Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Potato Protein Structures
2.2. Determination of Trypsin Inhibitory Activity in PPI
2.3. Gastrointestinal Simulated Semi-Dynamic in Vitro Digestion
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Size Exclusion Chromatography
2.7. Degree of Hydrolysis
2.8. Free Amino Acid Analysis by Triple Quadrupole Mass Spectrometry
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microstructural Characterization
3.2. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.3. Determination of Trypsin Inhibitory Activity in PPI/Processing Effect on Trypsin Inhibitory Activity in Potato Protein Isolates
3.4. Peptide Size Distribution and Degree of Hydrolysis during Gastric Emptying and Intestinal Digestion
3.5. Free Amino Acid Analysis by Triple Quadrupole Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Drulyte, D.; Orlien, V. The effect of processing on digestion of legume proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlant-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The nutritional value and biological activity of concentrated protein fraction of potato juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef] [Green Version]
- Ralet, M.C.; Guéguen, J. Fractionation of potato proteins: Solubility, thermal coagulation and emulsifying properties. LWT 2000, 33, 380–387. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Luo, Z.; Tamer, T.M. Stability of trypsin inhibitor isolated from potato fruit juice against pH and heating treatment and in vitro gastrointestinal digestion. Food Chem. 2020, 328, 127152. [Google Scholar] [CrossRef]
- Liyanage, R.; Han, K.-H.; Watanabe, S.; Shimada, K.-I.; Sekikawa, M.; Ohba, K.; Tokuji, Y.; Ohnishi, M.; Shibayama, S.; Nakamori, T.; et al. Potato and soy peptide diets modulate lipid metabolism in rats. Biosci. Biotechnol. Biochem. 2008, 72, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-Inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Cook, A.; Raskin, I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int. J. Obes. 2011, 35, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.G.; Guan, D.; Green, G.M.; Phillips, W.T. Treatment with oral proteinase inhibitor slows gastric emptying and acutely reduces glucose and insulin levels after a liquid meal in type II diabetic patients. Diabetes Care 1994, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mu, T.; Sun, H.; Chen, J.; Zhang, M. Comparative study of potato protein concentrates extracted using ammonium sulfate and isoelectric precipitation. Int. J. Food Prop. 2017, 20, 2113–2127. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Qayum, A.; Xiuxiu, Z.; Liu, L.; Hussain, K.; Yue, P.; Yue, S.; Koko, M.Y.; Hussain, A.; Li, X. Potato protein: An emerging source of high quality and allergy free protein, and its possible future based products. Food Res. Int. 2021, 148, 110583. [Google Scholar] [CrossRef]
- Schoenbeck, I.; Graf, A.; Leuthold, M.; Pastor, A.; Beutel, S.; Scheper, T. Purification of high value proteins from particle containing potato fruit juice via direct capture membrane adsorption chromatography. J. Biotechnol. 2013, 168, 693–700. [Google Scholar] [CrossRef]
- AVEBE. Potato Protein Isolates. Google Patents WO2014011042A1, 16 January 2014. Available online: https://patents.google.com/patent/WO2014011042A1/en (accessed on 18 May 2022).
