Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
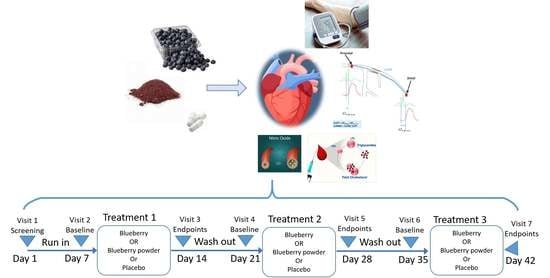
2.2. Treatment
2.3. Total Polyphenol Analysis
2.4. Biological Sampling
2.5. Anthropometric Measurements
2.6. Whole Body Measurements of Cardiovascular Health
2.7. Clinical Chemistry Assessment
2.8. Statistical Analysis
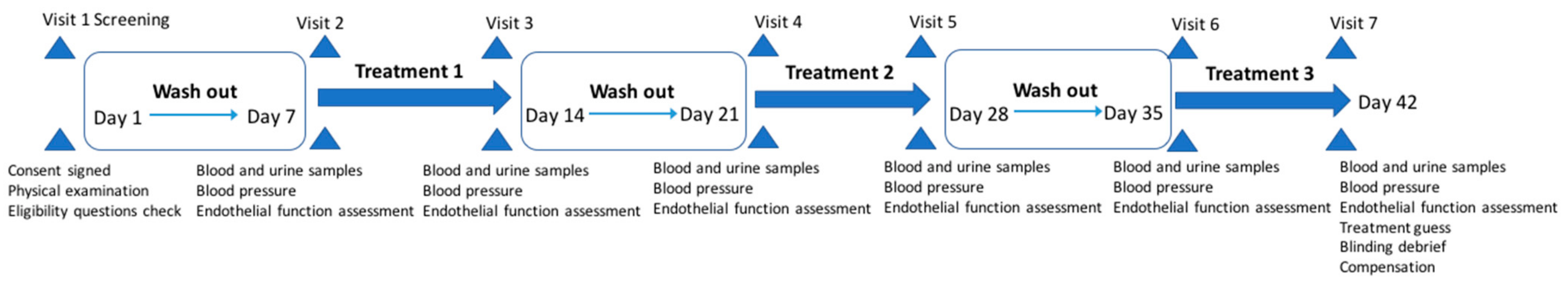
2.9. Study Procedure
3. Results
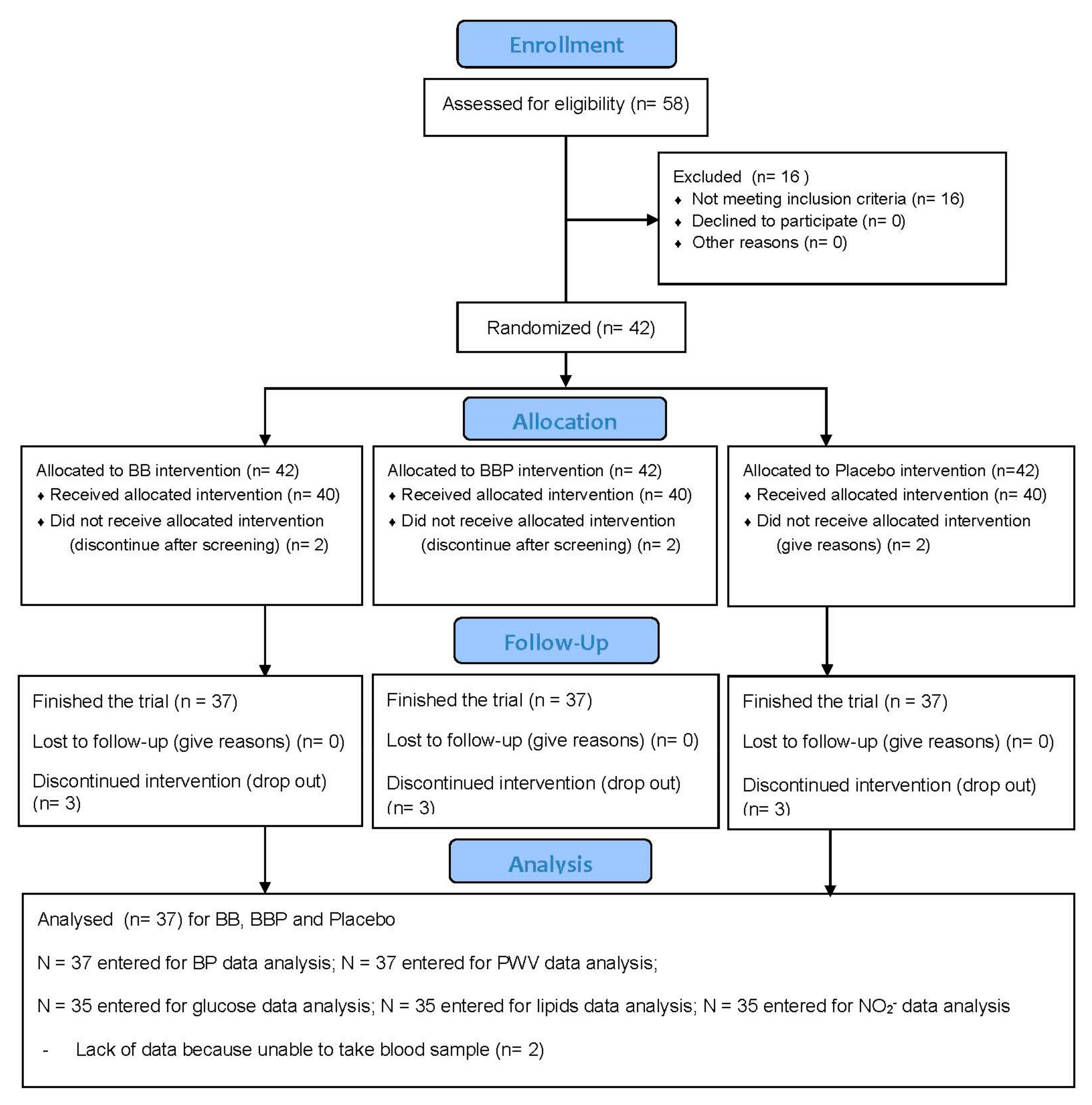
3.1. Participants
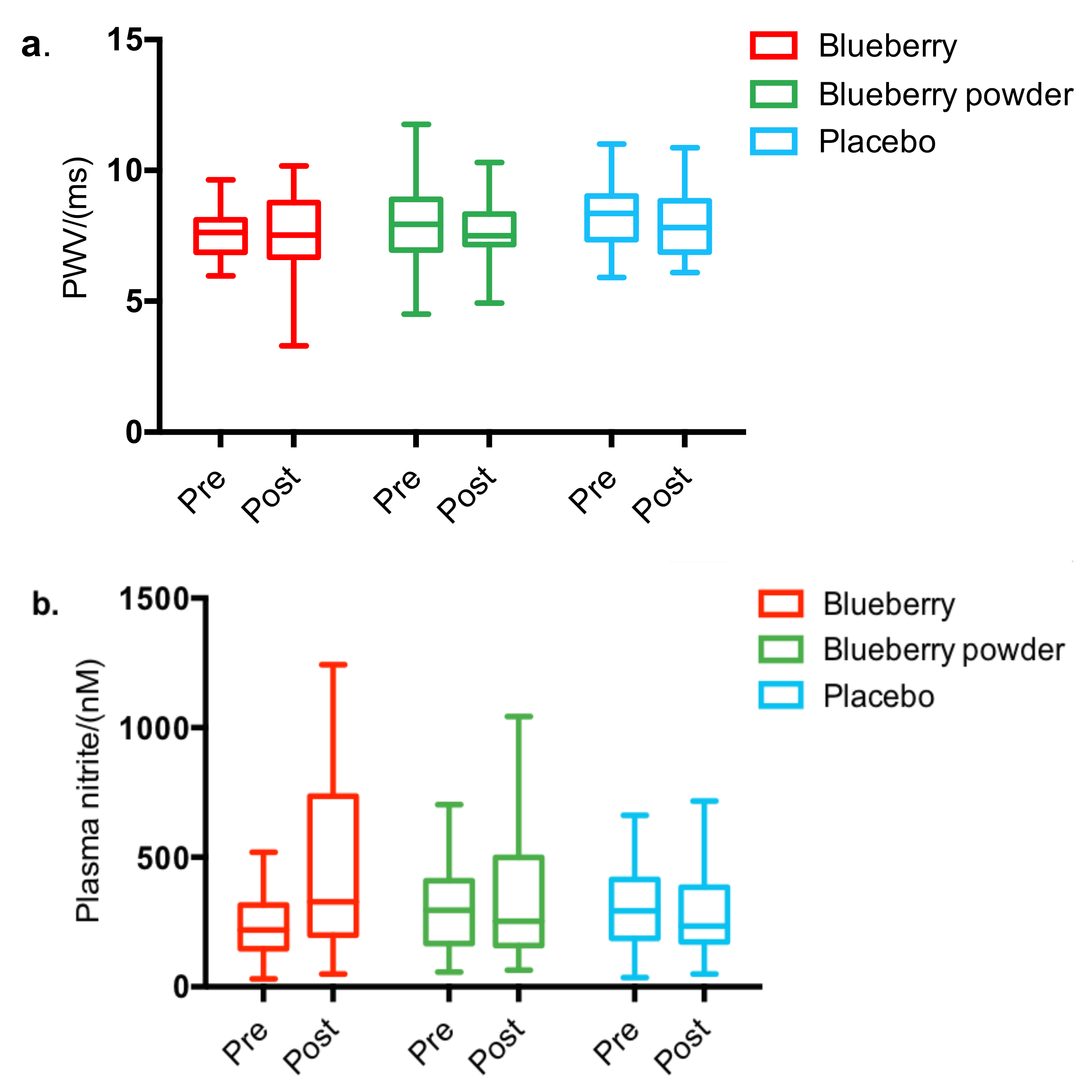
3.2. Whole Body Measurements of Cardiovascular Health
3.3. Clinical Chemistry Assessment
4. Discussion
4.1. Principal Findings
4.2. Effect of Blueberry Interventions on Cardiovascular Health
4.3. Comparison between Whole Blueberry and Blueberry Powder
4.4. Study Limitations and Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruan, Y.; Guo, Y.; Zheng, Y.; Huang, Z.; Sun, S.; Kowal, P.; Shi, Y.; Wu, F. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE Wave 1. BMC Public Health 2018, 18, 778. [Google Scholar] [CrossRef] [Green Version]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Li, H.; Horke, S.; Förstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.H. Nitrite and nitric oxide as potential diagnostic markers in acute vascular diseases. J. Neurol. Neurophysiol. 2011, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function from research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Gates, P.E.; Strain, W.D.; Shore, A.C. Human endothelial function and microvascular ageing. Exp. Physiol. 2009, 94, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Bearden, M.M.; Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef]
- Bondonno, N.; Bondonno, C.P.; Blekkenhorst, L.; Considine, M.; Maghzal, G.; Stocker, R.; Woodman, R.; Ward, N.; Hodgson, J.M.; Croft, K. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Mol. Nutr. Food Res. 2017, 62, 1700674. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle modulators of neuroplasticity: How physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2020, 60, 615–639. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of anthocyanins on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef]
- Wu, X.; Wang, T.T.Y.; Prior, R.L.; Pehrsson, P.R. Prevention of atherosclerosis by berries: The case of blueberries. J. Agric. Food Chem. 2018, 66, 9172–9188. [Google Scholar] [CrossRef]
- CBI; Ministry of Foreign Affairs. The European Market Potential for Fresh Blueberries. 2020. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/blueberries/market-potential#:~:text=In%202018%20the%20United%20Kingdom,estimated%200.7%20kg%20per%20capita (accessed on 1 March 2020).
