Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hericium erinaceus Mycelium
2.2. Anterior Cruciate Ligament Transection (ACLT) Animal Model
2.3. Micro-Computed Tomography (μ-CT) Measurements
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analysis
3. Results
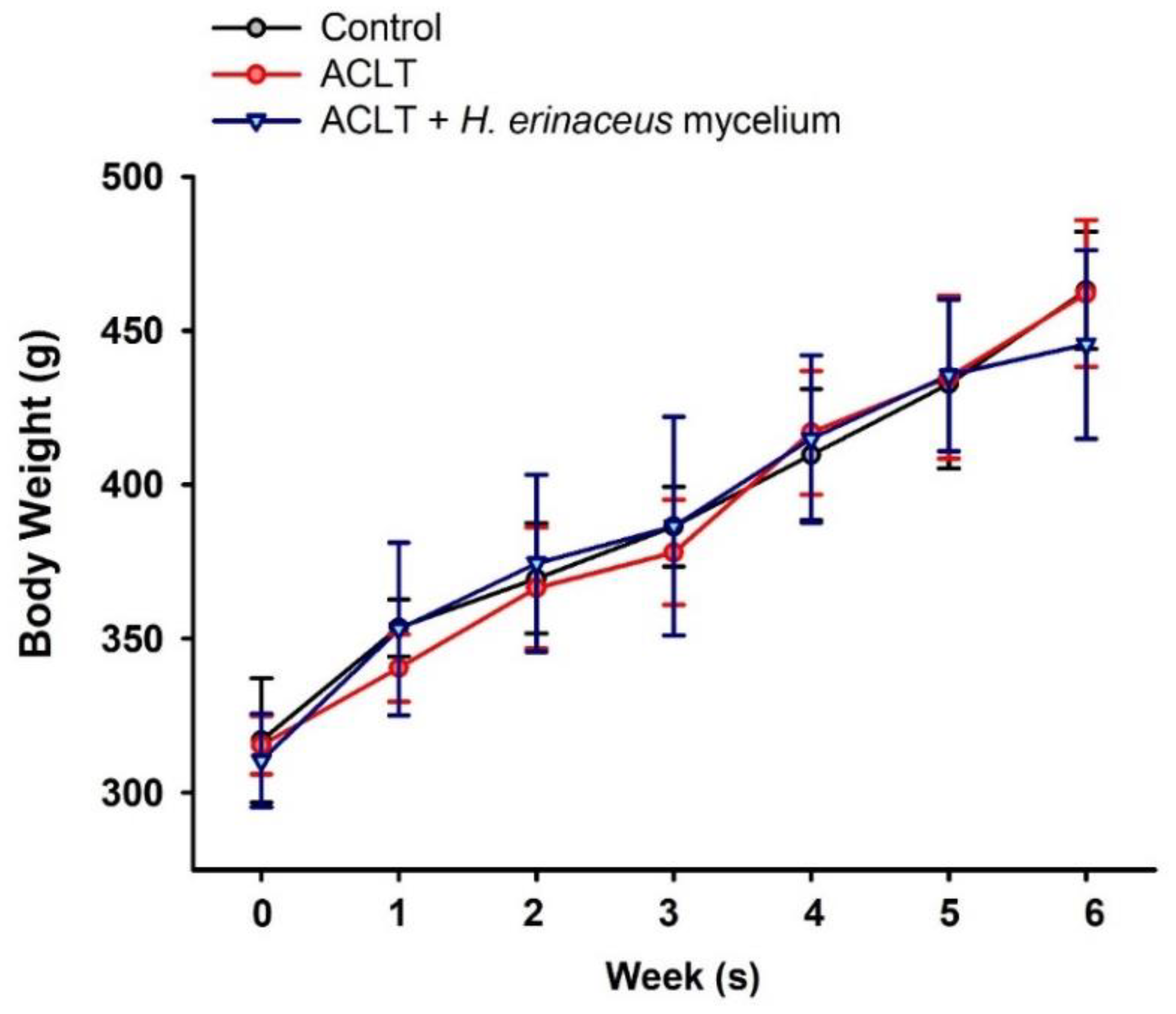
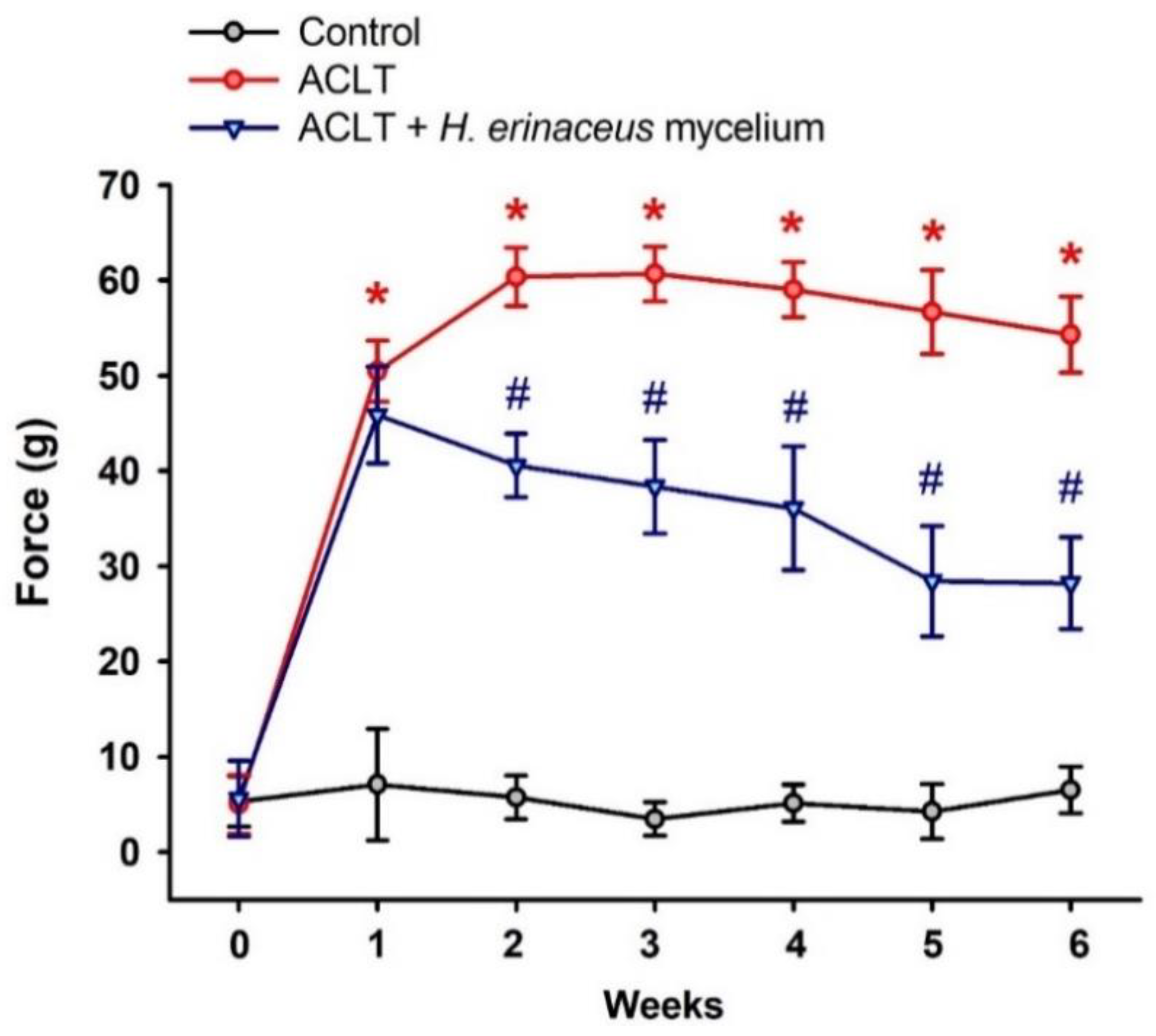
3.1. H. erinaceus Mycelium Reduces ACLT-Induced Weight-Bearing Asymmetry and Pain
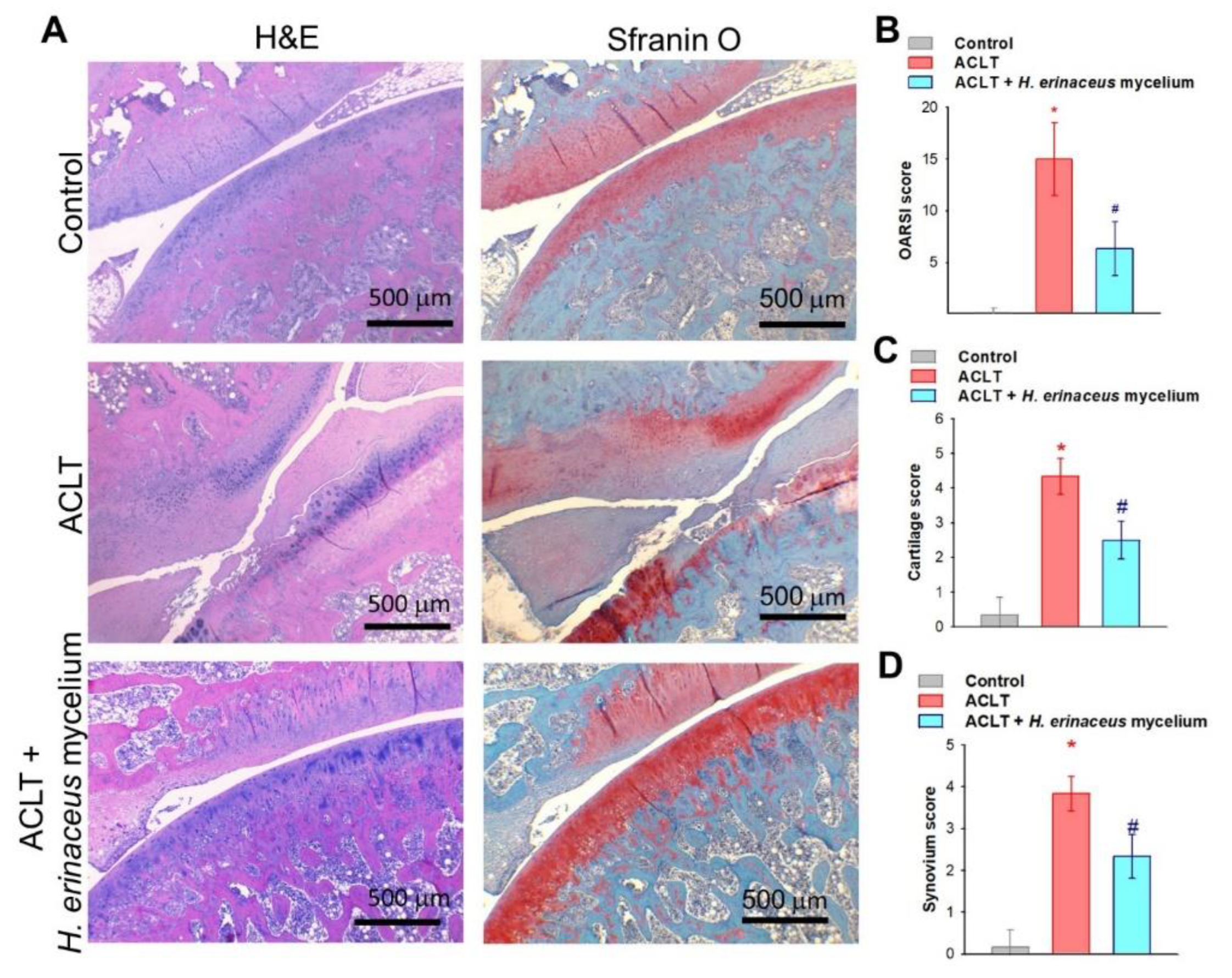
3.2. H. erinaceus Mycelium Improves Bone and Cartilage Architecture in ACLT Rats
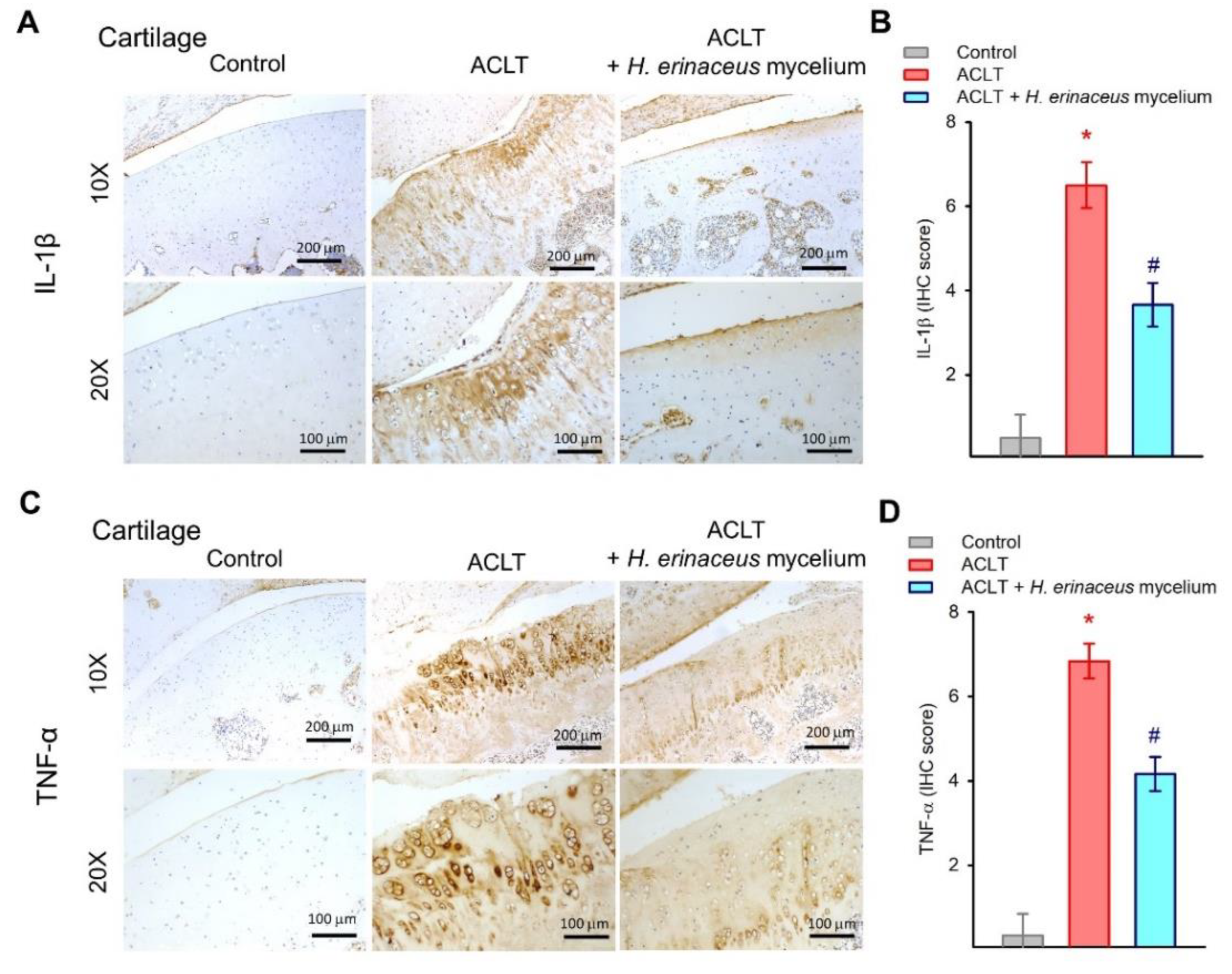
3.3. H. erinaceus Mycelium Suppresses Proinflammatory Cytokine Upregulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [Green Version]
- Abolhasani, M.; Halabchi, F.; Afsharnia, E.; Moradi, V.; Ingle, L.; Shariat, A.; Hakakzadeh, A. Effects of kinesiotaping on knee osteoarthritis: A literature review. J. Exerc. Rehabil. 2019, 15, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef]
- Su, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Lee, H.-P.; Lo, L.-C.; Huang, W.-C.; Wu, Y.-C.; Hsieh, C.-L.; Tang, C.-H. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef]
- Law, Y.Y.; Lee, W.F.; Hsu, C.J.; Lin, Y.Y.; Tsai, C.H.; Huang, C.C.; Wu, M.H.; Tang, C.H.; Liu, J.F. miR-let-7c-5p and miR-149-5p inhibit proinflammatory cytokine production in osteoarthritis and rheumatoid arthritis synovial fibroblasts. Aging 2021, 13, 17227–17236. [Google Scholar] [CrossRef]
- Liu, S.C.; Tsai, C.H.; Wu, T.Y.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Soya-cerebroside reduces IL-1 beta-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agr. Immunol. 2019, 30, 620–632. [Google Scholar] [CrossRef]
