Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Populations
2.2. Information of Dietary BCAAs Intake
2.3. BP Measurements and the Definition of Hypertension
2.4. Measurements of Confounders
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
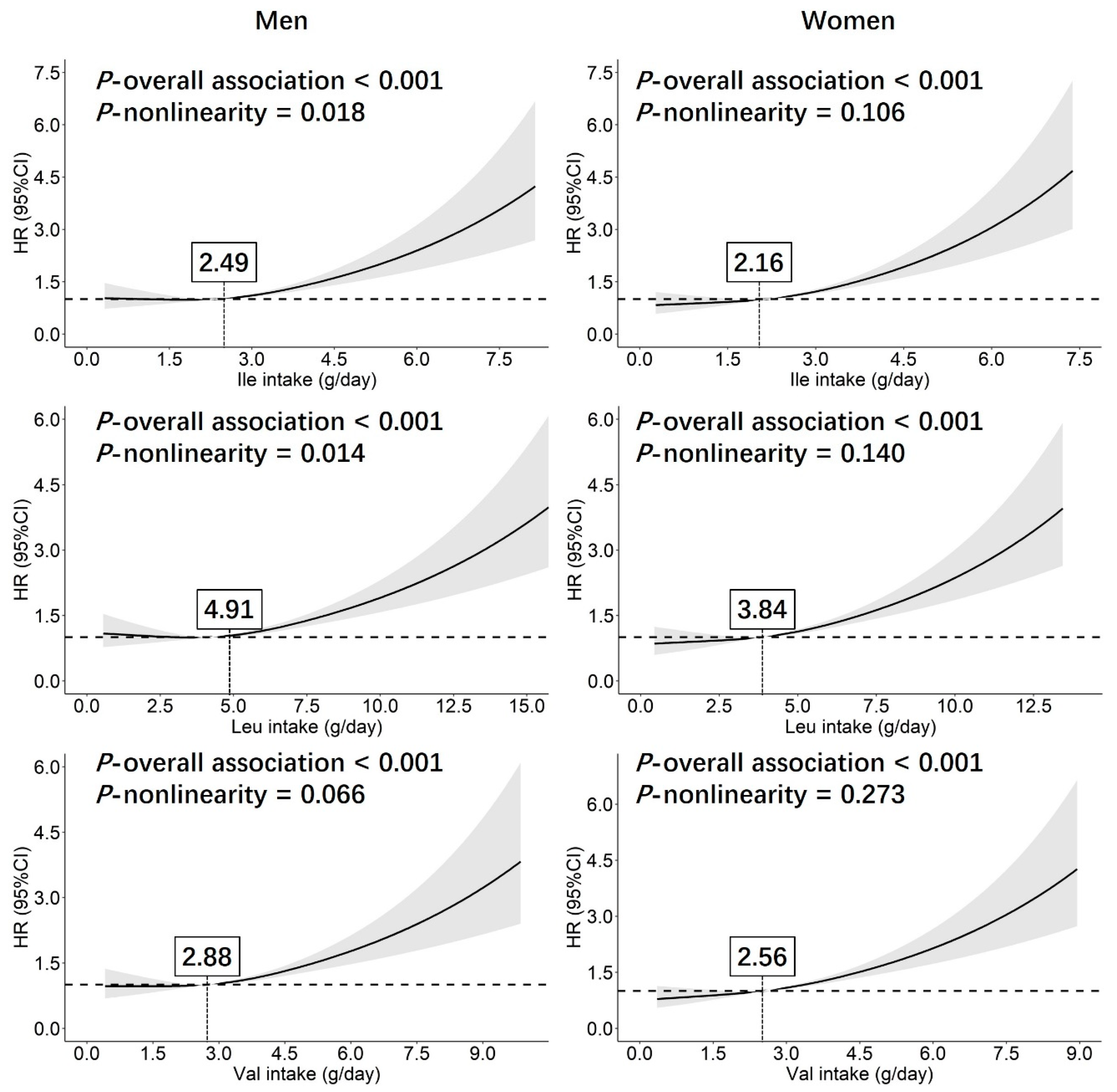
3.2. Associations of BP Values with Dietary BCAAs Intake
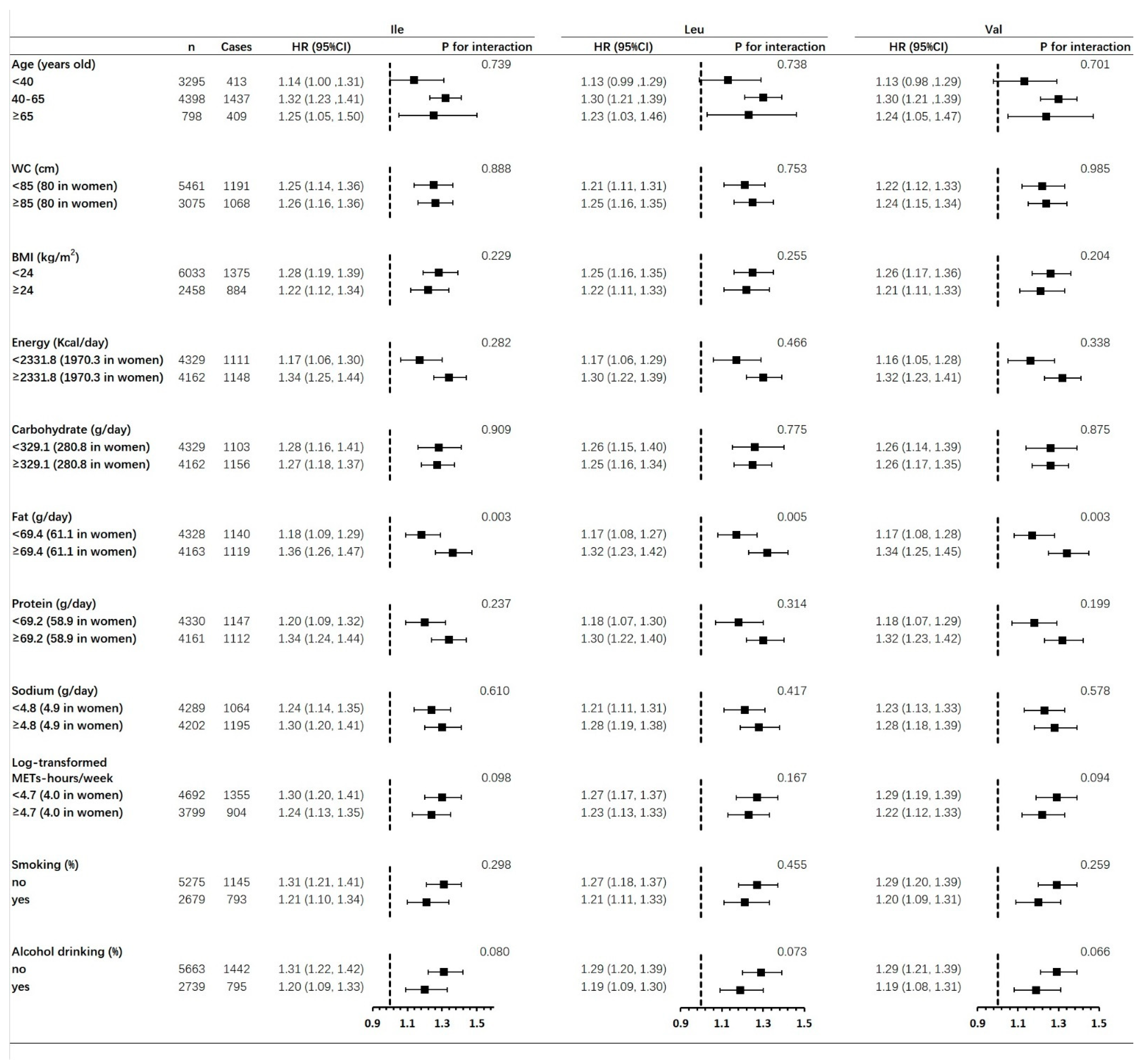
3.3. Associations of Hypertension Risk with Dietary BCAAs Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shou, J.; Chen, P.J.; Xiao, W.H. The Effects of BCAAs on Insulin Resistance in Athletes. J. Nutr. Sci. Vitaminol. 2019, 65, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef]
- Isanejad, M.; LaCroix, A.Z.; Thomson, C.A.; Tinker, L.; Larson, J.C.; Qi, Q.; Qi, L.; Cooper-DeHoff, R.M.; Phillips, L.S.; Prentice, R.L.; et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br. J. Nutr. 2017, 117, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okekunle, A.P.; Wu, X.; Duan, W.; Feng, R.; Li, Y.; Sun, C. Dietary Intakes of Branched-Chained Amino Acid and Risk for Type 2 Diabetes in Adults: The Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases Study. Can. J. Diabetes 2018, 42, 484–492.e487. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Bradley, D.; Eagon, J.C.; Patterson, B.W.; Klein, S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on gastrointestinal metabolism of ingested glucose. Am. J. Clin. Nutr. 2016, 103, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunny, N.E.; Kalavalapalli, S.; Bril, F.; Garrett, T.J.; Nautiyal, M.; Mathew, J.T.; Williams, C.M.; Cusi, K. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E311–E319. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.Y.; Wang, S.M.; Sun, L.; Liu, J.M.; Li, H.X.; Sui, X.F.; Wang, M.; Xiu, H.L.; Wang, S.; He, Q.; et al. Association of branched-chain amino acids with coronary artery disease: A matched-pair case-control study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 937–942. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Wang, T.J. Branched-Chain Amino Acids and Cardiovascular Disease: Does Diet Matter? Clin. Chem. 2016, 62, 545–547. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef] [Green Version]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.Y.