Effects of Postdischarge High-Protein Oral Nutritional Supplements and Resistance Training in Malnourished Surgical Patients: A Pilot Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Intervention
2.3. Control Group
2.4. Outcomes
2.5. Measurements
2.6. Compliance
2.7. Statistical Analysis
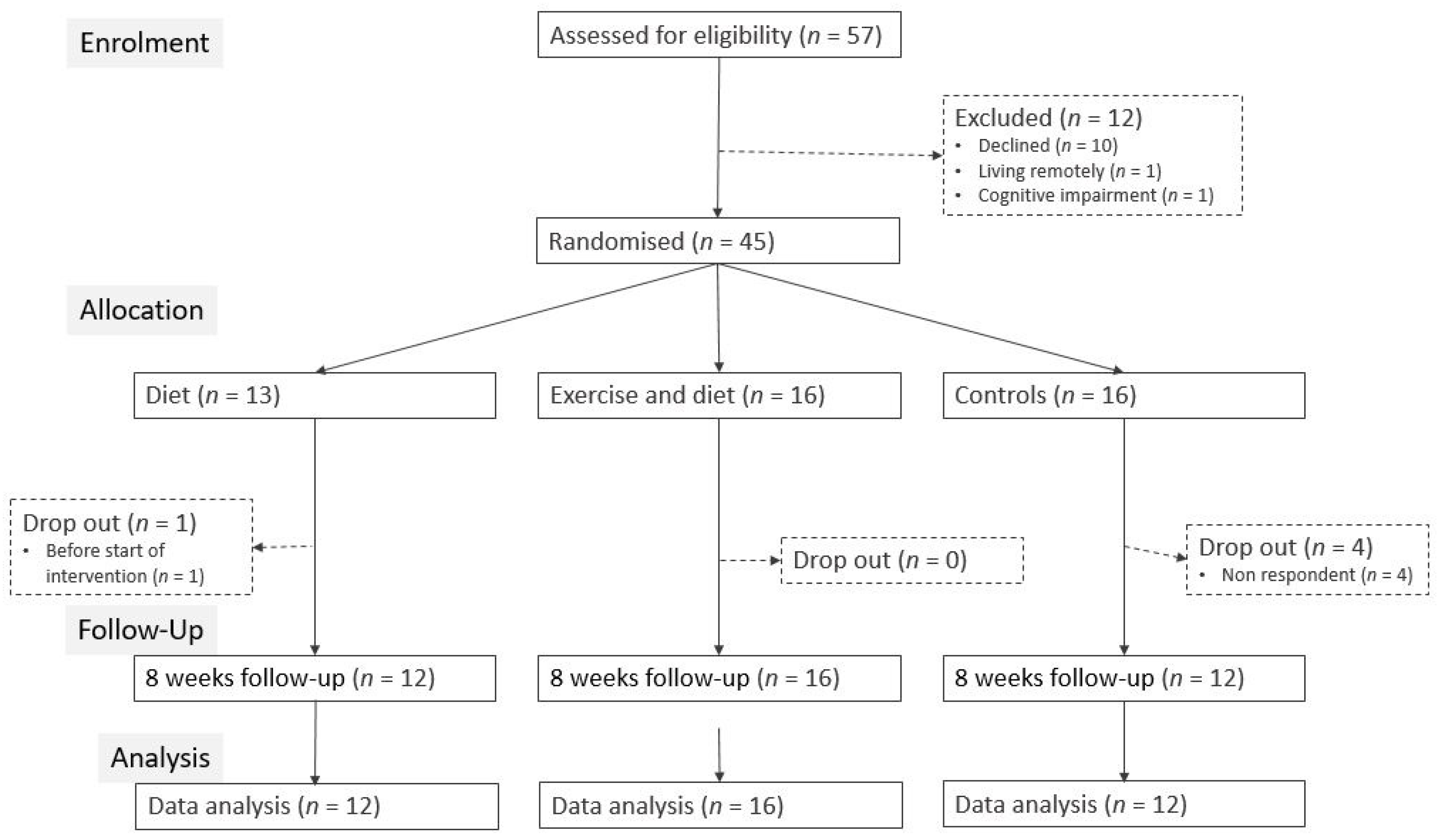
3. Results
3.1. Intention to Treat Analysis
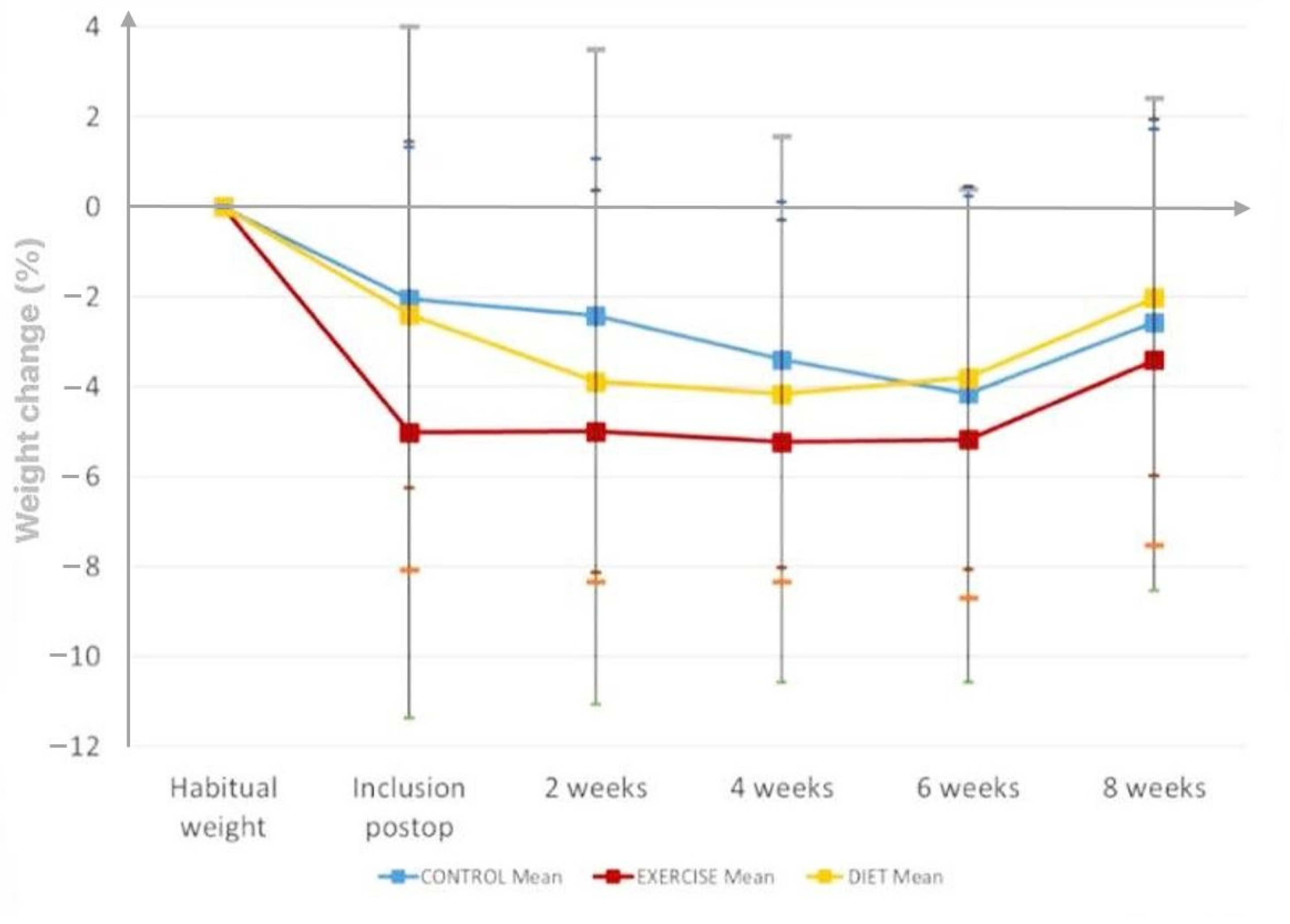
3.1.1. Weight Change during the Intervention Period
3.1.2. Energy and Protein Intake
3.1.3. Other Outcomes
3.2. Per Protocol Analyses
3.2.1. Training Compliance
3.2.2. ONS Compliance
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Note
References
- Beaton, J.; Carey, S.; Solomon, M.; Young, J. Preoperative and postoperative nutritional status of patients following pelvic exenteration surgery for rectal cancer. e-ESPEN J. 2013, 8, e164–e168. [Google Scholar] [CrossRef]
- Bell, J.; Bauer, J.; Capra, S.; Pulle, C.R. Barriers to nutritional intake in patients with acute hip fracture: Time to treat malnutrition as a disease and food as a medicine? Can. J. Physiol. Pharmacol. 2013, 91, 489–495. [Google Scholar] [CrossRef]
- Fettes, S.B.; Davidson, H.I.M.; Richardson, R.A.; Pennington, C.R. Nutritional status of elective gastrointestinal surgery patients pre- and post-operatively. Clin. Nutr. 2002, 21, 249–254. [Google Scholar] [CrossRef]
- Lidder, P.G.; Lewis, S.; Duxbury, M.; Thomas, S. Systematic review of postdischarge oral nutritional supplementation in patients undergoing GI Surgery. Nutr. Clin. Pract. 2009, 24, 388–394. [Google Scholar] [CrossRef]
- Hessov, I. Kirurgi og ernæring. Ugeskr. Læger 2003, 165, 4952–4955. [Google Scholar]
- Leopold, S.S.; Casnellie, M.T.; Warme, W.J.; Dougherty, P.J.; Wingo, S.T.; Shott, S. Endogenous cortisol production in response to knee arthroscopy and total knee arthroplasty. J. Bone Jt. Surg. Am. 2003, 85, 2163–2167. [Google Scholar] [CrossRef]
- Hessov, I.; Bekker Jeppesen, P. Klinisk Ernæring, 5th ed.; Munksgaard, Danmark, 2011; ISBN 9788762809840. Available online: https://nexs.ku.dk/ansatte/?pure=en%2Fpublications%2Fernaering-af-det-syge-barn(3b79acb5-7a8e-49e7-9907-d9cfed03b63c)%2Fexport.html (accessed on 19 May 2022).
