64 ± 13.49 | 28.00 ± 0.00 | 5.67 ± 9.81 |
| Age (Years) | Sex (n = 83) | Hand Grip Strength (kg) | Maximum Occlusal Force (kN) | Masticatory Performance (mg/dL) | Number of Chewing Cycles (N) | Chewing Time (s) | Chewing Rate (s/N) | Swallowing Threshold (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| 5 | M (n = 4) | 7.50 ± 1.29 | 0.21 ± 0.04 | 86.25 ± 12.89 | 18.25 ± 10.72 | 15.75 ± 8.26 | 0.89 ± 0.13 | 83.00 ± 28.48 |
| F (n = 4) | 5.75 ± 0.50 * | 0.24 ± 0.08 | 87.50 ± 3.79 | 31.00 ± 1.41 | 26.25 ± 2.22 * | 0.85 ± 0.07 | 108.50 ± 12.97 | |
| 6 | M (n = 5) | 8.90 ± 1.43 | 0.24 ± 0.05 | 99.00 ± 22.03 | 16.80 ± 1.79 | 15.60 ± 2.19 | 0.93 ± 0.14 | 85.20 ± 13.14 |
| F (n = 6) | 9.00 ± 1.26 | 0.22 ± 0.04 | 100.83 ± 15.72 | 16.17 ± 1.47 | 16.67 ± 3.61 | 1.02 ± 0.16 | 84.83 ± 8.11 | |
| 7 | M (n = 4) | 10.50 ± 1.00 | 0.27 ± 0.08 | 104.75 ± 17.73 | 23.50 ± 7.55 | 20.00 ± 6.68 | 0.85 ± 0.08 | 100.75 ± 14.08 |
| F (n = 6) | 9.30 ± 2.92 | 0.27 ± 0.08 | 100.33 ± 30.79 | 25.33 ± 4.41 | 24.67 ± 5.43 | 0.97 ± 0.11 | 126.33 ± 38.14 | |
| 8 | M (n = 3) | 11.00 ± 4.77 | 0.36 ± 0.10 | 101.67 ± 48.40 | 31.33 ± 8.96 | 28.33 ± 3.06 | 0.98 ± 0.43 | 127.67 ± 43.78 |
| F (n = 3) | 11.17± 2.25 | 0.30 ± 0.01 | 153.00 ± 49.24 | 25.00 ± 6.56 | 22.00 ± 3.61 | 0.90 ± 0.10 | 160.67 ± 54.60 | |
| 9 | M (n = 4) | 15.25 ± 6.54 | 0.37 ± 0.05 | 114.50 ± 10.34 | 36.00 ± 8.60 | 25.50 ± 3.11 | 0.73 ± 0.10 | 129.00 ± 6.06 |
| F (n = 4) | 11.00 ± 2.71 | 0.34 ± 0.06 | 146.50 ± 40.62 | 25.00 ± 2.58 | 21.25 ± 5.56 | 0.84 ± 0.15 | 144.00 ± 21.82 | |
| 10 | M (n = 3) | 24.33 ± 0.58 | 0.37 ± 0.06 | 171.33 ± 12.66 | 22.67 ± 1.53 | 19.00 ± 1.00 | 0.84 ± 0.04 | 162.00 ± 5.00 |
| F (n = 5) | 10.30 ± 1.57 * | 0.33 ± 0.05 | 122.60 ± 34.78 | 26.60 ± 7.89 | 22.20 ± 7.01 | 0.83 ± 0.05 | 122.80 ± 38.30 | |
| 11 | M (n = 4) | 18.63 ± 6.94 | 0.36 ± 0.09 | 159.00 ± 59.35 | 25.75 ± 4.86 | 21.50 ± 7.14 | 0.87 ± 0.34 | 155.00 ± 48.19 |
| F (n = 3) | 11.67 ± 0.58 | 0.32 ± 0.05 | 106.33 ± 17.47 | 19.33 ± 0.58 | 17.00 ± 1.00 | 0.88 ± 0.03 | 85.00 ± 12.49 | |
| 12 | M (n = 3) | 29.50 ± 5.63 | 0.46 ± 0.05 | 176.67 ± 7.57 | 16.67 ± 3.21 | 14.33 ± 2.89 | 0.86 ± 0.03 | 150.33 ± 35.80 |
| F (n = 3) | 16.00 ± 4.36 * | 0.34 ± 0.04 * | 130.67 ± 9.07 * | 20.33 ± 4.93 | 17.33 ± 4.93 | 0.85 ± 0.03 | 120.33 ± 7.64 | |
| 13 | M (n = 3) | 30.17 ± 11.51 | 0.49 ± 0.06 | 136.33 ± 47.72 | 22.00 ± 5.00 | 17.00 ± 1.73 | 0.79 ± 0.13 | 147.33 ± 54.50 |
