Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fatty-Acid Profile and Nutritional Quality of Buriti Oil
2.3. Analyses of Quality and Identity of the Buriti Oil
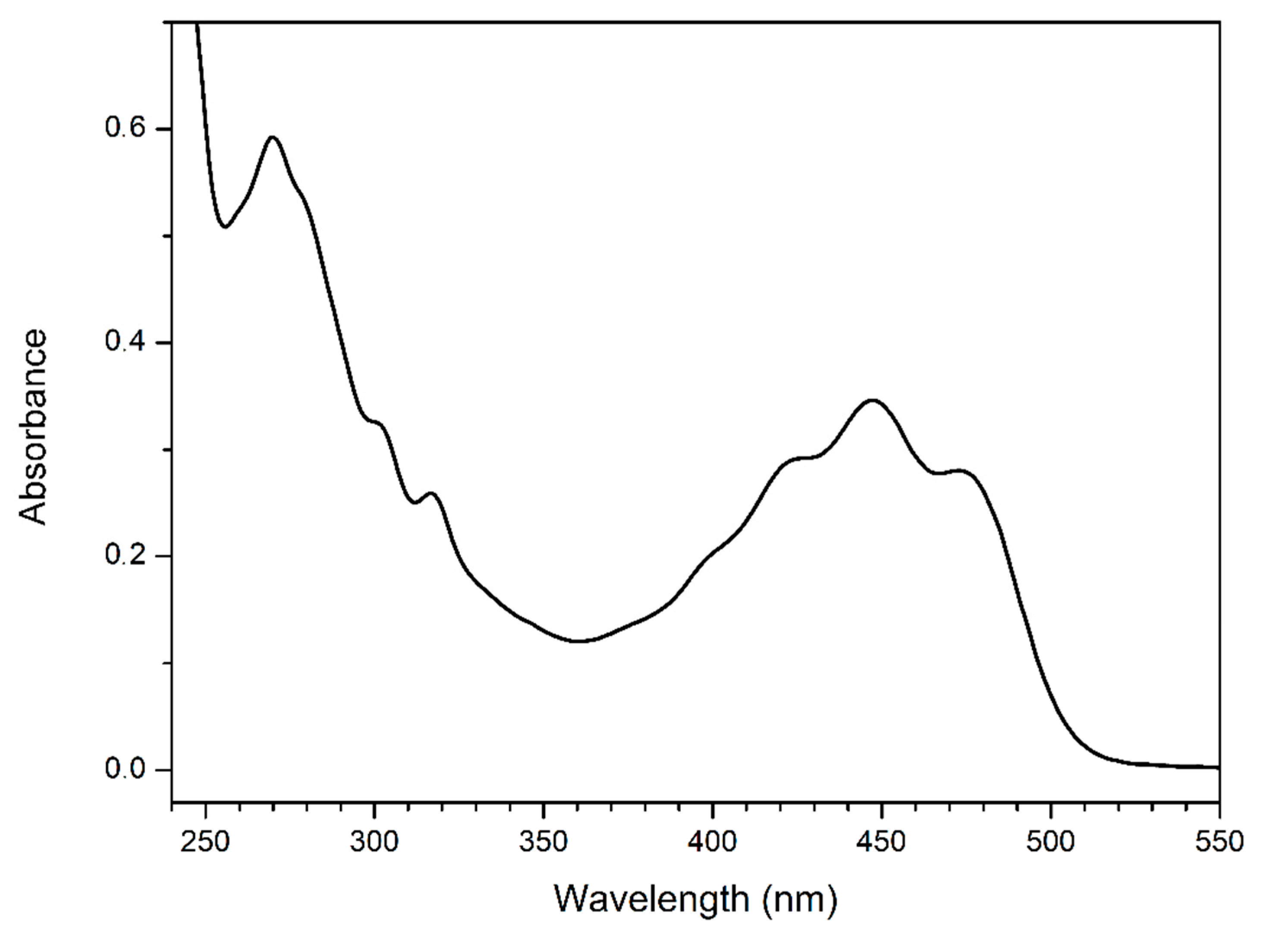
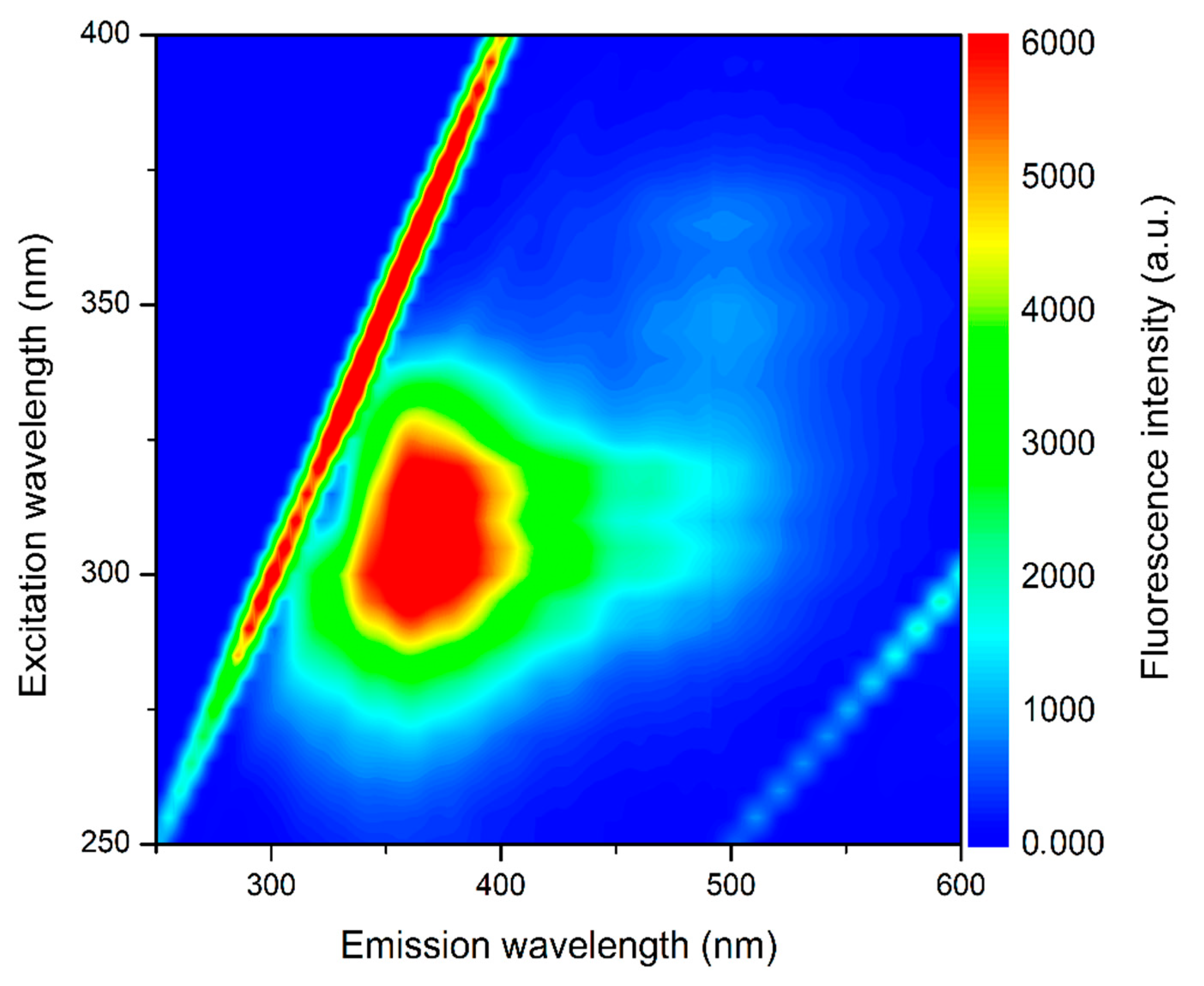
2.4. Optical Analyses of Buriti Oil: UV–Vis Absorption and Fluorescence Emission—Excitation Matrix
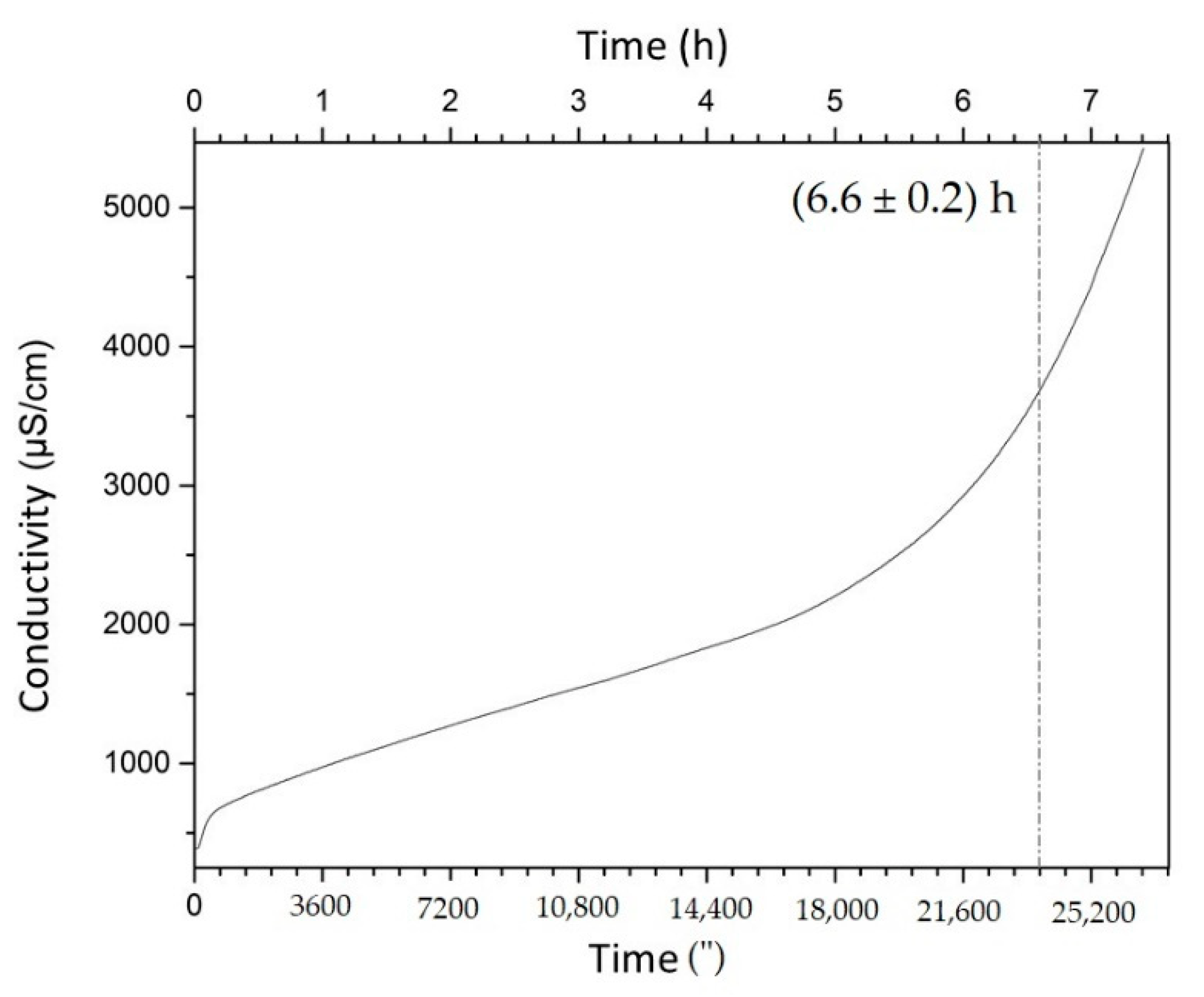
2.5. Oxidative Stability: Rancimat Test
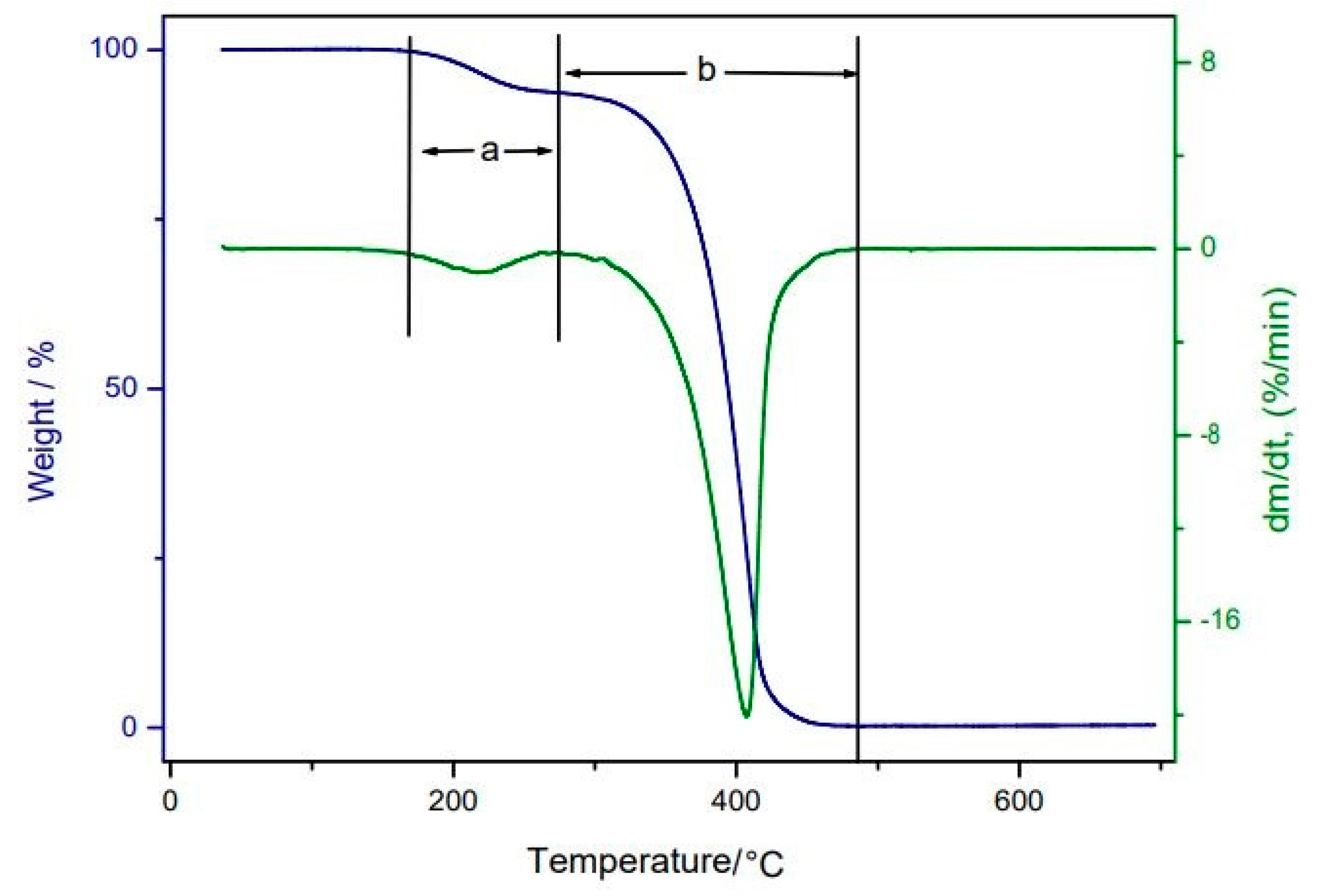
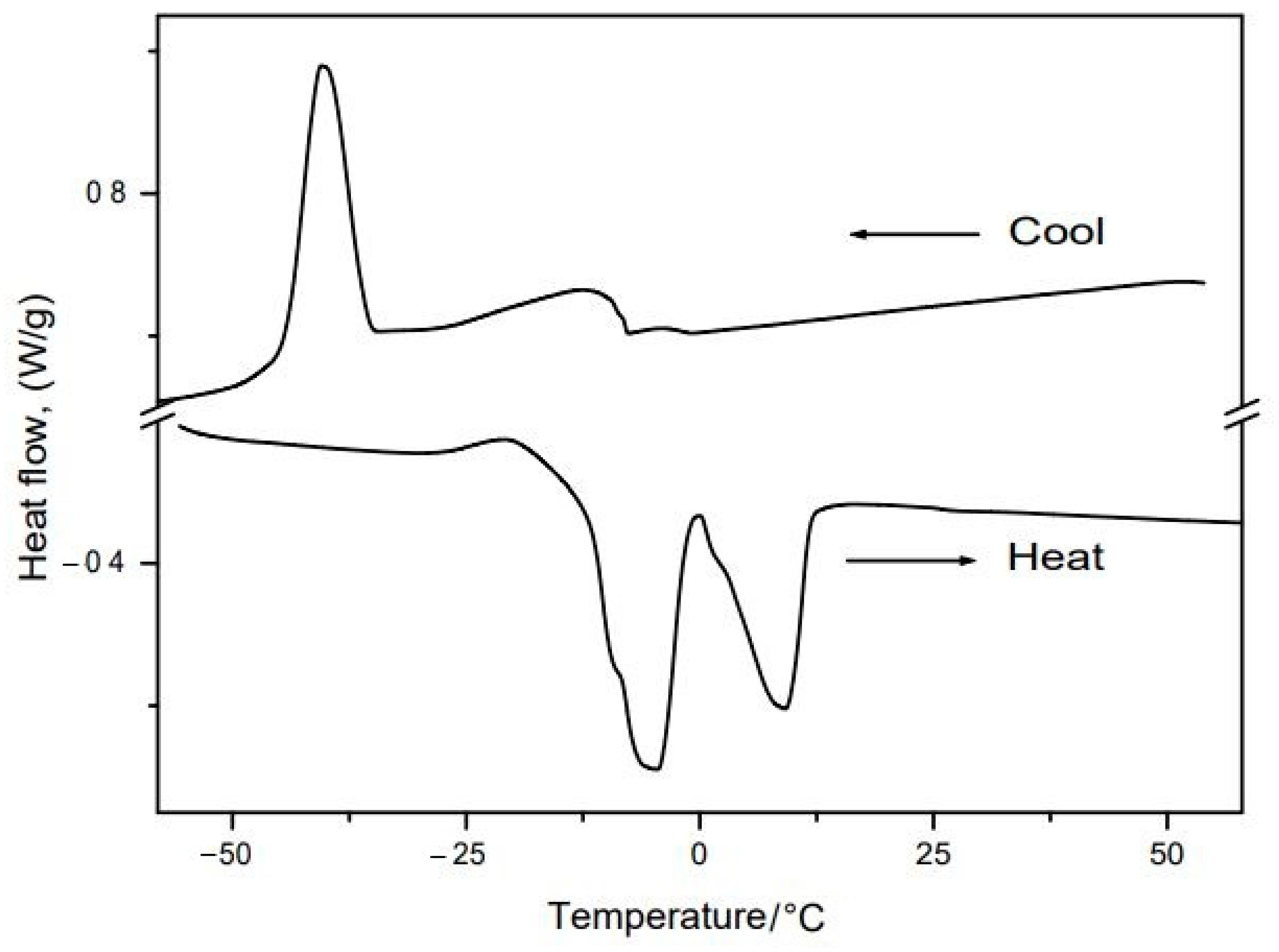
2.6. Thermic Analyses: Thermogravimetry/Derived Thermogravimetry (TG/DTG) and Differential Scanning Calorimetry (DSC)
2.7. Antioxidant Activity: ORAC (Oxygen Radical Absorbance Capacity) Lipophilic, ABTS (2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) and FRAP (Ferric Reducing Antioxidant Power) Methods
2.8. Total Phenols (or Phenolic Compounds)
2.9. β-Carotene
2.10. Coloration
2.11. Experimental Design
2.11.1. Animal Groups
2.11.2. Euthanasia and Autopsy Sampling
2.11.3. Serum Parameters
2.11.4. Histology of the Liver
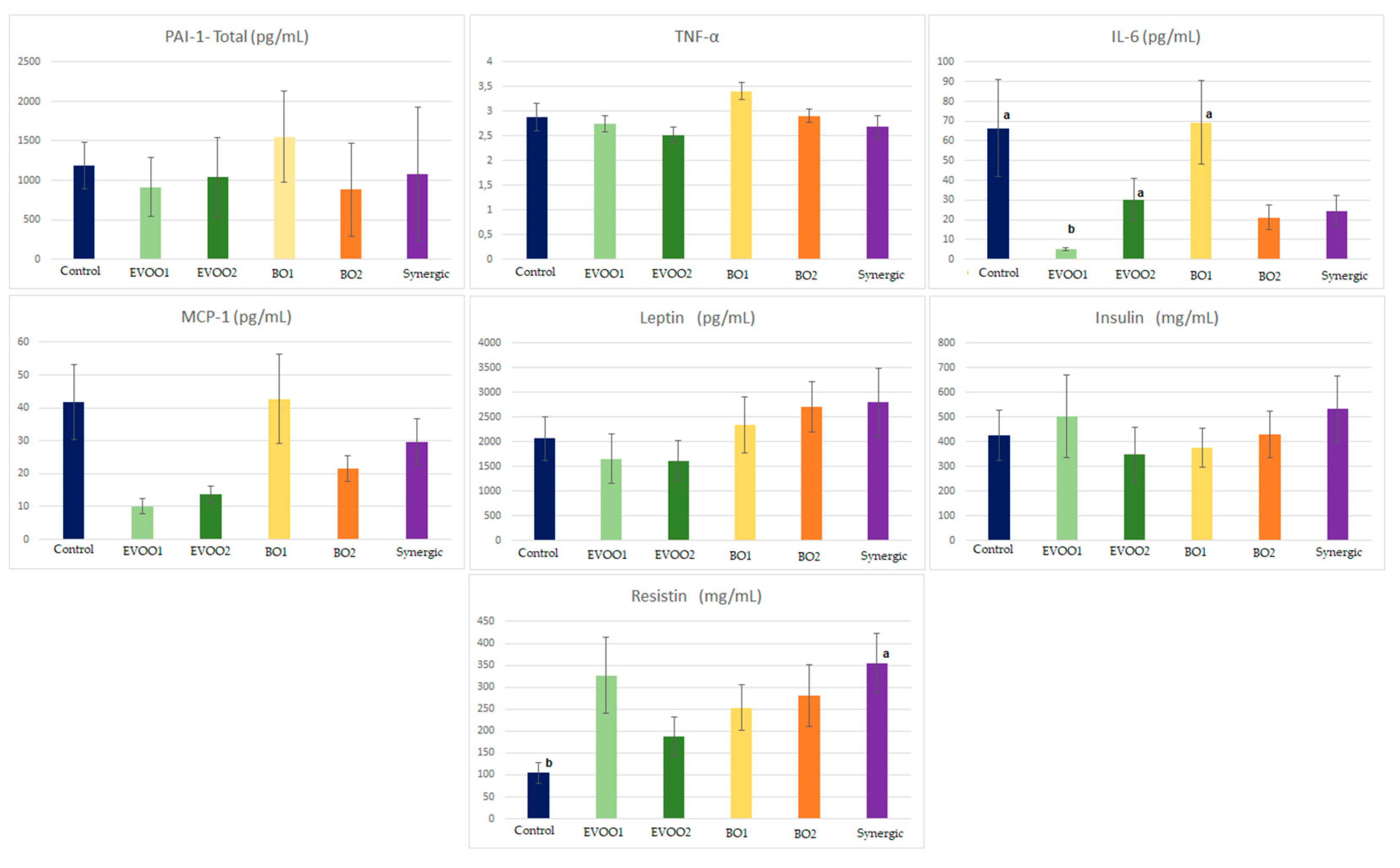
2.11.5. Cytokines
2.11.6. Statistical Analyses
3. Results and Discussion
3.1. Profile of Fatty Acids and Nutritional Quality of Buriti Oil
3.2. Analyses of Quality and Identity of the Buriti Oil
3.3. Optical Analyses: UV–Vis Absorption and Emission—Excitation Fluorescence Matrix
3.4. Oxidative Stability: Rancimat
