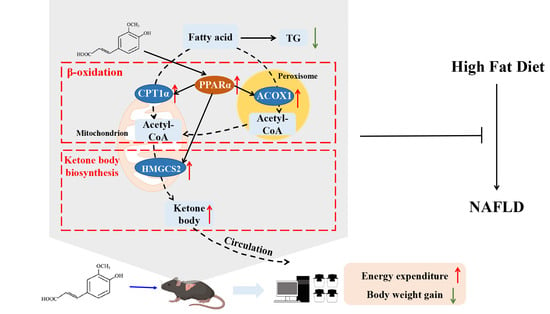
Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiment
2.3. Dosage Information
2.4. Metabolic Cage Studies
2.5. Metabolic Assay
2.6. Biochemical Analysis of Serum and Liver Samples
2.7. Liver Histopathology
2.8. Quantitative Real-Time PCR
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. FA Reduces Hepatic Steatosis in HFD-Fed Mice
3.2. FA Reduces Expansion of Adipose Tissue and Body Weight Gain in HFD-Fed Mice
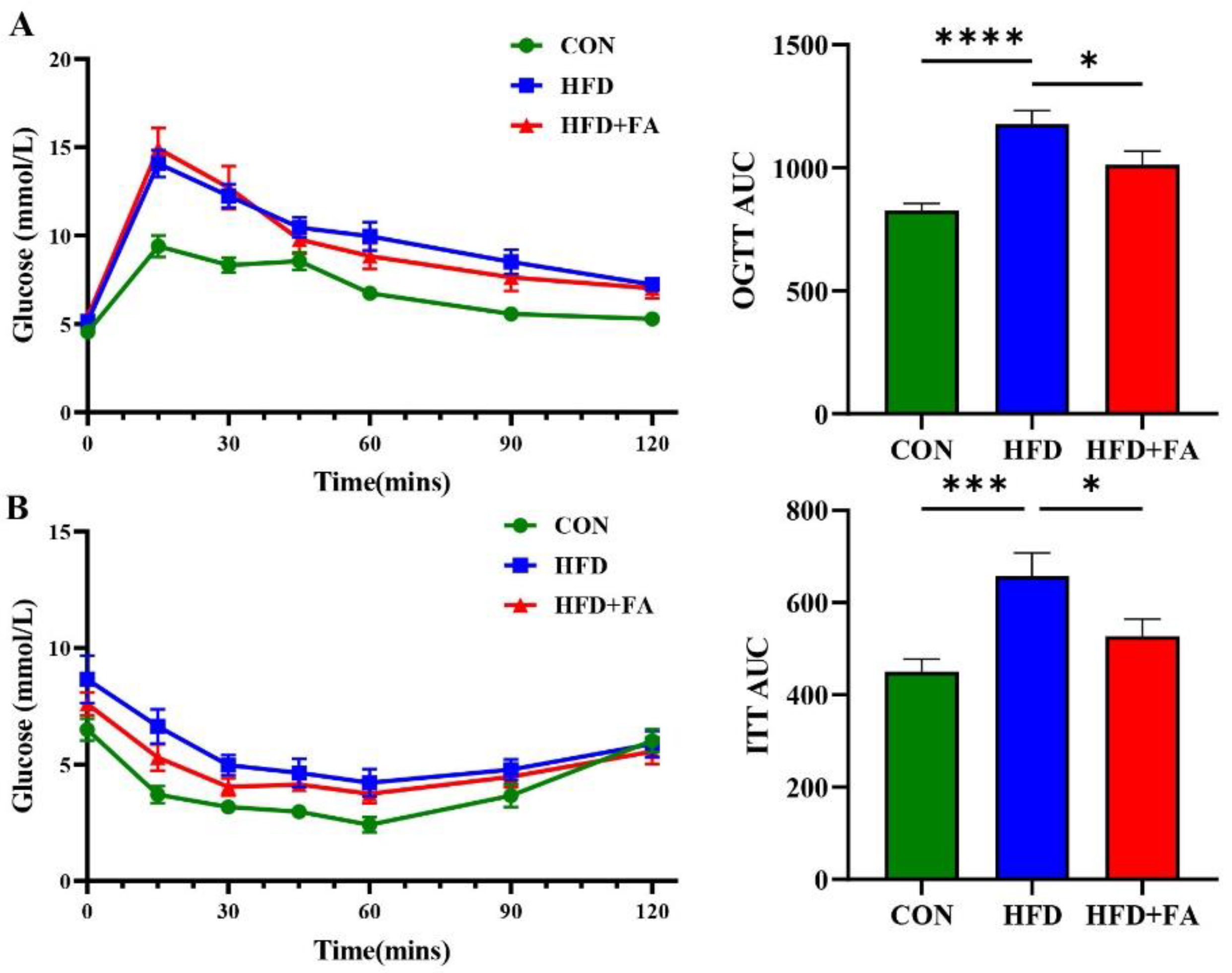
3.3. FA Improves Insulin Sensitivity and Glucose Tolerance
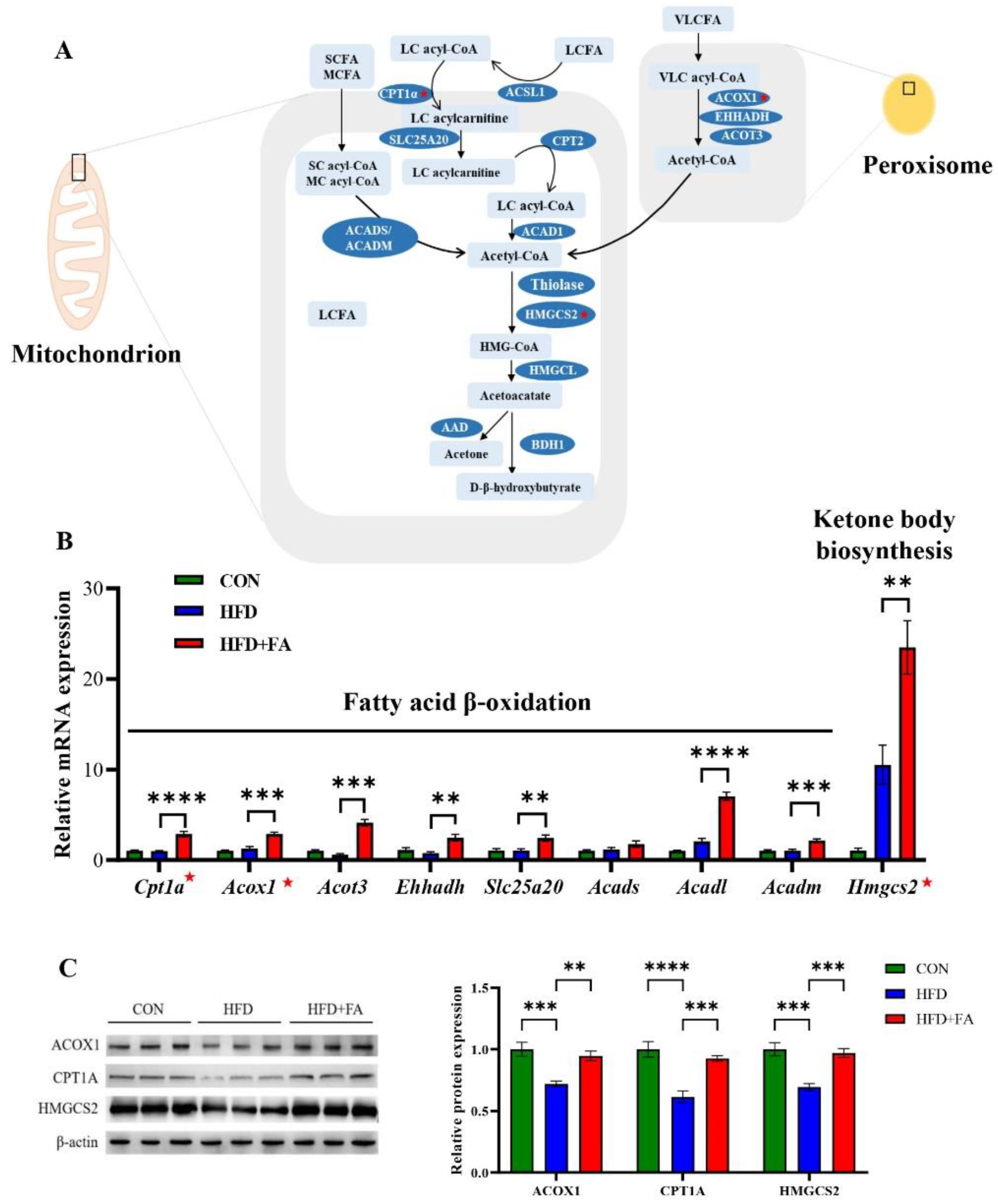
3.4. FA Increased Fatty Acid Oxidation and Ketone Body Biosynthesis in Liver
3.5. FA Activates PPARα in Liver
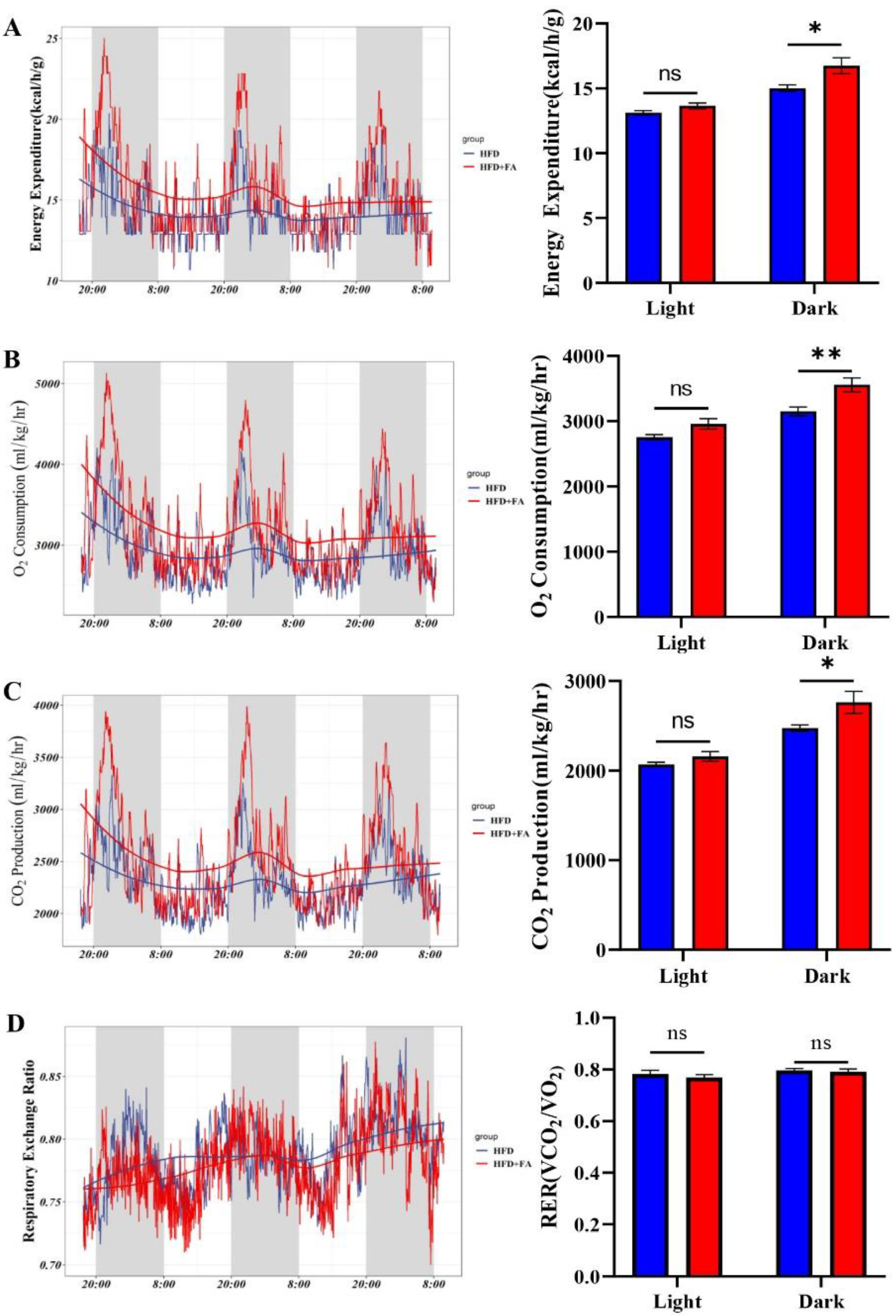
3.6. FA Increases Energy Expenditure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. Impact of the Co-Administration of N-3 Fatty Acids and Olive Oil Components in Preclinical Nonalcoholic Fatty Liver Disease Models: A Mechanistic View. Nutrients 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, K.; Yao, W.; Zheng, R.; He, Q.; Xia, J.; Li, J.; Shao, Y.; Zhang, L.; Huang, L.; et al. Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J. Hepatol. 2021, 74, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of Natural Products on Fructose-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Cai, J.; Li, H. Emerging Molecular Targets for Treatment of Nonalcoholic Fatty Liver Disease. Trends Endocrinol. Metab. 2019, 30, 903–914. [Google Scholar] [CrossRef]
- Zammit, V.A. Carnitine palmitoyltransferase 1: Central to cell function. IUBMB Life 2008, 60, 347–354. [Google Scholar] [CrossRef]
- Setoyama, D.; Fujimura, Y.; Miura, D. Metabolomics reveals that carnitine palmitoyltransferase-1 is a novel target for oxidative inactivation in human cells. Genes Cells 2013, 18, 1107–1119. [Google Scholar] [CrossRef]