- Del Bo’, C.; Porrini, M.; Fracassetti, D.; Campolo, J.; Klimis-Zacas, D.; Riso, P. A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: A randomized-controlled trial. Food Funct. 2014, 5, 3107–3116. [Google Scholar] [CrossRef] [Green Version]
- Del Bo, C.; Deon, V.; Campolo, J.; Lanti, C.; Parolini, M.; Porrini, M.; Klimis-Zacas, D.; Riso, P. A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: Two randomized, controlled, crossover pilot studies. Food Funct. 2017, 8, 4108–4117. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [Green Version]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Bennett, J.A. The consolidated standards of reporting trials (CONSORT): Guidelines for reporting randomized trials. Nurs. Res. 2005, 54, 128–132. [Google Scholar] [CrossRef]
- Hatami, T.; Emami, S.A.; Miraghaee, S.S.; Mojarrab, M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of artemisia biennis willd. Iran. J. Pharm. Res. IJPR 2014, 13, 551–559. [Google Scholar]
- Ee, C. Understanding Blood Pressure Reading: Measuring Guide and Tips. Available online: https://www.homage.com.my/health/high-blood-pressure-reading/ (accessed on 1 March 2020).
- Total Cholesterol|Reagent|Randox Laboratories. Available online: https://www.randox.com/total-cholesterol/ (accessed on 1 March 2020).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Measurement of plasma nitrite by chemiluminescence without interference of S-, N-nitroso and nitrated species. Free Radic. Biol. Med. 2007, 42, 1146–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, P.J.; Van Der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre-and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, S.; Hong, S.J.; Choi, S.C.; Choi, J.-H.; Kim, J.-H.; Park, C.-Y.; Cho, J.Y.; Lee, T.-B.; Kwon, J.-W.; et al. Black raspberry extract increased circulating endothelial progenitor cells and improved arterial stiffness in patients with metabolic syndrome: A randomized controlled trial. J. Med. Food 2016, 19, 346–352. [Google Scholar] [CrossRef]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Dodd, G.F.; Williams, C.M.; Butler, L.T.; Spencer, J.P. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr. Health Aging 2019, 5, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.N.; Han, S.N.; Ha, T.J.; Kim, H.-K. Black soybean anthocyanins attenuate inflammatory responses by suppressing reactive oxygen species production and mitogen activated protein kinases signaling in lipopolysaccharide-stimulated macrophages. Nutr. Res. Pract. 2017, 11, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.R.; Gochenaur, K.E. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A Review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; DEL Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2012, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DEL Bo’, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L.) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef]
- Nyberg, S.; Gerring, E.; Gjellan, S.; Vergara, M.; Lindström, T.; Nystrom, F.H. Effects of exercise with or without blueberries in the diet on cardio-metabolic risk factors: An exploratory pilot study in healthy subjects. Upsala J. Med. Sci. 2013, 118, 247–255. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Pallas, L.A. Drying Methods and Their Effects on Bioactive Compounds and Quality of Georgia Blueberries. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2011. [Google Scholar]
- Salehi, F. Recent applications of powdered fruits and vegetables as novel ingredients in biscuits: A review. Nutrire 2019, 45, 1. [Google Scholar] [CrossRef]
- Langer, S.; Kennel, A.; Lodge, J.K. The influence of juicing on the appearance of blueberry metabolites 2 h after consumption: A metabolite profiling approach. Br. J. Nutr. 2018, 119, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Kearney, M. Prevention and treatment of CVD: A new priority for the NHS. Heart 2019, 105, 1924. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental vitamins and minerals for CVD prevention and treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.M.; Fuhrmann, J.C.; van Dorsten, F.A.; Rein, D.; Peters, S.; van Velzen, E.J.; Hollebrands, B.; Draijer, R.; van Duynhoven, J.; Garczarek, U. Impact of short-term intake of red wine and grape polyphenol extract on the human metabolome. J. Agric. Food Chem. 2012, 60, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.; Ma, S.; Ploeger, B. Clinical trial simulation: A review. Clin. Pharmacol. Ther. 2010, 88, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Hallare, J.; Gerriets, V. Half Life. Available online: https://europepmc.org/article/nbk/nbk5544982020 (accessed on 1 March 2020).