- Williams, D.G.A.; Ohnuma, T.; Krishnamoorthy, V.; Raghunathan, K.; Sulo, S.; Cassady, B.A.; Hegazi, R.; Wischmeyer, P.E. Impact of early postoperative oral nutritional supplement utilization on clinical outcomes in colorectal surgery. Perioper. Med. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Alley, D.E.; Koster, A.; Mackey, D.; Cawthon, P.; Ferrucci, L.; Simonsick, E.M.; Yu, B.; Hardy, S.; Goodpaster, B.; Sarkisian, C.; et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J. Am. Geriatr. Soc. 2010, 58, 2085–2091. [Google Scholar] [CrossRef] [Green Version]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef]
- Holubar, S.D.; Miller, T.E.; Thiele, R.H.; Gupta, R.; Bennett-Guerrero, E.; Feldman, L.S.; Wischmeyer, P.E.; Pryor, A.; Bergamaschi, R.; Abola, R.E.; et al. American Society for Enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef]
- Williams, D.G.A.; Ohnuma, T.; Krishnamoorthy, V.; Raghunathan, K.; Sulo, S.; Cassady, B.A.; Hegazi, R.; Wischmeyer, P.E. Postoperative Utilization of Oral Nutrition Supplements in Surgical Patients in US Hospitals. J. Parenter. Enter. Nutr. 2021, 45, 596–606. [Google Scholar] [CrossRef]
- Williams, D.G.A.; Molinger, J.; Wischmeyer, P.E. The malnourished surgery patient: A silent epidemic in perioperative outcomes? Curr. Opin. Anaesthesiol. 2019, 32, 405–411. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Zamarrón, I.; Arrieta, F.; Vázquez, C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: A randomized clinical trial. JPEN J. Parenter. Enteral Nutr. 2008, 32, 120–128. [Google Scholar] [CrossRef]
- Kong, S.H.; Lee, H.J.; Na, J.R.; Kim, W.G.; Han, D.S.; Park, S.H.; Hong, H.; Choi, Y.; Ahn, H.S.; Suh, Y.S.; et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: A prospective randomized trial. Surgery 2018, 164, 1263–1270. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery after Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Crickmer, M.; Dunne, C.P.; O’Regan, A.; Coffey, J.C.; Dunne, S.S. Benefits of post-operative oral protein supplementation in gastrointestinal surgery patients: A systematic review of clinical trials. World J. Gastrointest. Surg. 2016, 8, 521. [Google Scholar] [CrossRef]