3.5. Thermic Analyses: TG/DTG and DSC
3.6. Antioxidant Activity: ORAC, ABTS, and FRAP Methods
3.7. Total Phenolic Compounds
3.8. β-Carotene
3.9. Coloration
3.10. Experimental Design
3.10.1. Weight Gain and Food Ingestion
3.10.2. Weights of Liver and Visceral Fats, and Adiposity Index
3.10.3. Biochemical Parameters
3.10.4. Hepatic Histology
3.10.5. Quantification of Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Becker, F.S.; Damiani, C.; Melo, A.A.M.; Borges, P.R.S.; Vilas Boas, E.V.B. Incorporation of buriti endocarp flour in gluten-free whole cookies as potential source of dietary fiber. Plant. Foods Hum. Nutr. 2014, 69, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Darnet, S.H.; Silva, L.H.M.; Rodrigues, A.M.C.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of buriti (Mauritia flexuosa) and patawa (Oenocarpus bataua) fruit pulp from the Amazon region. Food Sci. Technol. 2004, 2, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Freire, J.A.P.; Barros, K.B.N.T.; Lima, L.K.F.; Martins, J.M.; Araújo, Y.C.; Oliveira, G.L.S.; Aquino, J.S.; Ferreira, P.M.P. Phytochesmitry profile, nutritional properties and pharmacological activities of Mauritia flexuosa. J. Food. Sci. 2016, 81, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Koolen, H.H.F.; Silva, F.M.A.; Gozzo, F.C.; Souza, A.Q.L.; Souza, A.D.L. Antioxidant, antimicrobial activities and