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-Chain Amino Acids as Critical Switches in Health and Disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Teymoori, F.; Asghari, G.; Mirmiran, P.; Azizi, F. Dietary amino acids and incidence of hypertension: A principle component analysis approach. Sci. Rep. 2017, 7, 16838. [Google Scholar] [CrossRef] [Green Version]
- Flores-Guerrero, J.L.; Connelly, M.A.; Shalaurova, I.; Garcia, E.; Bakker, S.J.L.; Dullaart, R.P.F. A metabolomic index based on lipoprotein subfractions and branched chain amino acids is associated with incident hypertension. Eur. J. Intern. Med. 2021, 94, 56–63. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Hase, R.; Takahashi, H.; Ishimaru, Y.; Watanabe, R.; Saito, H.; Shimokawa, J.; Yamamoto, H.; Kikuchi, S.; et al. Plasma Branched-Chain and Aromatic Amino Acids in Relation to Hypertension. Nutrients 2020, 12, 3791. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Welch, A.; Chowienczyk, P.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Inversely Associated with Arterial Stiffness and Central Blood Pressure in Women. J. Nutr. 2015, 145, 2130–2138. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; Hivert, M.F.; Chavarro, J.E.; Oken, E. Branched Chain Amino Acids, Androgen Hormones, and Metabolic Risk Across Early Adolescence: A Prospective Study in Project Viva. Obesity 2018, 26, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Groothof, D.; Connelly, M.A.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. Concentration of Branched-Chain Amino Acids Is a Strong Risk Marker for Incident Hypertension. Hypertension 2019, 74, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Ge, K. The transition of Chinese dietary guidelines and food guide pagoda. Asia Pac. J. Clin. Nutr. 2011, 20, 439–446. [Google Scholar] [PubMed]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. 1), 2–7. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- Zhai, F.; Guo, X.; Popkin, B.M.; Ma, L.; Wang, Q.; Yu, W.; Jin, S.; Ge, K. Evaluation of the 24-h individual recall method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Shen, Q.; Li, C.; Dai, Z.; He, T. The Visceral Adipose Index in Relation to Incidence of Hypertension in Chinese Adults: China Health and Nutrition Survey (CHNS). Nutrients 2020, 12, 805. [Google Scholar] [CrossRef] [Green Version]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar]