| F (n = 3) | 21.50 ± 2.18 | 0.35 ± 0.08 | 139.67 ± 65.25 | 27.00 ± 1.73 | 20.00 ± 1.73 | 0.74 ± 0.02 | 140.67 ± 53.13 | |
| 14 | M (n = 3) | 38.00 ± 3.00 | 0.44 ± 0.01 | 143.67 ± 21.96 | 12.00 ± 5.57 | 11.24 ± 5.28 | 0.93 ± 0.02 | 65.33 ± 16.65 |
| F (n = 4) | 24.75 ± 2.75 * | 0.38 ± 0.10 | 167.50 ± 31.42 | 19.50 ± 2.38 | 18.75 ± 2.50 | 0.97 ± 0.17 | 152.75 ± 37.19 * | |
| 15 | M (n = 3) | 43.67 ± 6.43 | 0.52 ± 0.06 | 169.33 ± 61.04 | 18.67 ± 9.02 | 12.00 ± 4.00 | 0.71 ± 0.25 | 117.67 ± 28.87 |
| F (n = 3) | 21.67 ± 1.53 * | 0.47 ± 0.03 | 164.33 ± 21.01 | 25.00 ± 5.20 | 21.33 ± 3.79 | 0.86 ± 0.04 | 186.00 ± 18.73 * |
| Age | Height | Body Weight | Number of Erupted Teeth | DMFT Index | Hand Grip Strength | Maximum Occlusal Force | Masticatory Performance | Number of Chewing Cycles | Chewing Time | Chewing Rate | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||||||
| Height | 0.871 ** | 1 | |||||||||
| Body weight | 0.833 ** | 0.916 ** | 1 | ||||||||
| Number of erupted teeth | 0.885 ** | 0.815 ** | 0.778 ** | 1 | |||||||
| DMFT index | −0.195 | −0.207 | −0.148 | −0.232 * | 1 | ||||||
| Hand grip strength | 0.795 ** | 0.848 ** | 0.903 ** | 0.762 ** | −0.216 * | 1 | |||||
| Maximum occlusal force | 0.760 ** | 0.738 ** | 0.722 ** | 0.734 ** | −0.339 ** | 0.791 ** | 1 | ||||
| Masticatory performance | 0.580 ** | 0.471 ** | 0.498 ** | 0.587 ** | −0.375 ** | 0.611 ** | 0.659 ** | 1 | |||
| Number of chewing cycles | −0.104 | −0.101 | −0.236 * | −0.026 | −0.064 | −0.203 | 0.049 | −0.028 | 1 | ||
| Chewing time | −0.238 * | −0.295 ** | −0.396 ** | −0.156 | −0.052 | −0.423 ** | −0.193 | −0.217 * | 0.801 ** | 1 | |
| Chewing rate | −0.211 | −0.287 ** | −0.227 * | −0.208 | 0.015 | −0.312 ** | −0.390 ** | −0.317 ** | −0.379 ** | 0.226 * | 1 |
| SW | 0.362 ** | 0.225 * | 0.130 | 0.432 ** | −0.369 ** | 0.271 * | 0.493 ** | 0.727 ** | 0.465 ** | 0.351 ** | −0.259 * |
| Participants (n = 83) | Normal (%) (n = 67) | Developmental Failure (%) (n = 16) | χ2 | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 36 (53.7) | 8 (50.0) | ||
| Male | 31 (46.3) | 8 (50.0) | ||
| ― | 1.000 † | |||
| Degree of obesity | ||||
| Normal | 34 (50.7) | 5 (31.3) | ||
| Underweight/severely underweight | 27 (40.3) | 5 (31.3) | ||
| Overweight/obese | 6 (9.0) | 6 (37.5) | ||
| 8.598 | 0.014 ‡ | |||
| Skipping breakfast | ||||
| Less than two times a week | 48 (71.6) | 10 (62.5) | ||
| Two times or more a week | 19 (28.4) | 6 (37.5) | ||