- Beattie, A.H.; Prach, A.T.; Baxter, J.P.; Pennington, C.R. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000, 46, 813–818. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.S.; Avelar, A.; Schoenfeld, B.J.; Fleck, S.J.; Souza, M.F.; Padilha, C.S.; Cyrino, E.S. Analysis of the training load during a hypertrophy-type resistance training programme in men and women. Eur. J. Sport Sci. 2014, 1–9. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, L.; Cholewa, J.M.; Naimo, M.A.; Zhi, X.I.A.; Magagnin, D.; de Sá, R.B.D.P.; Streck, E.L.; da Silva Teixeira, T.; Zanchi, N.E. Synergistic effects of resistance training and protein intake: Practical aspects. Nutrition 2014, 30, 1097–1103. [Google Scholar] [CrossRef]
- Miller, M.D.; Crotty, M.; Whitehead, C.; Bannerman, E.; Daniels, L.A. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture: A randomized controlled trial. Clin. Rehabil. 2006, 20, 311–323. [Google Scholar] [CrossRef]
- Kondrup, J. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Patursson, P.; Beermann, T.; Steffensen, T.; Muhic, A.; Holtug, K.; Andersen, J.R. P118 Malnutrition during abdominal radiotherapy for cancer and the effect of supplement containing fish-oil. Clin. Nutr. Suppl. 2009, 4, 75. [Google Scholar] [CrossRef]
- Andersen, H.K.; Lewis, S.J.; Thomas, S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst. Rev. 2006, 4, CD004080. [Google Scholar] [CrossRef]
- De Morton, N.A.; Brusco, N.K.; Wood, L.; Lawler, K.; Taylor, N.F. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: An observational study. J. Physiother. 2011, 57, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Dansk Selskab for Almen Medicin. Klinisk Vejledning for Almen Praksis. Den Ældre Patient; Dansk Selskab for Almen Medicin: Copenhagen, Denmark, 2012. [Google Scholar]
- Frankenfield, D.C.; Muth, E.R.; Rowe, W.A. The Harris-Benedict studies of human basal metabolism: History and limitations. J. Am. Diet. Assoc. 1998, 98, 439–445. [Google Scholar] [CrossRef]
- Pedersen, A.N.; Ovesen, L. Anbefalinger for den Danske Institutionskost, 4th ed.; Pedersen, A.N., Ovesen, L., Eds.; Fødevarestyrelsen: København, Danmark, 2009; ISBN 9788792395290. [Google Scholar]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Tailoring nutrition therapy to illness and recovery. Crit. Care 2017, 21, 15–25. [Google Scholar] [CrossRef]