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.C.; Brennan, L.; Pujos-Guillot, E.; Sébédio, J.e.; Scalbert, A.; Fagan, A.; Higgins, D.G.; Gibney, M.J. Influence of acute phytochemical intake on human urinary metabolomic profiles. Am. J. Clin. Nutr. 2007, 86, 1687–1693. [Google Scholar] [CrossRef]
- Crichton, G.E.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef] [Green Version]
- Miraghajani, M.; Momenyan, S.; Arab, A.; Dehkordi, A.H.; Symonds, M.E. Blueberry and cardiovascular disease risk factors: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 53, 102389. [Google Scholar] [CrossRef]
- Choices, N. How to have a balanced diet-Live Well-NHS Choices. Men’s Health 2014, 18, 39. [Google Scholar]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Baker, L.; Meldrum, K.K.; Wang, M.; Sankula, R.; Vanam, R.; Raiesdana, A.; Tsai, B.; Hile, K.; Brown, J.W.; Meldrum, D.R. The role of estrogen in cardiovascular disease. J. Surg. Res. 2003, 115, 325–344. [Google Scholar] [CrossRef]
| Blueberry (per 160 g) 1 | Blueberry Powder (per 20 g) 2 | Control (per 1 g) 3 | ||
|---|---|---|---|---|
| Energy (kcal) | 120.00 | 69.20 | 0.00 | |
| Fat (g) | 0.32 | 1.30 | 0.00 | |
| From Saturates (g) | 0.00 | 0.08 | 0.00 | |
| Total Carbohydrates (g) | 23.20 | 13.90 | 1.00 | |
| From Sugars (g) | 22.40 | 7.78 | 0.00 | |
| Protein (g) | 0.96 | 1.40 | 0.00 | |
| Total Polyphenol Analysis (TPC) 4 | Gallic Acid Equivalence (mg/d) 4 | 220.48 | 288.43 | 0.00 |
| Variables | Value 1 |
|---|---|
| Age (years) | 25.86 ± 6.81 |
| BMI (kg/m2) | 23.15 ± 3.12 |
| Gender | 13 male, 24 female |
| Ethnicity | 1 Black |
| 2 Indian Asian | |
| 3 Chinese Asian | |
| 31 White European | |
| Fruit and vegetable intake (portions/day) | 2.13 ± 0.85 |
| Berry intake (portions/day) 2 | 0.06 ± 1.61 |
| Pre | Post | |||||||
|---|---|---|---|---|---|---|---|---|
| Blueberry | Blueberry Powder | Placebo | Significance | Blueberry | Blueberry Powder | Placebo | Significance | |
| Energy (kcal) a | 1580.697 (425.250) | 1485.929 (406.151) | 1487.353 (453.500) | p ≥ 0.581 | 1583.227 (424.395) | 1490.961 (409.881) | 1489.849 (453.103) | p ≥ 0.591 |
| Total Carbohydrates (g) | 170.112 (50.693) | 161.009 (57.216) | 163.453 (68.156) | p ≥ 0.806 | 170.372 (51.247) | 177.028 (86.868) | 163.619 (68.344) | p ≥ 0.735 |
| Fat (g) | 57.512 (22.635) | 57.926 (22.238) | 55.923 (24.775) | p ≥ 0.932 | 58.650 (22.168) | 59.163 (21.634) | 57.388 (25.154) | p ≥ 0.948 |
| Protein (g) | 79.629 (48.500) | 65.235 (34.302) | 71.323 (37.794) | p ≥ 0.346 | 80.098 (48.638) | 63.435 (24.886) | 71.989 (37.826) | p ≥ 0.206 |