characterization oh phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Batista, J.S.; Olinda, R.G.; Medeiros, V.B.; Rodrigues, C.M.F.; Oliveira, A.F.; Paiva, E.S.; Freitas, C.I.A.; Medeiros, A.C. Atividade antibacteriana e cicatrizante do óleo de buriti (Mauritia flexuosa L.). Cienc. Rural 2012, 42, 136–141. [Google Scholar] [CrossRef]
- Falcão, A.O.; Speranza, P.; Ueta, T.; Martins, I.M.; Macedo, G.A.; Macedo, J.A. Antioxidant potential and modulatory effects of restructured lipids from Amazonian palms on liver cells. Food Technol. Biotechnol. 2017, 54, 553–561. [Google Scholar] [CrossRef]
- Speranza, P.; Falcão, A.O.; Macedo, J.; Silva, L.; Rodrigues, A.M.; Macedo, G. Amazonian buriti oil: Chemical characterization and antioxidant potential. Grasas Aceites 2016, 67, e135. [Google Scholar] [CrossRef] [Green Version]
- Taipina, M.S.; Lamardo, L.C.A.; Campos, N.C.; Tavares, M.; Gonzalez, E.; Jorge, L.I.F.; Aued-Pimentel, S. Review of literature: Carotenoids, chemical composition and dietary reference intake of buriti fruits. Int. J. Nutr. 2013, 6, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Dorni, C.; Sharma, O.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef]