- Dorais, M.; Martel-Pelletier, J.; Raynauld, J.P.; Delorme, P.; Pelletier, J.P. Impact of oral osteoarthritis therapy usage among other risk factors on knee replacement: A nested case-control study using the Osteoarthritis Initiative cohort. Arthritis Res. Ther. 2018, 20, 172. [Google Scholar] [CrossRef]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complementary Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Xu, T.; Zou, X.; Mao, R.; Pi, X.; Wu, W.; Huang, L.; Yang, K.; Zeng, X.; et al. Digestive Characteristics of Hericium erinaceus Polysaccharides and Their Positive Effects on Fecal Microbiota of Male and Female Volunteers During in vitro Fermentation. Front. Nutr. 2022, 9, 858585. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine A Prevents Lipopolysaccharide-Mediated Glial Cell Activation to Protect Dopaminergic Neurons against Inflammatory Factor-Induced Cell Death In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Antibiofilm activity and bioactive phenolic compounds of ethanol extract from the Hericium erinaceus basidiome. J. Adv. Pharm. Technol. Res. 2022, 13, 111–116. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Qin, M. Effects of Extraction Conditions on Crude Polysaccharides and Antioxidant Activities of the Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1007–1018. [Google Scholar] [CrossRef]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Tang, C.; Liu, Y.; Li, Q.; Wang, W.; Zhou, S.; Zhang, Z.; Cui, F.; Yang, Y. Structural elucidation and immunomodulatory activity of a beta-D-glucan prepared by freeze-thawing from Hericium erinaceus. Carbohydr. Polym. 