- O’Doherty, A.F.; West, M.; Jack, S.; Grocott, M.P.W. Preoperative aerobic exercise training in elective intra-cavity surgery: A systematic review. Br. J. Anaesth. 2013, 110, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, A.J.; Webber, S.C.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.A.; Danyliw, A.; Sawant, A.; Dal Bello-Haas, V.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD010884. [Google Scholar] [CrossRef] [PubMed]
- Hubner, S.; Boron, J.B.; Koehler, K. The Effects of Exercise on Appetite in Older Adults: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 734267. [Google Scholar] [CrossRef]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Bastien Grigioni, S.; Déchelotte, P.; Coë Ffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Diet (n = 13) | Exercise + Diet (n = 16) | Control (n = 16) | |
|---|---|---|---|
| Sex | |||
| Female | 7 (53.8%) | 7 (43.8%) | 8 (50.0%) |
| Male | 6 (46.2%) | 9 (56.2%) | 8 (50.0%) |
| Age | 68.0 [56.0, 74.0] | 59.0 [37.3, 73.3] | 66.5 [54.8, 70.3] |
| Postoperative days at inclusion | 4.0 [3.0, 8.0] | 6.50 [3.0, 9.0] | 3.00 [3.0, 6.5] |
| Surgery | |||
| Minimal | 0 (0%) | 5 (31.2%) | 3 (18.8%) |
| Open | 13 (100%) | 11 (68.8%) | 13 (81.2%) |
| Specialty | |||
| Gastroenterology | 9 (69.2%) | 8 (50.0%) | 5 (31.2%) |
| Gynaecology | 0 (0%) | 0 (0%) | 1 (6.2%) |
| Orthopaedics | 4 (30.8%) | 8 (50.0%) | 9 (56.2%) |
| Urology | 0 (0%) | 0 (0%) | 1 (6.2%) |
| Habitual bodyweight (kg) | 89.1 (10.6) | 81.5 (21.5) | 77.8 (18.2) |
| Bodyweight (kg) | 86.7 (9.02) | 77.2 (20.6) | 76.3 (18.3) |
| BMI | 29.6 [28.0, 32.0] | 24.4 [23.6, 28.1] | 25.6 [22.7, 30.8] |
| Weight loss from habitual weight (%) | −2.35 (3.70) | −4.37 (5.41) | −1.53 (4.40) |
| Lean Body mass (kg) | 52.3 (7.8) | 51.7 (13.9) | 51.0 (11.9) |
| Fat mass (kg) | 35.7 (12.1) | 25.4 (13.2) | 25.7 (13.5) |
| Handgrip strength | 32.6 (9.72) | 35.0 (13.1) | 34.1 (15.3) |
| Nutritional Risk Score (NRS) | |||
| Total NRS ≥ 3 (n (%)) | 12 (92) | 14 (88) | 14 (88) |
| NRS nutritional score (0–3) | 1.1 (1.0) | 1.1 (0.7) | 1.5 (0.9) |
| NRS severity of disease (0–3) | 1.9 (0.3) | 1.6 (0.5) | 1.4 (0.5) |
| Total NRS score (0–7) | 3.4 (1.1) | 3.1 (0.6) | 3.3 (1.2) |
| Energy intake | |||
| Energy intake (kJ/d) | 8130 (3020) | 7590 (2600) | 8880 (2430) |
| Energy intake (% of requirements) | 81 (30) | 83 (29) | 98 (35) |