- Muralidharan, J.; Galiè, S.; Hernández-Alonso, P.; Bulló, M.; Salas-Salvadó, J. Plant-based fat, dietary patterns rich in vegetable fat and gut microbiota modulation. Front. Nutr. 2019, 6, 157. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, V.P.; Arruda, C.; El-Salam, M.A.; Bastos, J.K. Brazilian medicinal plants with corroborated anti-inflammatory activities: A review. Pharm. Biol. 2018, 56, 253–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid peroxidation: Chemical mechanism, biological implications and analytical determination. In Lipid Peroxidation; Catala, A., Ed.; IntechOpen: London, UK, 2012; pp. 3–30. [Google Scholar]
- Min, D.B.; Boff, J.M. Lipid oxidation of edible oil. In Food Lipids—Chemistry, Nutrition, and Biotechnology, 2nd ed.; Akoh, C.C., Min, D.B., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 353–381. [Google Scholar]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maya, R.L.; Rodriguez-Amaya, D.B. Avaliação de um método simples e econômico para a metilação de ácidos graxos com lipídeos de diversas espécies de peixes. Revi. Inst. Adolfo Lutz. 1993, 53, 27–53. [Google Scholar]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 19, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: Fatty and composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. American Oil Chemists’ Society, 4th ed.; AOCS Press: Urbana, IL, USA, 1990. [Google Scholar]
- EN14112:2003; Fatty Acid Methyl Esters (Fame)—Determination of Oxidation Stability (Accelerated Oxidation Test). European Committee for Standardization: Brussels, Belgium, 2003.