| Blueberry Intervention | Blueberry Powder Intervention | Placebo Intervention | Effects 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | △ | Pre | Post | △ | Pre | Post | △ | ||
| PWV, m/s | 7.630 (0.153) | 7.443 (0.239) | −0.187 | 7.993 (0.258) | 7.526 (0.234) | −0.467 | 8.265 (0.209) | 7.891 (0.244) | −0.374 | p = 0.567 |
| BP, mmHg | ||||||||||
| Systolic | 108.727 (1.411) | 108.704 (1.815) | −0.023 | 110.278 (1.711) | 111.395 (1.806) | 1.117 | 109.314 (1.691) | 109.732 (1.822) | 0.418 | p = 0.540 |
| Diastolic | 64.059 (1.440) | 63.369 (1.398) | −0.690 | 64.333 (1.482) | 64.048 (1.388) | −0.285 | 63.676 (1.395) | 64.626 (1.406) | 0.950 | p = 0.366 |
| Plasma biomarkers | ||||||||||
| TAG | 0.820 (0.053) | 0.840 (0.075) | 0.020 | 0.894 (0.059) | 0.918 (0.071) | 0.024 | 0.825 (0.061) | 0.880 (0.074) | 0.055 | p = 0.960 |
| Total cholesterol | 4.302 (0.171) | 4.557 (0.136) | 0.255 | 4.567 (0.195) | 4.533 (0.125) | −0.034 | 4.509 (0.161) | 4.324 (0.134) | −0.185 | p = 0.402 |
| LDL-C | 1.389 (0.147) | 2.829 (0.136) | 1.440 | 2.875 (0.138) | 2.911 (0.128) | 0.036 | 2.858 (0.128) | 2.688 (0.136) | −0.170 | p = 0.171 |
| HDL-C | 2.749 (0.064) | 1.439 (0.068) | −1.310 | 1.514 (0.093) | 1.443 (0.066) | −0.071 | 1.487 (0.080) | 1.516 (0.068) | 0.029 | p = 0.989 |
| Glucose | 5.755 (0.137) | 5.952 (0.155) | 0.197 | 5.818 (0.12) | 5.788 (0.145) | −0.030 | 5.854 (0.171) | 5.625 (0.155) | −0.229 | p = 0.659 |
| Nitrite/NO2−, nM | 236.690 (21.086) | 399.190 (47.030) | 155.733 | 310.371 (31.311) | 323.84 (45.19) | 27.368 | 305.95 (32.708) | 278.12 (45.249) | −74.967 | p = 0.184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gallegos, J.L.; Haskell-Ramsay, C.; Lodge, J.K. Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial. Nutrients 2022, 14, 2562. https://doi.org/10.3390/nu14132562
Wang Y, Gallegos JL, Haskell-Ramsay C, Lodge JK. Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial. Nutrients. 2022; 14(13):2562. https://doi.org/10.3390/nu14132562
Chicago/Turabian StyleWang, Yueyue, Jose Lara Gallegos, Crystal Haskell-Ramsay, and John K. Lodge. 2022. "Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial" Nutrients 14, no. 13: 2562. https://doi.org/10.3390/nu14132562
APA StyleWang, Y., Gallegos, J. L., Haskell-Ramsay, C., & Lodge, J. K. (2022). Effects of Blueberry Consumption on Cardiovascular Health in Healthy Adults: A Cross-Over Randomised Controlled Trial. Nutrients, 14(13), 2562. https://doi.org/10.3390/nu14132562