| Energy < 75% requirements (n (%)) | 2 (15) | 5 (31) | 4 (25) |
| Protein intake | |||
| Protein intake (g/d) | 73.8 (30.6) | 72.3 (33.4) | 82.8 (19.7) |
| Protein intake (g/kg/d) | 0.9 (0.36) | 1.1 (0.64) | 1.1 (0.49) |
| Protein intake (% of requirements) | 69 (28) | 73 (30) | 85 (26) |
| Protein < 75% requirements (n (%)) | 2 (15) | 6 (38) | 4 (25) |
| Change within Groups | Difference between Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet (n = 12) | p | Exercise + Diet (n = 16) | p | Control (n = 12) | p | Exercise + Diet vs. Diet | p | Diet vs. Control | p | Exercise + Diet vs. Control | p | |
| LBM (kg) | 0.2 (−1.7, 2.2) | 0.81 | 0.8 (−0.9, 2.5) | 0.36 | 0.1 (−1.9, 2.1) | 0.93 | 0.6 (−2.0, 3.2) | 0.66 | 0.2 (−2.7, 3.0) | 0.91 | 0.7 (−1.9, 3.0) | 0.59 |
| LBM % | 0.4 (−3.8, 4.6) | NS | 1.5 (−2.1, 5.2) | NS | 0.2 (−4.2, 4.6) | NS | ||||||
| Weight (kg) | 0.5 (−1.8, 2.8) | 0.68 | 1.4 (−0.6, 3.4) | 0.17 | 0.4 (−1.9, 2.7) | 0.75 | 0.9 (−2.1, 4.0) | 0.56 | 0.1 (−3.1, 3.4) | 0.95 | 1.0 (−2.0, 4.1) | 0.51 |
| Weight (%) | 0.5 (−2.7, 3.7) | NS | 2.0 (−0.8, 4.8) | NS | 0.7 (−2.5, 3.9) | NS | ||||||
| Handgrip strength (kg) | 1.2 (−1.5, 3.8) | 0.40 | 2.1 (−0.2, 4.5) | 0.08 | 1.6 (−1.1, 4.4) | 0.25 | 1.0 (−2.6, 4.6) | 0.59 | −0.5 (−4.3, 3.4) | 0.81 | 0.5 (−3.1, 4.2) | 0.78 |
| Fat mass (kg) | −0.4 (−1.8, 1.0) | 0.56 | −0.1 (−1.3, 1.2) | 0.91 | 0.4 (−1.1, 1.8) | 0.64 | 0.4 (−1.5, 2.2) | 0.71 | −0.8 (−2.8, 1.3) | 0.46 | −0.4 (−2.3, 1.5) | 0.67 |
| Energy intake (kJ/d) | 2311 (−177, 4798) | 0.07 | 2818 (−669, 4968) | 0.01 * | −1402 (−3808, 1004) | 0.25 | 507 (−2780, 3795) | 0.76 | 3713 (252, 7174) | 0.04 * | 4220 (994, 7447) | 0.01 * |
| Energy intake (% of requirements) | 25 (−7, 58) | 0.13 | 38 (9, 66) | 0.01 * | −16 (−48, 15) | 0.31 | 12 (−31, 56) | 0.58 | 42 (−4, 87) | 0.07 | 54 (11, 97) | 0.01 * |
| Protein intake (g/d) | 21.0 (−2.9, 44.8) | 0.09 | 20.1 (−0.5, 40.6) | 0.06 | −8.6(−31.7,14.5) | 0.47 | 00.9 (−32.4, 30.6) | 0.96 | 29.5 (−3.7, 62.7) | 0.08 | 28.6 (−2.3, 59.5) | 0.07 |
| Protein intake (% of requirements) | 20 (−8, 48) | 0.17 | 26 (1, 51) | 0.04 * | −9 (−36, 18) | 0.53 | 6 (−31, 44) | 0.74 | 29 (−11, 68) | 0.15 | 35 (−2, 72) | 0.06 |