- He, W.; Li, Q.; Yang, M.; Jiao, J.; Ma, X.; Zhou, Y.; Song, A.; Heymsfield, S.B.; Zhang, S.; Zhu, S. Lower BMI cutoffs to define overweight and obesity in China. Obesity 2015, 23, 684–691. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Duan, W.; Ren, X.; Wei, C.; Sun, C.; Li, Y. Eighteen-year alcohol consumption trajectories and their association with risk of type 2 diabetes and its related factors: The China Health and Nutrition Survey. Diabetologia 2019, 62, 970–980. [Google Scholar] [CrossRef] [Green Version]
- Zuo, H.; Shi, Z.; Yuan, B.; Dai, Y.; Hu, G.; Wu, G.; Hussain, A. Interaction between physical activity and sleep duration in relation to insulin resistance among non-diabetic Chinese adults. BMC Public Health 2012, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Siomkajlo, M.; Rybka, J.; Mierzchala-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific plasma amino acid disturbances associated with metabolic syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Mahbub, M.H.; Takahashi, H.; Hase, R.; Ishimaru, Y.; Sunagawa, H.; Amano, H.; Kobayashi-Miura, M.; Kanda, H.; Fujita, Y.; et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Environ. Health Prev. Med. 2017, 22, 35. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Fu, J.; Huang, A.; Fu, Z.; Yu, W.; et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 8173905. [Google Scholar] [CrossRef] [Green Version]
- Costeas, P.A.; Chinsky, J.M. Effects of insulin on the regulation of branched-chain alpha-keto acid dehydrogenase E1 alpha subunit gene expression. Biochem. J. 1996, 318, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Rietman, A.; Schwarz, J.; Tome, D.; Kok, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Teymoori, F.; Asghari, G.; Azizi, F. Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Arch. Iran. Med. 2019, 22, 182–188. [Google Scholar]
- Apro, W.; Blomstrand, E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol. 2010, 200, 237–248. [Google Scholar] [CrossRef]