- Chen, X.F.; Tian, M.X.; Sun, R.Q.; Zhang, M.L.; Zhou, L.S.; Jin, L.; Chen, L.L.; Zhou, W.J.; Duan, K.L.; Chen, Y.J.; et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018, 19, e45124. [Google Scholar] [CrossRef]
- Li, J.; Viswanadha, S.; Loor, J.J. Hepatic Metabolic, Inflammatory, and Stress-Related Gene Expression in Growing Mice Consuming a Low Dose of Trans-10, cis-12-Conjugated Linoleic Acid. J. Lipids 2012, 2012, 571281. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Satoh, A.; Tezuka, H.; Han, S.I.; Takei, K.; Iwasaki, H.; Yatoh, S.; Yahagi, N.; Suzuki, H.; Iwasaki, Y.; et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARalpha. Sci. Rep. 2016, 6, 39182. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Nicolosi, R.J.; Woolfrey, B.; Kritchevsky, D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J. Nutr. Biochem. 2007, 18, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Christensen, L.P.; Meyer, A.S.; Hansen, A. Content of phenolic acids and ferulic acid dehydrodimers in 17 rye (Secale cereale L.) varieties. J. Agric. Food Chem. 2000, 48, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Klepacka, J.; Fornal, L. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic Acid Ameliorates Atherosclerotic Injury by Modulating Gut Microbiota and Lipid Metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Wang, W.W.; Pan, Y.; Zhou, H.; Wang, L.; Chen, X.; Song, G.; Liu, J.X.; Li, A.K. Ferulic acid suppresses obesity and obesity-related metabolic syndromes in high fat diet-induced obese C57BL/6J mice. Food Agric. Immunol. 2018, 29, 1116–1125. [Google Scholar] [CrossRef]