2019, 222, 114996. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, H.K.; Kim, S.E.; Kim, M.J.; Kim, D.H.; Lee, H.S. Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients 2018, 10, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.S.; Chueh, S.H.; Chen, C.C.; Lee, L.Y.; Shiu, L.Y. Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), Modulates Purinoceptor-Coupled Calcium Signaling and Murine Nociceptive Behavior. Int. J. Med. Mushrooms 2017, 19, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Su, C.H.; Liu, S.C.; Chen, B.C.; Chang, J.W.; Tsai, C.H.; Huang, W.C.; Hsu, C.J.; Chen, W.C.; Wu, Y.C.; et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022, e14108. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.W.; Liu, S.C.; Tsay, G.J.; Tang, C.H.; Chang, H.H. The Traditional Chinese Medicine “Hu-Qian-Wan” Attenuates Osteoarthritis-Induced Signs and Symptoms in an Experimental Rat Model of Knee Osteoarthritis. Evid. Based Complement. Alternat. Med. 2022, 2022, 5367494. [Google Scholar] [CrossRef]
- Achudhan, D.; Liu, S.C.; Lin, Y.Y.; Lee, H.P.; Wang, S.W.; Huang, W.C.; Wu, Y.C.; Kuo, Y.H.; Tang, C.H. Antcin K inhibits VEGF-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022, 46, e14022. [Google Scholar] [CrossRef]
- Liu, S.C.; Tsai, C.H.; Wang, Y.H.; Su, C.M.; Wu, H.C.; Fong, Y.C.; Yang, S.F.; Tang, C.H. Melatonin abolished proinflammatory factor expression and antagonized osteoarthritis progression in vivo. Cell Death Dis. 2022, 13, 215. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Ko, C.Y.; Liu, S.C.; Wang, Y.H.; Hsu, C.J.; Tsai, C.H.; Wu, T.J.; Tang, C.H. miR-144-3p ameliorates the progression of osteoarthritis by targeting IL-1beta: Potential therapeutic implications. J. Cell Physiol. 2021, 236, 6988–7000. [Google Scholar] [CrossRef]
- Chen, W.C.; Lu, Y.C.; Kuo, S.J.; Lin, C.Y.; Tsai, C.H.; Liu, S.C.; Chen, Y.L.; Wang, S.W.; Tang, C.H. Resistin enhances IL-1beta and TNF-alpha expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways. FASEB J. 2020, 34, 13671–13684. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Lin, L.W.; Tsai, F.J.; Yang, J.S.; Li, T.M.; Tang, C.H. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agr. Immunol. 2020, 31, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agr. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Meng, H.; Wang, Y.; Peng, J.; Guo, Q.; Wang, A.; Lu, S. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Gao, W.; Wu, Z.; Zhou, T.; Qiu, X.; Wang, X.; Lian, C.; Peng, Y.; Liang, A.; Qiu, J.; et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Lee, C.W.; Xu, H.; Wang, Y.F.; Yung, P.S.H.; Jiang, Y.; Lee, O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021, 23, 110. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef]
- Liyis, B.G.de; Nolan, J.; Maharjana, M.A. Fibroblast Growth Factor Receptor 1-Bound Extracellular Vesicle as Novel Therapy for Osteoarthritis. BioMedicine 2022, 12, 1. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Huang, C.C.; Liu, S.C.; Lin, Y.Y.; Tang, C.H. Targeting CCN Proteins in Rheumatoid Arthritis and Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 4340. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Liu, S.C.; Su, C.M.; Wang, Y.H.; Tsai, C.H.; Tang, C.H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef] [Green Version]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Lu, Z.; Xu, H.; Shi, J.S.; Xu, Z.H. Anti-inflammatory activity of mycelial extracts from medicinal mushrooms. Int. J. Med. Mushrooms 2014, 16, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ulziijargal, E.; Mau, J.L. Nutrient compositions of culinary-medicinal mushroom fruiting bodies and mycelia. Int. J. Med. Mushrooms 2011, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.L.; Naidu, M.; Sabaratnam, V.; Wong, K.H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Naidu, M.; David, R.P.; Bakar, R.; Sabaratnam, V. Neuroregenerative potential of lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (higher Basidiomycetes), in the treatment of peripheral nerve injury (review). Int. J. Med. Mushrooms 2012, 14, 427–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorganic Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef]
- Kim, S. Antioxidant Compounds for the Inhibition of Enzymatic Browning by Polyphenol Oxidases in the Fruiting Body Extract of the Edible Mushroom Hericium erinaceus. Foods 2020, 9, 951. [Google Scholar] [CrossRef]
- Lee, K.F.; Tung, S.Y.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Post-Treatment with Erinacine A, a Derived Diterpenoid of H. erinaceus, Attenuates Neurotoxicity in MPTP Model of Parkinson’s Disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Lee, K.F.; Tung, S.Y.; Huang, W.S.