- Hinault, C.; Mothe-Satney, I.; Gautier, N.; Lawrence, J.C., Jr.; Van Obberghen, E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004, 18, 1894–1896. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, H.J.; Martinez-Lemus, L.A.; Sowers, J.R. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E201–E208. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Q.; Qin, L.; Ali, R.; Qyang, Y.; Tassabehji, M.; Pober, B.R.; Sessa, W.C.; Giordano, F.J.; Tellides, G. Rapamycin inhibits smooth muscle cell proliferation and obstructive arteriopathy attributable to elastin deficiency. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yu, J.; Yuan, R.; Li, N.; Li, C.; Zhang, X. mTOR inhibitor improves testosterone-induced myocardial hypertrophy in hypertensive rats. J. Endocrinol. 2022, 252, 179–193. [Google Scholar] [CrossRef]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Marrachelli, V.G.; Yang, W.Y.; Trenson, S.; Huang, Q.F.; Wei, F.F.; Thijs, L.; Van Keer, J.; Monleon, D.; Verhamme, P.; et al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur. J. Prev. Cardiol. 2019, 26, 22–32. [Google Scholar] [CrossRef]
- Wang, N.X.; Arcand, J.; Campbell, N.R.C.; Johnson, C.; Malta, D.; Petersen, K.; Rae, S.; Santos, J.A.; Sivakumar, B.; Thout, S.R.; et al. The World Hypertension League Science of Salt: A regularly updated systematic review of salt and health outcomes studies (September 2019 to December 2020). J. Hum. Hypertens. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- van Nielen, M.; Feskens, E.J.; Mensink, M.; Sluijs, I.; Molina, E.; Amiano, P.; Ardanaz, E.; Balkau, B.; Beulens, J.W.; Boeing, H.; et al. Dietary protein intake and incidence of type 2 diabetes in Europe: The EPIC-InterAct Case-Cohort Study. Diabetes Care 2014, 37, 1854–1862. [Google Scholar] [CrossRef] [Green Version]
- Tinker, L.F.; Sarto, G.E.; Howard, B.V.; Huang, Y.; Neuhouser, M.L.; Mossavar-Rahmani, Y.; Beasley, J.M.; Margolis, K.L.; Eaton, C.B.; Phillips, L.S.; et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women’s Health Initiative. Am. J. Clin. Nutr. 2011, 94, 1600–1606. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
| Men (n = 3995) | Women (n = 4496) | p-Value | |
|---|---|---|---|
| Age at baseline (years old) | 44.4 ± 14.2 | 44.8 ± 13.9 | 0.148 |
| Follow-up duration (years) | 4.68 ± 2.13 | 4.81 ± 2.16 | 0.005 |
| Weight at baseline (kg) | 63.4 ± 10.3 | 55.4 ± 8.87 | <0.001 |
| Height at baseline (cm) | 166.9 ± 6.6 | 155.0 ± 6.3 | <0.001 |