- De Melo, T.S.; Lima, P.R.; Carvalho, K.M.; Fontenele, T.M.; Solon, F.R.; Tome, A.R.; de Lemos, T.L.; da Cruz Fonseca, S.G.; Santos, F.A.; Rao, V.S.; et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Braz. J. Med. Biol. Res. 2017, 50, e5630. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and gamma-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.J.; Bohm, M.; Alvarez de Sotomayor, M.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Matsuda, M.; Mahankali, S.; Santini, E.; DeFronzo, R.A.; Ferrannini, E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 5098–5103. [Google Scholar] [CrossRef]
- Fan, J.G.; Cao, H.X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 81–87. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Ma, Y.C.; Chen, K.; Lv, L.; Wu, S.Y.; Guo, Z.J. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE(−/−) mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.W.; Yang, C.F.; Zhong, Y.J.; He, H.; Zhu, F.C.; Li, L. Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 Pathways in Rats and L-02 cells. Chem-Biol. Interact. 2018, 291, 180–189. [Google Scholar] [CrossRef]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta 2012, 1821, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Reddy, J.K.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 2006, 147, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Knauf, C.; Rieusset, J.; Foretz, M.; Cani, P.D.; Uldry, M.; Hosokawa, M.; Martinez, E.; Bringart, M.; Waget, A.; Kersten, S.; et al. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: Role in liver and brain. Endocrinology 2006, 147, 4067–4078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lakhia, R.; Yheskel, M.; Flaten, A.; Quittner-Strom, E.B.; Holland, W.L.; Patel, V. PPARalpha agonist fenofibrate enhances fatty acid beta-oxidation and attenuates polycystic kidney and liver disease in mice. Am. J. Physiol. Renal Physiol. 2018, 314, F122–F131. [Google Scholar] [CrossRef]