- Ou, B.; Tony, C.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Comunicado Técnico—Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre ABTS+; EMBRAPA: Fortaleza, Brazil, 2007. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Comunicado Técnico—Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pelo Método de Redução do Ferro (FRAP); EMBRAPA: Fortaleza, Brazil, 2006. [Google Scholar]
- Swain, T.; Hills, W.E. The phenolics constituents of Prunus domestica: The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Lozada, M.I.O.; Oliveira, L.L.; Rodrigues, D.B. Comunicado Técnico—Metodologia para Determinação de Carotenoides Totais e β-caroteno em Óleo; EMBRAPA: Brasilia, Brazil, 2021. [Google Scholar]
- Minolta Corporation. Precise Color Communication: Color Control from Feeling to Instrumentation; Minolta Corporation Instrument Systems Division: Ramsey, NJ, USA, 1994. [Google Scholar]
- Figueiredo, P.R.L.; Oliveira, I.B.; Santana Neto, J.B.; Oliveira, J.A.; Ribeiro, L.B.; Viana, G.S.B.; Felipe, C.F.B.; Coutinho, H.D.M.; Menezes, I.R.A. Caryocar coriaceum Wittm. (Pequi) fixed oil presents hypolipemic and anti-inflammatory effects in vivo and in vitro. J. Ethnopharmacol. 2016, 191, 87–94. [Google Scholar] [CrossRef]
- Silva, G.T.; Fernandes, C.D.P.; Hiane, P.A.; Freitas, K.C.; Figueiredo, P.S.; Inada, A.C.; Filiú, W.F.; Maldonade, I.R.; Nunes, Â.A.; Oliveira, L.C.S.; et al. Caryocar brasiliense Cambess. Pulp Oil supplementation reduces total cholesterol, LDL-c, and Non-HDLc- in animals. Molecules 2020, 25, 4530. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martínez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.A.; Phillips, S.J. Detection of obesity WTLs on mouse chromosomes 1 and 8 by selective DNA pooling. Genomics 1996, 34, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.H.; Hagen, P.B. An enzymatic method for the estimation of glycerol in blood. J. Biochem. Physiol. 1962, 40, 1129–1139. [Google Scholar]
- Fleg, H.M. An investigation of the determination of serum cholesterol by an enzymatic method. Ann. Clin. Biochem. 1973, 10, 79–84. [Google Scholar]
- Carey, R.N.; Feldbruegge, C.; Westgard, J.O. Evaluation of the adaptation of the glucose oxidase/peroxidase-3-methyl-2-benzothiazolinone hydrazine-N, N-dimethylaniline procedure to the Technicon “SMA 12/60”, and comparation with other automated method for glucose. Clin. Chem. 1974, 20, 595–602. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Freitas, M.L.F.; Chisté, R.C.; Polachini, T.C.; Sardella, L.A.C.Z.; Aranha, C.P.M.; Ribeiro, A.P.B.; Nicoletti, V.R. Quality characteristics and thermal behavior of buriti (Mauritia flexuosa L.) oil. Grasas Aceites 2017, 68, e220. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.B.; Oliveira, W.S.; Araújo, R.L.; França, A.C.H.; Pertuzatti, P.B. Buriti (Mauritia flexuosa L.) pulp oil as an immunomodulator against enteropathogenic Escherichia coli. Ind. Crops Prod. 2020, 149, 112330. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Yoshida, M.; Chizawa, Y.; Adachi, S. Induction periods for lipid crystallization of various vegetable oils. J. Oleo Sci. 2019, 68, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Pardauil, J.J.R.; Molfetta, F.A.; Braga, M.; Souza, L.K.C.; Filho, G.N.R.; Zamian, J.R. Characterization, thermal properties and phase transitions of Amazonian vegetable oils. J. Therm. Anal. Calorim. 2017, 127, 1221–1229. [Google Scholar] [CrossRef]
- Aremu, M.O.L.; Ibrahim, H.; Andrew, C. Comparative studies on the lipid composition of bool pum (Haematostaphis Barteri) pulp and seed oils. Open Biochem. J. 2017, 11, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.F.; Lescano, C.H.; Arrigo, J.S.; Cardoso, C.A.L.; Coutinho, J.P.; Moslaves, I.S.B.; Ximenes, T.V.N.; Kadri, M.C.T.; Weber, S.C.; Perdomo, R.T.; et al. Anti-inflammatory, antiproliferative and cytoprotective potential of the Attalea phalerata Mart. Ex Spreng. pulp oil. PLoS ONE 2018, 13, e0195678. [Google Scholar] [CrossRef]
- Aoqui, M. Caracterização Do óleo da Polpa de Macaúba (Acrocomia aculeata (Jacq.) Lodd. Ex Mart.) e Azeite de Oliva (Olea europaea L.) Virgem Extra e Seus Efeitos Sobre Dislipidemia e Outros Parâmetros Sanguíneos, Tecido Hepático e Mutagênese em Ratos Wistar. Master’s Thesis, Universidade Católica Dom Bosco, Campo Grande, Mato Grosso do Sul, Brazil, June 2012. [Google Scholar]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Edible Fats and Oils Not Covered by Individual Standards. Available online: https://www.fao.org/3/y2774e/y2774e03.htm (accessed on 31 March 2022).