| QOL:SF-12-1, general health, scale | −0.2 (−0.7, 0.3) | 0.47 | −0.5 (−1.0, 0.0) | 0.03 * | −0.9 (−1.4, −0.3) | <0.01 * | −0.3 (−1.0, 0.4) | 0.40 | 0.7 (−0.1, 1.4) | 0.08 | 0.4 (−0.3, 1.1) | 0.31 |
| QOL:SF-12-8, pain interference, scale | −0.3 (−1.2, 0.5) | 0.43 | −1.4 (−2.1, −0.6) | <0.01 * | −1.0 (−1.9, −0.2) | 0.01 * | −1.0 (−2.2, 0.1) | 0.07 | 0.7 (−0.5, 1.9) | 0.26 | −0.3 (−1.5, 0.8) | 0.50 |
| Sit-to-stand, times | 3.2 (0.5, 5.9) | 0.02 * | 6.0 (3.5, 8.4) | <0.01 * | 6.9 (4.1, 9.7) | <0.01 * | 2.8 (−0.9, 6.4) | 0.14 | −3.7 (−7.6, 0.2) | 0.06 | −1.0 (−4.6, 2.7) | 0.61 |
| DEMMI, score | 17 (6, 28) | <0.01 * | 18 (8, 28) | <0.01 * | 15 (4, 27) | <0.01 * | 1 (−14, 16) | 0.88 | 2 (−14, 18) | 0.82 | 3 (−12, 18) | 0.71 |
| Surgery-related side effect: pain, scale (1 no pain, 6 very strong pain) | −1.0 (−1.9, 0.0) | 0.05 * | −1.5 (−2.4, −0.7) | <0.01 * | −0.2 (−1.1, 0.7) | 0.66 | −0.5 (−1.8, 0.7) | 0.40 | −0.8 (−2.1, 0.6) | 0.27 | −1.3 (−2.6, 0.0) | 0.04 * |
| Surgery-related side effect: self-reported food intake less than usual (6 all the time, 1 never) | −2.2 (−3.4, −0.9) | <0.01 * | −2.1 (−3.45, −0.93) | <0.01 * | −2.6 (−3.7, −1.4) | <0.01 * | 0.1 (−1.6, 1.7) | 0.95 | 0.4 (−1.4, 2.1) | 0.68 | 0.4 (−1.2, 2.1) | 0.61 |
| Surgery-related side effect: Poor appetite (6 all the time, 1 never) | −1.2 (−2.4, −0.0) | 0.04 * | −1.0 (−2.1, 0.0) | 0.06 | −2.2 (−3.3, −1.1) | <0.01 * | 0.2 (−1.4, 1.8) | 0.80 | 1.0 (−0.6, 2.6) | 0.22 | 1.2 (−0.3, 2.8) | 0.12 |
| Surgery-related side effect: nausea (6 all the time, 1 never) | −0.5 (−1.7, 0.6) | 0.35 | −0.3 (−1.4. 0.7) | 0.51 | −1.6 (−2.7, −0.5) | <0.01 * | 0.2 (−1.3, 1.7) | 0.80 | 1.0 (−1.3, 1.7) | 0.20 | 1.2 (−0.3, 2.7) | 0.11 |
| Surgery-related side effect: use of painkillers (6 all the time, 1 never) | −1.6 (−2.6, −0.6) | <0.01 * | −2.29 (−3.2, −1.4) | <0.01 * | −2.0 (−3.0, −1.1) | <0.01 * | −0.7 (−2.0, 0.7) | 0.32 | 0.4 (−1.0, 1.8) | 0.55 | −0.3 (−1.6, 1.1) | 0.71 |
| Within Group | Between Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise + Diet (>75% Training Attendance | Diet (>75% ONS Compliance) | Exercise + Diet (>75% ONS Compliance) | Exercise + Diet (>75% ONS Compliance) vs. Diet | p | Diet (>75% ONS Compliance) vs. Control | p | Exercise + Diet (>75% ONS Compliance) vs. Control | p | |
| LBM (kg) | 1.7(−0.5, 3.9) | 2.1 (−0.4, 5.6) | 1.9 (0.0, 3.7) * | −0.2 (−4.2, 3.7) | 0.92 | 2.0 (−1.8, 5.8) | 0.30 | 1.8 (−0.6, 4.2) | 0.14 |