- Yoon, M.; Jeong, S.; Nicol, C.J.; Lee, H.; Han, M.; Kim, J.J.; Seo, Y.J.; Ryu, C.; Oh, G.T. Fenofibrate regulates obesity and lipid metabolism with sexual dimorphism. Exp. Mol. Med. 2002, 34, 481–488. [Google Scholar] [CrossRef]
- Liu, Z.G.; Qiao, Q.L.; Sun, Y.L.; Chen, Y.W.; Ren, B.; Liu, X.B. Sesamol ameliorates diet-induced obesity in C57BL/6J mice and suppresses adipogenesis in 3T3-L1 cells via regulating mitochondria-lipid metabolism. Mol. Nutr. Food Res. 2017, 61, 1600717. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhang, Y.; Chu, S.; Huo, Y.; Zhao, J.; Wan, C. Huangjinya Black Tea Alleviates Obesity and Insulin Resistance via Modulating Fecal Metabolome in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2020, 64, e2000353. [Google Scholar] [CrossRef]
- Rong, S.; Zhao, S.; Kai, X.; Zhang, L.; Zhao, Y.; Xiao, X.; Bao, W.; Liu, L. Procyanidins extracted from the litchi pericarp attenuate atherosclerosis and hyperlipidemia associated with consumption of a high fat diet in apolipoprotein-E knockout mice. Biomed. Pharmacother. 2018, 97, 1639–1644. [Google Scholar] [CrossRef]
- Goedeke, L.; Bates, J.; Vatner, D.F.; Perry, R.J.; Wang, T.; Ramirez, R.; Li, L.; Ellis, M.W.; Zhang, D.; Wong, K.E.; et al. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents. Hepatology 2018, 68, 2197–2211. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.B.; Mohiuddin, S.S. Biochemistry, Ketone Metabolism; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone body metabolism and its defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Frias, F.T.; Rocha, K.C.E.; de Mendonca, M.; Murata, G.M.; Araujo, H.N.; de Sousa, L.G.O.; de Sousa, E.; Hirabara, S.M.; Leite, N.C.; Carneiro, E.M.; et al. Fenofibrate reverses changes induced by high-fat diet on metabolism in mice muscle and visceral adipocytes. J. Cell. Physiol. 