- Jaramillo, J.E.C.C.; Bautista, M.O.; Solano, O.A.A.; Achenie, L.E.K.; Barrios, A.F.G. Impact of the mode of extraction on the lipidomic profile of oils obtained from selected Amazonian fruits. Biomolecules 2019, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y. Analytical methods to evaluate the quality of edible fats and oils: The JOCS standard methods for analysis of fats, oils and related materials (2013) and advanced methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aquino, J.S.; Pessoa, D.C.N.; Araújo, K.L.G.V.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.; Stamford, T.L.M. Refining of buriti oil (Mauritia flexuosa) originated from the Brazilian cerrado: Physicochemical, thermaloxidative and nutritional implication. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Wanasundara, P.K.J.P.D. Extraction and analysis of lipids. In Food Lipids—Chemistry, Nutrition, and Biotechnology, 2nd ed.; Akoh, C.C., Min, D.B., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 151–186. [Google Scholar]
- Gomez, N.F.; Abonia, R.; Cadavid, H.; Vargas, I.H. Chemical and spectroscopic characterization of a vegetable oils used as dielectric coolant in distribution transformers. J. Braz. Chem. Soc. 2011, 22, 2292–2303. [Google Scholar] [CrossRef]
- Magalhães, K.F.; Caires, A.R.L.; Silva, M.S.; Alcantra, G.B.; Oliveira, S.L. Endogenous fluorescence of biodiesel and products thereof: Investigation of the molecules responsible for this effect. Fuel 2014, 119, 120–128. [Google Scholar] [CrossRef]
- Artuç, G.Ö. Quantitative determination of α-tocopherol in pharmaceutical soft capsule by spectrophotometry. Experimed 2020, 10, 72–76. [Google Scholar] [CrossRef]
- Bakhouche, K.; Dhaouadi, Z.; Lahmar, S.; Hammoutène, D. TDDFT prediction of UV-vis absorption and emission spectra of tocopherols in different media. J. Mol. Model. 2015, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.P.; Souza, A.F.; Lescano, C.H.; Caires, A.R.L.; Muzzi, R.M. Thermal oxidation analysis of forage turnip (Raphanus sativus L. var. oleiferus Metzg.) oil. J. Am. Oil Chem. Soc. 2015, 92, 403–408. [Google Scholar] [CrossRef]
- Silva, V.D.; Conceição, J.N.; Oliveira, I.P.; Lescano, C.H.; Muzzi, R.M.; Filho, O.P.S.; Conceição, E.C.; Casagrande, G.A.; Caires, A.R.L. Oxidative stability of Baru (Dipteryz alata Vogel) oil monitored by fluorescence and absorption spectroscopy. J. Spectroc. 2015, 2015, 803705. [Google Scholar] [CrossRef] [Green Version]
- Sikorska, E.; Gliszczynska-Swiglo, A.; Khmelinskii, I.; Sikorski, M. Synchronous fluorescence spectroscopy of edible vegetable oils. Quantification of tocopherols. J. Agric. Food Chem. 2005, 53, 6988–6994. [Google Scholar] [CrossRef]
- Ciconini, G. Caracterização de Frutos e Óleo de Polpa de Macaúba dos Biomas Cerrado e Pantanal do Estado de Mato Grosso do Sul, Brasil. Master’s Thesis, Mestrado em Biotecnologia Aplicada à Saúde, Universidade Católica Dom Bosco, Campo Grande, Mato Grosso do Sul, Brazil, 2012. [Google Scholar]
- Alexandre, E.C.F.; Silveira, E.V.; Castro, C.F.S.; Sales, J.F.; Oliveira, L.C.S.; Viana, L.H.; Barbosa, L.C.A. Synthesis, characterization and study of the thermal behavior of methylic and ethylic biodiesel produced from tucumã (Astrocaryum huaimi Mart.) seed oil. Fuel 2015, 161, 233–238. [Google Scholar] [CrossRef]
- Misutsu, M.Y.; Cavalheiro, L.F.; Ricci, T.G.; Viana, L.H.; Oliveira, S.C.; Machulek Junior, A.; Oliveira, L.C.S. Thermoanalytical methods in verifying the quality of biodiesel. In Biofuels—Status and Perspective; Biernat, K., Ed.; IntechOpen: London, UK, 2015; pp. 251–269. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants 2019, 8, 179–222. [Google Scholar] [CrossRef] [Green Version]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f) from the Cerrado and Amazon biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef]
- Nascimento-Silva, N.R.R.; Silva, F.A.; Silva, M.R. Physicochemical composition and antioxidants of buriti (Mauritia flexuosa Linn. F.)—Pulp and sweet. J. Bioenergy Food Sci. 2020, 7, 2792019. [Google Scholar] [CrossRef]
- Aquino, J.S.; Soares, J.K.B.; Magnani, M.; Stamford, T.C.M.; Mascarenhas, R.J.; Tavares, R.L.; Stamford, T.L.M. Effects of dietary Brazilian pal oil (Mauritia flexuosa L.) on cholesterol profile and vitamin A and E status of rats. Molecules 2015, 20, 9054–9070. [Google Scholar] [CrossRef] [Green Version]