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; et al. Induction Apoptosis of Erinacine A in Human Colorectal Cancer Cells Involving the Expression of TNFR, Fas, and Fas Ligand via the JNK/p300/p50 Signaling Pathway with Histone Acetylation. Front. Pharmacol. 2019, 10, 1174. [Google Scholar] [CrossRef]
- Huang, H.T.; Ho, C.H.; Sung, H.Y.; Lee, L.Y.; Chen, W.P.; Chen, Y.W.; Chen, C.C.; Yang, C.S.; Tzeng, S.F. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci. Rep. 2021, 11, 6551. [Google Scholar] [CrossRef]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-Y.; Fang, C.-J.; Chen, Y.-W.; Chen, W.-P.; Lee, L.-Y.; Chen, C.-C.; Lin, Y.-Y.; Liu, S.-C.; Tsai, C.-H.; Huang, W.-C.; et al. Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis. Nutrients 2022, 14, 2605. https://doi.org/10.3390/nu14132605
Yang S-Y, Fang C-J, Chen Y-W, Chen W-P, Lee L-Y, Chen C-C, Lin Y-Y, Liu S-C, Tsai C-H, Huang W-C, et al. Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis. Nutrients. 2022; 14(13):2605. https://doi.org/10.3390/nu14132605
Chicago/Turabian StyleYang, Shang-Yu, Chi-Jung Fang, Yu-Wen Chen, Wan-Ping Chen, Li-Ya Lee, Chin-Chu Chen, Yen-You Lin, Shan-Chi Liu, Chun-Hao Tsai, Wei-Chien Huang, and et al. 2022. "Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis" Nutrients 14, no. 13: 2605. https://doi.org/10.3390/nu14132605
APA StyleYang, S.-Y., Fang, C.-J., Chen, Y.-W., Chen, W.-P., Lee, L.-Y., Chen, C.-C., Lin, Y.-Y., Liu, S.-C., Tsai, C.-H., Huang, W.-C., Wu, Y.-C., & Tang, C.-H. (2022). Hericium erinaceus Mycelium Ameliorates In Vivo Progression of Osteoarthritis. Nutrients, 14(13), 2605. https://doi.org/10.3390/nu14132605