| ― | 0.333 † | |||
| Eating between meals | ||||
| Less than once a day | 31 (46.3) | 2 (12.5) | ||
| Once or more a day | 36 (53.7) | 14 (87.5) | ||
| ― | 0.021 † | |||
| Physical activity | ||||
| 30 min or more a day | 38 (56.7) | 2 (12.5) | ||
| Less than 30 min a day | 8 (11.9) | 3 (18.8) | ||
| None | 21 (31.3) | 11 (68.8) | ||
| 10.379 | 0.006 ‡ | |||
| Self-assessed sleep quality | ||||
| Good | 66 (98.5) | 16 (100) | ||
| Poor | 1 (1.5) | 0 (0.0) | ||
| ― | 1.000 † |
| Normal (n = 67) | Developmental Failure (n = 16) | |
|---|---|---|
| Age (years) | 9.93 ± 3.09 | 7.88 ± 3.07 * |
| Height (m) | 1.37 ± 0.18 | 1.28 ± 0.23 |
| Body weight (kg) | 33.41 ± 15.23 | 29.59 ± 17.10 |
| Number of erupted teeth (N) | 23.88 ± 2.96 | 21.81 ± 2.66 * |
| DMFT index | 4.69 ± 3.91 | 7.31 ± 4.73 * |
| Hand grip strength (kg) | 17.14 ± 10.39 | 12.99 ± 9.36 |
| Maximum occlusal force (kN) | 0.35 ± 0.10 | 0.27 ± 0.08 * |
| Masticatory performance (mg/dL) | 134.19 ± 40.75 | 97.94 ± 24.97 * |
| Number of chewing cycles (N) | 24.64 ± 6.95 | 15.69 ± 3.93 * |
| Chewing time (s) | 20.91 ± 5.56 | 14.42 ± 3.97 * |
| Chewing rate (s/N) | 0.86 ± 0.16 | 0.92 ± 0.13 |
| Swallowing threshold (mg/dL) | 134.40 ± 35.61 | 73.94 ± 10.49 * |
| Independent Variables | Category | Adjusted Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Degree of obesity | Normal | 1 | ― |
| Underweight/severely underweight | 1.278 (0.323–5.055) | 0.727 | |
| Overweight/obese | 5.343 (1.168–24.437) | 0.031 | |
| Eating between meals | Less than once a day | 1 | ― |
| Once or more a day | 4.934 (1.004–24.244) | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, Y. Effects of Developmental Failure of Swallowing Threshold on Obesity and Eating Behaviors in Children Aged 5–15 Years. Nutrients 2022, 14, 2614. https://doi.org/10.3390/nu14132614
Fujita Y. Effects of Developmental Failure of Swallowing Threshold on Obesity and Eating Behaviors in Children Aged 5–15 Years. Nutrients. 2022; 14(13):2614. https://doi.org/10.3390/nu14132614
Chicago/Turabian StyleFujita, Yuko. 2022. "Effects of Developmental Failure of Swallowing Threshold on Obesity and Eating Behaviors in Children Aged 5–15 Years" Nutrients 14, no. 13: 2614. https://doi.org/10.3390/nu14132614
APA StyleFujita, Y. (2022). Effects of Developmental Failure of Swallowing Threshold on Obesity and Eating Behaviors in Children Aged 5–15 Years. Nutrients, 14(13), 2614. https://doi.org/10.3390/nu14132614