- Arfaoui, M.O.; Renaud, J.; Ghazghazi, H.; Boukhchina, S.; Mayer, P. Variation in oil content, fatty acid and phytosterols profile of Onopordum acanthium L. during seed development. Nat. Prod. Res. 2014, 28, 2293–2300. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Lucchetti, S.; Forte, V.; Pastore, G. α -tocopherol, β-carotene, lutein, squalene and secoiridoids in sevem monocutival Italian extra-virgin olive oils. Int. J. Food Sci. Nutr. 2017, 68, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Donnini, S.; Calderone, V.; Zinnai, A.; Taglieri, I.; Venturi, F.; Testai, L. The nutraceutical value of olive oil and its bioactive constituents on the cardiovascular system. Focusing on main strategies to slow down its quality decay during production and storage. Nutrients 2019, 11, 1962. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra virgin olive oil: Lesson from nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydos, L.R.; Amaral, L.A.; Jocobowski, A.C.; Soua, R.S.; Parissotto, E.B.; Menezes, M.B.; Bittencourt Júnior, F.F.; Fernandes, E.S.; Silva, I.S.; Portugal, L.C.; et al. Buriti pulp oil did not improve high-fat-diet-induced metabolic disorders in C57bl/6 mice. J. Anim. Physiol. Anim. Nutr. 2020, 105, 364–375. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Y.; Zhang, L.; Guo, Q. Oxidative stress and liver disease. Hepatol. Res. 2012, 42, 741–749. [Google Scholar] [CrossRef]
- Bersh-Ferreira, A.C.; Sampaio, G.R.; Gehringer, M.O.; Torres, E.A.F.S.; Ross-Fernandes, M.B.; Silva, J.T.; Torreglosa, C.R.; Kovacs, C.; Alves, R.; Magnoni, C.D.; et al. Association between plasma fatty acids and inflammatory markers in patients with and without insulin resistance and in secondary prevention of cardiovascular disease, a cross-sectional study. Nutr. J. 2018, 17, 26. [Google Scholar] [CrossRef]
- Domenico, M.D.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The role of oxidative stress and hormones in controlling obesity. Front. Endocrinol. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Ikeoka, D.; Mader, J.K.; Pieber, T.R. Adipose tissue, inflammation and cardiovascular disease. Rev. Assoc. Med. Bras. 2010, 56, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Y.; Wang, P.; Miao, C.Y. Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2011, 38, 888–896. [Google Scholar] [CrossRef]
- Wruck, C.J.; Streetz, K.; Pavic, G.; Götz, M.; Tohidnezhad, M.; Brandenburg, L.O.; Varoga, D.; Eickelberg, O.; Hardegen, T.; Trautwein, C.; et al. Nrf2 induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J. Biol. Chem. 2011, 286, 4493–4499. [Google Scholar] [CrossRef] [Green Version]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food. Funct. 2019, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Pine, G.M.; Batugedara, H.M.; Nair, M.G. Here, there and everywhere: Resistin-like molecules in infection, inflammation, and metabolic disorders. Cytokine 2018, 110, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Fatty Acids | Buriti Oil (%) |
|---|---|
| Saturated | |
| Butyric, C4:0 | 0.09 ± 0.01 |
| Myristic, C14:0 | 0.05 ± 0.01 |
| Pentadecanoic, C15:0 | 0.02 ± 0.01 |
| Palmitic, C16:0 | 17.86 ± 0.00 |
| Heptadecanoic, C17:0 | 0.07 ± 0.02 |
| Stearic, C18:0 | 1.07 ± 0.01 |
| Arachidic, C20:0 | 0.08 ± 0.01 |
| ∑ TOTAL | 19.24 |
| Monounsaturated | |
| Palmitoleic, C16:1 | 0.18 ± 0.02 |
| Cis-10-heptadecanoic, C17:1 | 0.05 ± 0.01 |
| Oleic, C18:1 | 76.38 ± 0.03 |
| Gadoleic, C20:1 | 0.03 ± 0.00 |
| Erucic, C22:1 | 0.03 ± 0.01 |
| Nervonic, C24:1 | 0.02 ± 0.01 |
| ∑ TOTAL | 76.69 |
| Polyunsaturated | |
| Linoleic, C18:2 | 0.01 ± 0.00 |
| α-linolenic, C18:3 | 0.83 ± 0.01 |
| γ-linolenic, C18:3 | 0.37 ± 0.02 |
| Cis-11,14-eicosadienoic, C20:2 | 0.04 ± 0.01 |
| Arachidonic, C20:4 | 0.02 ± 0.01 |
| Eicosapentanoic, C20:5 | 0.03 ± 0.01 |
| Cis-13,16-docosadienoic, C22:2 | 0.03 ± 0.01 |
| Cis-4,7,10,13,16,19-docosahexaenoic, C22:6 | 0.02 ± 0.00 |
| ∑ TOTAL | 1.35 |
| Indices | Buriti Oil |
|---|---|
| Atherogenic | 0.23 |
| Thrombogenic | 0.15 |
| Hypocholesterolemic/hypercholesterolemic | 4.31 |
| Indices | Buriti Oil |
|---|---|
| Acidity (mgKOH/g) | 4.70 ± 0.18 |
| Peroxide (mEq2/kg) | 2.13 ± 0.30 |
| Refraction at 40 °C | 1.47 ± 0.00 |
| Iodide (gI2/100 g) | 87.56 ± 0.77 |
| Saponification (mgKOH/g) | 188.62 ± 3.31 |
| Relative density (mg/mL) | 0.91 ± 0.00 |
| Parameters | Buriti Oil |
|---|---|
| ORAC (µmol TE/100 mL) | 1.55 ± 0.03 |
| ABTS (µmol TE/100 mL) | 1758.02 ± 6.97 |
| FRAP (µmol TE/100 mL) | 164.86 ± 2.41 |
| Parameters | Buriti Oil |
|---|---|
| L* | 43.87 ± 0.00 |
| C* | 34.72 ± 0.00 |
| Hue (°) | 72.99 ± 0.00 |
| a* | 26.73 ± 0.00 |
| b* | 22.16 ± 0.00 |
| Parameters | C (n = 12) | EVOO1 (n = 12) | EVOO2 (n = 12) | BO1 (n = 12) | BO2 (n = 12) | Synergic (n = 12) |
|---|---|---|---|---|---|---|
| Final weight | 45.05 ± 1.38 | 45.16 ± 1.39 | 44.44 ± 1.33 | 47.77 ± 1.52 | 45.00 ± 0.88 | 46.61 ± 0.98 |
| Daily food ingestion | 5.85 ± 0.13 | 5.58 ± 0.03 | 5.72 ± 0.05 | 5.76 ± 0.06 | 5.83 ± 0.05 | 5.78 ± 0.08 |
| Parameters | C (n = 12) | EVOO1 (n = 12) | EVOO2 (n = 12) | BO1 (n = 12) | BO2 (n = 12) | Synergic (n = 12) |
|---|---|---|---|---|---|---|
| Liver | 1.82 ± 0.06 | 1.77 ± 0.06 | 1.84 ± 0.07 | 2.03 ± 0.11 | 1.74 ± 0.03 | 1.91 ± 0.06 |