| LBM (%) | 3.2(−1.7, 8.0) | 4.1 (−3.3, 11.5) | 3.7 (−0.3, 7.6) | −1.5 (−10.1, 7.0) | 0.71 | 3.1 (−5.0, 11.2) | 0.42 | 1.6 (−4.4, 7.6) | 0.58 |
| Weight (kg) | 2.9 (0.3, 5.4) * | 1.4 (−4.2, 6.9) | 2.0 (−0.9, 5.0) | 0.7 (−5.6, 7.0) | 0.83 | 0.9 (−5.1, 6,9) | 0.76 | 1.6 (−2.1, 5.4) | 0.39 |
| Weight (%) | 0.5(−2.8, 3.8) | 1.4 (−7.2, 10.0) | 3.1 (−1.5, 7.7) | −0.2 (−9.6, 9.2) | 0.97 | −0.7 (−9.5, 8.2) | 0.88 | −0.8 (−7.4, 5.7) | 0.79 |
| Diet (n = 13) | Exercise + Diet (n = 16) | |
|---|---|---|
| ONS compliance (%) | 50.2 [26.6, 71.9] | 69.6 [40.8, 88.5] |
| ONS per day (cans) | 1.0 [0.5, 1.4] | 1.4 [0.8, 1.8] |
| ONS compliance > 75% | ||
| Yes | 2 (15.4%) | 7 (43.8%) |
| No | 10 (76.9%) | 9 (56.2%) |
| Training attendance (%) | NA | 98.8 [67.3, 100.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patursson, P.; Møller, G.; Thomsen, B.B.; Olsen, E.; Mortensen, J.; Andorsdóttir, G.; Mohr, M.; Andersen, J.R. Effects of Postdischarge High-Protein Oral Nutritional Supplements and Resistance Training in Malnourished Surgical Patients: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 2599. https://doi.org/10.3390/nu14132599
Patursson P, Møller G, Thomsen BB, Olsen E, Mortensen J, Andorsdóttir G, Mohr M, Andersen JR. Effects of Postdischarge High-Protein Oral Nutritional Supplements and Resistance Training in Malnourished Surgical Patients: A Pilot Randomized Controlled Trial. Nutrients. 2022; 14(13):2599. https://doi.org/10.3390/nu14132599
Chicago/Turabian StylePatursson, Poula, Grith Møller, Bjartur Bernhardson Thomsen, Eyðfinnur Olsen, Jann Mortensen, Guðrið Andorsdóttir, Magni Mohr, and Jens Rikardt Andersen. 2022. "Effects of Postdischarge High-Protein Oral Nutritional Supplements and Resistance Training in Malnourished Surgical Patients: A Pilot Randomized Controlled Trial" Nutrients 14, no. 13: 2599. https://doi.org/10.3390/nu14132599
APA StylePatursson, P., Møller, G., Thomsen, B. B., Olsen, E., Mortensen, J., Andorsdóttir, G., Mohr, M., & Andersen, J. R. (2022). Effects of Postdischarge High-Protein Oral Nutritional Supplements and Resistance Training in Malnourished Surgical Patients: A Pilot Randomized Controlled Trial. Nutrients, 14(13), 2599. https://doi.org/10.3390/nu14132599