2018, 233, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Pan, D.; Liu, H.; Liu, M.; Shi, X.; Chu, X.; Lu, J.; Zhu, M.; Xia, B.; Wu, J. Diosmetin Protects Against Obesity and Metabolic Dysfunctions Through Activation of Adipose Estrogen Receptors in Mice. Mol. Nutr. Food Res. 2021, 65, e2100070. [Google Scholar] [CrossRef]



| Gene (Transcript) | Sequences (5′-3′) | Products Length/bp |
|---|---|---|
| Actb (NM_007393.5) | GGCTCCTAGCACCATGAAG | 190 |
| CGCAGCTCAGTAACAGTCC | ||
| Cpt1a (NM_013495.2) | TGACGGCTATGGTGTTTC | 124 |
| CATGGCTTGTCTCAAGTG | ||
| Ppara (NM_011144.6) | TCAAGGCCCGGGTCATACTC | 164 |
| CTGGCAGCAGTGGAAGAATC | ||
| Acox1 (NM_001377522.1) | GCCTGCTGTGTGGGTATGTCATT | 150 |
| GTCATGGGCGGGTGCAT | ||
| Hmgcs2 (NM_008256.4) | GTGTCCCGTCTAATGGAG | 180 |
| GAGACACCCAGGATTCAC | ||
| Acot3 (NM_001346701.1) | TGGTCCTGTGTGGGCTCT | 107 |
| TGCCCTCCGAGTGAGTGT | ||
| Ehhadh (NM_023737.3) | GGAACCCCCACCCGAAAG | 129 |
| GATGCGACCCAGTGGCTT | ||
| Fgf21 (NM_020013.4) | GGTGTCAAAGCCTCTAGGTTTC | 139 |
| CATGGGCTTCAGACTGGTACAC | ||
| Lipe (NM_010719.5) | TCCTGGAACTAAGTGGACGCAAG | 93 |
| CAGACACACTCCTGCGCATAGAC | ||
| Pnpla2 (NM_001163689.1) | AAAGATCGAATTCTAGAGCACC | 189 |
| CCACTCCAACAAGCGGA | ||
| Pygl (NM_133198.2) | CCGGAGACCGTTCTGTGC | 104 |
| CTCTACGCCCACGATGCC | ||
| Gck (NM_010292.5) | CTGGGAGGAACCAACTTCAG | 160 |
| CAGAGATGCACTCAGAGATG | ||
| Dgat2 (NM_026384.3) | GGCTACGTTGGCTGGTAACT | 197 |
| TCTTCAGGGTGACTGCGTTC | ||
| Slc2a2 (NM_031197.2) | AGATTGGGCCAGGTCCAATCC | 132 |
| ACTGGAAGCAGAGGGCGATGAC | ||
| Slc25a20 (NM_020520.5) | GGCTTTGCAGGGATCTTC | 142 |
| AGGTGACTCCTTCTTCTC | ||
| Acads (NM_007383.3) | GTGCTGCCATGTTGAAAG | 142 |