| Epidydimal | 1.34 ± 0.13 | 1.52 ± 0.11 | 1.62 ± 0.14 | 1.62 ± 0.14 | 1.53 ± 0.13 | 1.76 ± 0.11 |
| Mesenteric | 0.75 ± 0.08 ab | 0.78 ± 0.09 b | 0.47 ± 0.04 a | 0.61 ± 0.06 ab | 0.67 ± 0.06 ab | 0.71 ± 0.05 ab |
| Retroperitoneal | 0.49 ± 0.04 | 0.56 ± 0.04 | 0.44 ± 0.04 | 0.60 ± 0.05 | 0.57 ± 0.04 | 0.60 ± 0.04 |
| Perirenal | 0.21 ± 0.02 ab | 0.22 ± 0.02 ab | 0.14 ± 0.01 a | 0.20 ± 0.01 ab | 0.20 ± 0.01 ab | 0.25 ± 0.02 b |
| Adiposity index | 6.32 ± 0.43 | 6.53 ± 0.38 | 5.50 ± 0.45 | 6.55 ± 0.35 | 6.70 ± 0.40 | 7.21 ± 0.33 |
| Parameters | C (n = 12) | EVOO1 (n = 12) | EVOO2 (n = 12) | BO1 (n = 12) | BO2 (n = 12) | Synergic (n = 12) |
|---|---|---|---|---|---|---|
| Triglycerides | 210.57±12.30 | 171.62 ± 7.34 | 182.63 ± 8.50 | 194.61 ± 14.24 | 180.40 ± 7.58 | 182.18 ± 7.32 |
| Glucose | 172.38 ± 28.94 | 173.15 ± 15.87 | 163.85 ± 13.02 | 162.78 ± 14.97 | 180.85 ± 17.65 | 168.87 ± 15.90 |
| Total cholesterol | 1.59.47 ± 5.81 | 165.80 ± 8.40 | 150.18 ± 7.55 | 150.67 ± 7.51 | 160.46 ± 7.86 | 158.13 ± 4.75 |
| HDL-c | 91.90 ± 3.17 a | 92.05 ± 4.16 ab | 82.61 ± 4.97 a | 90.75 ± 5.05 ab | 101.48 ± 5.45 b | 106.85 ± 4.33 b |
| Non-HDL-c | 77.57 ± 3.59 b | 78.88 ± 6.40 b | 64.80 ± 5.22 ab | 59.90 ± 4.09 a | 62.33 ± 4.20 ab | 51.11 ± 2.04 a |
| Variables | C (n = 12) | EVOO1 (n = 12) | EVOO2 (n = 12) | BO1 (n = 12) | BO2 (n = 12) | Synergic (n = 12) |
|---|---|---|---|---|---|---|
| Steatosis (p = 0.41) | ||||||
| <5% | 100.00 (12) | 100.00 (12) | 100.00 (12) | 91.70 (11) | 100.00 (12) | 100.00 (12) |
| 5 a 33% | 0.00 (0) | 0.00 (0) | 0.00 (0) | 8.30 (1) | 0.00 (0) | 0.00 (0) |
| Localization of steatosis * | ||||||
| Zone 1 | 0.00 (0) | 0.00 (0) | 0.00 (0) | 8.30 (1) | 0.00 (0) | 8.30 (1) |
| Zone 3 | 0.00 (0) | 8.30 (1) | 0.00 (0) | 8.30 (1) | 0.00 (0) | 0.00 (0) |
| Not applicable | 100.00 (12) | 91.70 (11) | 100.00 (12) | 83.30 (10) | 100.00 (12) | 91.70 (11) |
| Microvesicular steatosis * | ||||||
| Absent | 100.00 (12) | 100.00 (12) | 100.00 (12) | 100.00 (12) | 100.00 (12) | 100.00 (12) |
| Lobular inflammation (p = 0.09) | ||||||
| No focus | 100.00 (12) | 75.00 (9) | 75.00 (9) | 50.00 (6) | 50.00 (6) | 83.30 (10) |
| <2 foci/field | 0.00 (0) | 8.30 (1) | 25.00 (3) | 41.70(5) | 41.70 (5) | 16.70 (2) |
| 2–4 foci/field | 0.00 (0) | 16.70 (2) | 0.00 (0) | 8.30 (1) | 8.30 (1) | 0.00 (0) |
| Ballooning (p = 0.01) | ||||||
| Absent | 58.30 (7) b | 8.30(1) a | 58.30(7) b | 33.30 (4) ab | 33.30 (4) ab | 25.00 (3) ab |
| Few cells | 41.70 (5) | 58.30 (7) | 41.70 (5) | 25.00 (3) | 66.70 (8) | 58.30 (7) |
| Many cells | 0.00 (0) | 33.30 (4) | 0.00 (0) | 41.70 (5) | 0.00 (0) | 16.70 (2) |
| Mallory hyaline (p = 0.41) | ||||||
| Absent | 100.00 (12) | 100.00 (12) | 100.00 (12) | 91.70 (11) | 100.00 (12) | 100.00 (12) |
| Rare | 0.00 (0) | 0.00 (0) | 0.00 (0) | 8.30 (1) | 0.00 (0) | 0.00 (0) |
| Apoptosis (p = 0.40) | ||||||
| Absent | 100.00 (12) | 75.00 (9) | 91.70 (11) | 75.00 (9) | 83.30 (10) | 91.70 (11) |
| Rare hepatocytes | 0.00 (0) | 25.00 (3) | 8.30 (1) | 25.00 (3) | 16.70 (2) | 8.30 (1) |
| Nuclear glycogenation (p = 0.41) | ||||||
| None to rare | 100.00 (12) | 91.70 (11) | 100.00 (12) | 100.00 (12) | 100.00 (12) | 100.00 (12) |
| Much | 0.00 (0) | 8.30 (1) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelino, G.; Hiane, P.A.; Pott, A.; de Oliveira Filiú, W.F.; Caires, A.R.L.; Michels, F.S.; Júnior, M.R.M.; Santos, N.M.S.; Nunes, Â.A.; Oliveira, L.C.S.; et al. Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model. Nutrients 2022, 14, 2547. https://doi.org/10.3390/nu14122547
Marcelino G, Hiane PA, Pott A, de Oliveira Filiú WF, Caires ARL, Michels FS, Júnior MRM, Santos NMS, Nunes ÂA, Oliveira LCS, et al. Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model. Nutrients. 2022; 14(12):2547. https://doi.org/10.3390/nu14122547
Chicago/Turabian StyleMarcelino, Gabriela, Priscila Aiko Hiane, Arnildo Pott, Wander Fernando de Oliveira Filiú, Anderson R. L. Caires, Flavio S. Michels, Mário R. Maróstica Júnior, Nathalia M. S. Santos, Ângela A. Nunes, Lincoln C. S. Oliveira, and et al. 2022. "Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model" Nutrients 14, no. 12: 2547. https://doi.org/10.3390/nu14122547
APA StyleMarcelino, G., Hiane, P. A., Pott, A., de Oliveira Filiú, W. F., Caires, A. R. L., Michels, F. S., Júnior, M. R. M., Santos, N. M. S., Nunes, Â. A., Oliveira, L. C. S., Cortes, M. R., Maldonade, I. R., Cavalheiro, L. F., Nazário, C. E. D., Santana, L. F., Di Pietro Fernandes, C., Negrão, F. J., Tatara, M. B., de Faria, B. B., ... de Cássia Avellaneda Guimarães, R. (2022). Characterization of Buriti (Mauritia flexuosa) Pulp Oil and the Effect of Its Supplementation in an In Vivo Experimental Model. Nutrients, 14(12), 2547. https://doi.org/10.3390/nu14122547