| BMI at baseline (kg/m2) | 22.5 ± 3.8 | 22.6 ± 3.7 | 0.168 |
| WC at baseline (cm) | 81.7 ± 9.3 | 78.1 ± 9.1 | <0.001 |
| Total physical activity at baseline (log-transformed METs-hours/week) | 4.02 ± 1.95 | 3.35 ± 1.87 | <0.001 |
| Energy intake at baseline (Kcal/day) | 2390.9 ± 685.6 | 2036.1 ± 610.3 | <0.001 |
| Carbohydrate intake at baseline (g/day) | 347.9 ± 115.3 | 296.0 ± 100.7 | <0.001 |
| Fat intake at baseline (g/day) | 74.1 ± 39.3 | 66.5 ± 38.0 | <0.001 |
| Protein intake at baseline (g/day) | 72.5 ± 26.1 | 62.7 ± 23.0 | <0.001 |
| Salt intake (g/day) | 9.0 ± 12.8 | 9.4 ± 13.8 | 0.217 |
| Sodium intake (g/day) | 5.9 ± 7.1 | 6.0 ± 7.4 | 0.409 |
| Ile intake during the follow-up (g/day) | 2.44 ± 1.16 | 2.25 ± 1.06 | <0.001 |
| Leu intake during the follow-up (g/day) | 4.39 ± 2.18 | 4.05 ± 2.01 | <0.001 |
| Val intake during the follow-up (g/day) | 2.88 ± 1.37 | 2.65 ± 1.26 | <0.001 |
| SBP at baseline (mmHg) | 117.3 ± 10.4 | 113.5 ± 11.9 | <0.001 |
| DBP at baseline (mmHg) | 76.3 ± 7.5 | 73.9 ± 8.1 | <0.001 |
| SBP at the end of follow-up (mmHg) | 123.6 ± 15.4 | 120.5 ± 17.0 | <0.001 |
| DBP at the end of follow-up (mmHg) | 80.8 ± 10.5 | 77.6 ± 10.5 | <0.001 |
| Change of SBP during the follow-up (mmHg) | 6.3 ± 15.5 | 6.9 ± 16.5 | 0.117 |
| Change of DBP during the follow-up (mmHg) | 4.4 ± 10.8 | 3.7 ± 11.0 | 0.006 |
| Urban residents (%) | 35.1 | 35.4 | 0.839 |
| Han ethnicity (%) | 86.6 | 86.6 | 0.716 |
| Education (%) | <0.001 | ||
| Illiteracy | 7.7 | 19.4 | |
| Primary school | 19.3 | 21.3 | |
| Middle school | 73 | 59.3 | |
| High school or above | 32.5 | 23.1 | |
| Smoking (%) | 63.8 | 3.7 | <0.001 |
| Alcohol drinking (%) | 59.3 | 8.9 | <0.001 |
| T2DM diagnosed at baseline (%) | 0.8 | 1 | 0.517 |
| Anti-hypertensive treatment (%) | 3.1 | 4.5 | 0.005 |
| Incidence of hypertension (%) | 28.9 | 24.6 | <0.001 |
| Men (n = 3646) | Women (n = 4115) | p-Value for Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p-Value | R2 | Coefficient | 95% CI | p-Value | R2 | ||
| SBP | |||||||||
| Ile | 2.55 | 1.84, 3.25 | <0.001 | 0.102 | 1.82 | 1.16, 2.49 | <0.001 | 0.194 | 0.183 |
| Leu | 2.39 | 1.70, 3.07 | <0.001 | 0.100 | 1.80 | 1.15, 2.45 | <0.001 | 0.195 | 0.287 |
| Val | 2.40 | 1.71, 3.09 | <0.001 | 0.100 | 1.74 | 1.09, 2.39 | <0.001 | 0.194 | 0.249 |