| GGCATCTCTGTCACATAC | ||
| Acadm (NM_007382.5) | GGAGGCTATGGATTCAAC | 119 |
| ATGTGCTCACGAGCTATG | ||
| Acadl (NM_007381.4) | GGTACATGTGGGAGTACC | 100 |
| CTTGCGATCAGCTCTTTC | ||
| Fasn (NM_007988.3) | TAAAGCATGACCTCGTGATGAA | 230 |
| GAAGTTCAGTGAGGCGTAGTAG | ||
| Scd1 (NM_009127.4) | GAACACCCATCCCGAGAGT | 176 |
| TGTAAGAACTGGAGATCTCTTGGA | ||
| Acaca (NM_133360.2) | AGTGATGGTGGCCTGCTCTTG | 150 |
| AGCAGACGGTGAGCGCATTA |
| Group | CON | HFD | HFD+FA | |
|---|---|---|---|---|
| Ingredient (g/kg Diet) | TP23402 | TP23400 | ||
| Casein | 195 | 195 | ||
| Maltodextrin | 56 | 225 | ||
| Sucrose | 55 | 89 | ||
| Corn starch | 479 | 0 | ||
| Soybean Oil | 33 | 33 | ||
| Lard | 25 | 301 | ||
| Cellulose | 69 | 69 | ||
| Mineral Mix, M1020 | 68 | 68 | ||
| Vitamin Mix, V1010 | 14 | 14 | ||
| L-Cystine | 3 | 3 | ||
| Choline Bitartrate | 3 | 3 | ||
| TBHQ | 0.067 | 0.067 | ||
| Total | 1000 | 1000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Li, M.; Yang, Q.; Zhang, Y.; Liu, F.; Gong, L.; Han, L.; Wang, M. Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet. Nutrients 2022, 14, 2530. https://doi.org/10.3390/nu14122530
Luo Z, Li M, Yang Q, Zhang Y, Liu F, Gong L, Han L, Wang M. Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet. Nutrients. 2022; 14(12):2530. https://doi.org/10.3390/nu14122530
Chicago/Turabian StyleLuo, Zhixin, Mengqian Li, Qiong Yang, Yuhong Zhang, Fang Liu, Lan Gong, Lin Han, and Min Wang. 2022. "Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet" Nutrients 14, no. 12: 2530. https://doi.org/10.3390/nu14122530
APA StyleLuo, Z., Li, M., Yang, Q., Zhang, Y., Liu, F., Gong, L., Han, L., & Wang, M. (2022). Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet. Nutrients, 14(12), 2530. https://doi.org/10.3390/nu14122530