| DBP | |||||||||
| Ile | 1.27 | 0.78, 1.76 | <0.001 | 0.041 | 0.97 | 0.52, 1.42 | <0.001 | 0.076 | 0.429 |
| Leu | 1.21 | 0.73, 1.69 | <0.001 | 0.040 | 0.96 | 0.52, 1.41 | <0.001 | 0.076 | 0.546 |
| Val | 1.19 | 0.70, 1.67 | <0.001 | 0.039 | 0.92 | 0.48, 1.37 | <0.001 | 0.076 | 0.526 |
| Change of SBP | |||||||||
| Ile | 2.46 | 1.74, 3.19 | <0.001 | 0.070 | 1.31 | 0.60, 2.02 | <0.001 | 0.064 | 0.008 |
| Leu | 2.30 | 1.59, 3.00 | <0.001 | 0.068 | 1.33 | 0.63, 2.02 | <0.001 | 0.064 | 0.020 |
| Val | 2.38 | 1.67, 3.10 | <0.001 | 0.069 | 1.29 | 0.59, 1.98 | <0.001 | 0.064 | 0.012 |
| Change of DBP | |||||||||
| Ile | 1.16 | 0.64, 1.69 | <0.001 | 0.045 | 0.76 | 0.27, 1.26 | 0.003 | 0.038 | 0.344 |
| Leu | 1.10 | 0.59, 1.61 | <0.001 | 0.044 | 0.82 | 0.33, 1.30 | 0.001 | 0.039 | 0.495 |
| Val | 1.20 | 0.69, 1.71 | <0.001 | 0.046 | 0.83 | 0.35, 1.32 | 0.001 | 0.039 | 0.399 |
| Men (n = 3995) | Women (n = 4496) | p-Value for Interaction | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Model 1 | |||||||
| Ile | 1.23 | 1.16, 1.29 | <0.001 | 1.23 | 1.17, 1.29 | <0.001 | 0.803 |
| Leu | 1.22 | 1.16, 1.28 | <0.001 | 1.18 | 1.13, 1.24 | <0.001 | 0.700 |
| Val | 1.22 | 1.16, 1.28 | <0.001 | 1.22 | 1.16, 1.28 | <0.001 | 0.806 |
| Model 2 | |||||||
| Ile | 1.18 | 1.10, 1.27 | <0.001 | 1.22 | 1.14, 1.31 | <0.001 | 0.449 |
| Leu | 1.18 | 1.10, 1.26 | <0.001 | 1.21 | 1.13, 1.30 | <0.001 | 0.397 |
| Val | 1.18 | 1.10, 1.27 | <0.001 | 1.22 | 1.13, 1.30 | <0.001 | 0.376 |
| Model 3 | |||||||
| Ile | 1.24 | 1.14, 1.35 | <0.001 | 1.29 | 1.19, 1.40 | <0.001 | 0.328 |
| Leu | 1.23 | 1.13, 1.33 | <0.001 | 1.27 | 1.18, 1.38 | <0.001 | 0.283 |
| Val | 1.23 | 1.13, 1.33 | <0.001 | 1.28 | 1.18, 1.38 | <0.001 | 0.265 |
| Men (n = 3995) | Women (n = 4496) | p-Value for Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases (Incidence) | HR | 95% CI | p-Value | n | Cases (Incidence) | HR | 95% CI | p-Value | |||
| Ile (g/day) | Ile (g/day) | |||||||||||
| Q1 (<1.66) | 1001 | 272 (27.2) | 0.85 | 0.48, 1.51 | 0.575 | Q1 (<1.57) | 1132 | 257 (22.7) | 1.21 | 0.63, 2.31 | 0.567 | 0.709 |
| Q2 (1.66–2.32) | 994 | 230 (23.1) | 1.28 | 0.42, 3.90 | 0.665 | Q2 (1.57–2.15) | 1119 | 246 (22.0) | 1.99 | 0.72, 5.52 | 0.187 | 0.384 |
| Q3 (2.32–3.06) | 997 | 267 (26.8) | 1.60 | 0.70, 3.67 | 0.265 | Q3 (2.15–2.87) | 1128 | 255 (22.6) | 1.84 | 0.80, 4.25 | 0.154 | 0.933 |
| Q4 (≥3.06) | 1003 | 386 (38.5) | 1.14 | 1.01, 1.29 | 0.041 | Q4 (≥2.87) | 1117 | 346 (31.0) | 1.36 | 1.20, 1.54 | <0.001 | 0.117 |
| Leu (g/day) | Leu (g/day) | |||||||||||
| Q1 (<2.94) | 998 | 272 (27.3) | 0.94 | 0.66, 1.33 | 0.939 | Q1 (<2.76) | 1118 | 259 (23.2) | 1.17 | 0.81, 1.70 | 0.400 | 0.503 |
| Q2 (2.94–4.12) | 1004 | 238 (23.7) | 0.84 | 0.48, 1.47 | 0.835 | Q2 (2.76–3.82) | 1127 | 241 (21.4) | 2.06 | 1.10, 3.85 | 0.024 | 0.020 |
| Q3 (4.12–5.54) | 997 | 264 (26.5) | 1.16 | 0.74, 1.81 | 0.530 | Q3 (3.82–5.15) | 1133 | 255 (22.5) | 1.05 | 0.67, 1.64 | 0.839 | 0.950 |
| Q4 (≥5.54) | 996 | 381 (38.3) | 1.09 | 1.03, 1.15 | 0.003 | Q4 (≥5.15) | 1118 | 349 (31.2) | 1.15 | 1.08, 1.23 | <0.001 | 0.191 |
| Val (g/day) | Val (g/day) | |||||||||||
| Q1 (<1.96) | 999 | 263 (26.3) | 0.93 | 0.56, 1.54 | 0.928 | Q1 (<1.85) | 1122 | 250 (22.3) | 1.27 | 0.71, 2.26 | 0.419 | 0.745 |
| Q2 (1.96–2.71) | 1000 | 237 (23.7) | 0.63 | 0.26, 1.53 | 0.305 | Q2 (1.85–2.52) | 1123 | 236 (21.0) | 1.86 | 0.72, 4.85 | 0.203 | 0.149 |
| Q3 (2.71–3.61) | 991 | 282 (28.5) | 2.49 | 1.30, 4.76 | 0.006 | Q3 (2.52–3.37) | 1126 | 267 (23.7) | 0.86 | 0.42, 1.76 | 0.671 | 0.023 |
| Q4 (≥3.61) | 1005 | 373 (37.1) | 1.15 | 1.03, 1.27 | 0.009 | Q4 (≥3.37) | 1125 | 351 (31.2) | 1.25 | 1.13, 1.39 | <0.001 | 0.475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, C.; Zhang, Y.; Jiang, X.; Liang, Y.; Wang, H.; Li, Y.; Sun, G. Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population. Nutrients 2022, 14, 2582. https://doi.org/10.3390/nu14132582
Liu Y, Zhang C, Zhang Y, Jiang X, Liang Y, Wang H, Li Y, Sun G. Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population. Nutrients. 2022; 14(13):2582. https://doi.org/10.3390/nu14132582
Chicago/Turabian StyleLiu, Yuyan, Chengwen Zhang, Yuan Zhang, Xuheng Jiang, Yuanhong Liang, Huan Wang, Yongfang Li, and Guifan Sun. 2022. "Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population" Nutrients 14, no. 13: 2582. https://doi.org/10.3390/nu14132582
APA StyleLiu, Y., Zhang, C., Zhang, Y., Jiang, X., Liang, Y., Wang, H., Li, Y., & Sun, G. (2022). Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population. Nutrients, 14(13), 2582. https://doi.org/10.3390/nu14132582