- Wu, Y.; Li, W.; Martin, G.J.O.; Ashokkumar, M. Mechanism of low frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 130057. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.-L.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food—An international consensus. Food Funct. 2020, 11, 1702. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zaeim, D.; Mulet-Cabrero, A.; Read, S.A.; Liu, W.; Han, J.; Wilde, P.J. Effect of oil droplet size on the gastric digestion of milk protein emulsions using a semi-dynamic gastric model. Food Hydrocoll. 2022, 124, 107278. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T. A standardised static in vitro digestion method suitable for food- An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Fernández-García, E.; Lérida-Carvajal, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Andlinger, D.J.; Röscheisen, P.; Hengst, C.; Kulozik, U. Influence of pH, temperature and protease inhibitors on kinetics and mechanism of thermally induced aggregation of potato proteins. Foods 2021, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Van Koningsveld, G.A.; Gruppen, H.; de Jongh, H.H.J.; Wijngaards, G.; van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Effects of Ph and heat treatments on the structure and solubility of potato proteins in different preparations. J. Agric. Food. Chem 2001, 49, 4889–4897. [Google Scholar] [CrossRef]
- Tanger, C.; Andlinger, D.J.; Brümer-Rolf, A.B.; Engel, J.; Kulozik, U. Quantification of protein-protein interactions in highly denatured whey and potato protein gels. MethodsX 2021, 8, 101243. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Sunds, A.V.; Larsen, L.B.; Hammershøj, M. Gel properties of potato protein and the isolated fractions of patatin and protease inhibitors—Impact of drying method, protein concentration, pH and ionic strength. Food Hydrocoll. 2019, 96, 246–258. [Google Scholar] [CrossRef]
- Elahi, R.; Mu, T.H. High hydrostatic pressure (HHP)-Induced structural modification of patatin and its antioxidant activities. Molecules 2017, 22, 438. [Google Scholar] [CrossRef] [Green Version]
- Melnikov, S.M.; Stoyanov, S.D.; Kovacs, E.M.; Arnaudov, L.; De Groot, P.; Schuring, E.A.; Wiseman, S.A.; Mela, D.J.; Peters, H.P. Sustained hunger suppression from stable liquid foams. Obesity 2014, 22, 2131–2136. [Google Scholar] [CrossRef]
- Ménard, O.; Famelart, M.H.; Deglaire, A.; Le Gouar, Y.; Guérin, S.; Malbert, C.H.; Dupont, D. Gastric emptying and dynamic in vitro digestion of drinkable yogurts: Effect of viscosity and composition. Nutrients 2018, 10, 1308. [Google Scholar] [CrossRef] [Green Version]
- Shelat, K.J.; Nicholson, T.; Flanagan, B.M.; Zhang, D.; Williams, B.A.; Gidley, M.J. Rheology an microstructure characterization of small intestinal digesta from pigs fed a red meat-containing Western-style diet. Food Hydrocoll. 2015, 45, 300–308. [Google Scholar] [CrossRef]
- Murray, K.; Placidi, E.; Schuring, E.A.H.; Hoad, C.; Koppenol, W.; Arnaudov, L.N.; Blom, W.; E Pritchard, S.; Stoyanov, S.D.; Gowland, P.A.; et al. Aerated drinks increase gastric volume and reduce appetite as assessed by MRI: A randomized, balanced, crossover trial. Am. J. Clin. Nutr. 2015, 101, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomolino, G.; Vincenzi, S.; Zannoni, S.; Marangon, M.; De Iseppi, A.; Curioni, A. Emulsifying activity of potato proteins in the presence of k-carrageenan at different pH conditions. Food Chem. X 2022, 13, 100232. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Blanco, G. Medical Biochemistry: Digestion-Absorption; Academic Press: Cambridge, MA, USA, 2017; Chapter 12. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.; Brodkorb, A.; Gómez-Mascaraque, L.; Corredig, M. Effect of heat treatment on the digestion behavior of pea and rice protein dispersions and their blends, studied using the semi-dynamic INFOGEST digestion method. Food Funct. 2021, 12, 8747–8759. [Google Scholar] [CrossRef] [PubMed]
- Peram, M.R.; Loveday, S.M.; Ye, A.Q.; Singh, H. In vitro gastric digestion of heat-induced aggregates of beta-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.K.; Oiseth, S.K.; Lundin, L.; Day, L. Influence of heat and shear induced protein aggregation on the in vitro digestion rate of whey proteins. Food Funct. 2014, 5, 2686–2698. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Control of protein digestion under simulated gastrointestinal conditions using biopolymer microgels. Food Res. Int. 2017, 100, 86–94. [Google Scholar] [CrossRef]
- Golding, M. Exploring and exploiting the role of food structure in digestion. In Interdisciplinary Approaches to Food Digestion; Gouseti, O., Bornhorst, G., Bakalis, S., Mackie, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Kim, M.Y.; Son, C.W.; Shim, H.J.; Lee, J.H.; Lee, K.J.; Sok, D.E.; Kim, H.C.; Yoon, W.K.; Kim, H.M.; Kim, M.R. Effect of cultivars and cooking methods on the trypsin inhibitor activities of potatoes. Food Sci. Biotechnol. 2008, 17, 161–165. [Google Scholar]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna-Saldivar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2017, 83, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Vanga, S.K.; Wang, J.; Raghavan, V. Effect of ultrasound and microwave processing on the structure, in-vitro digestibility and trypsin inhibitor activity of soymilk proteins. LWT 2020, 131, 109708. [Google Scholar] [CrossRef]
- Nagase, H.; Salvesen, G.S. Finding, Purification, and Characterization of Natural Protease Inhibitors in Proteolytic Enzymes: A Practical Approach, 2nd ed.; Beynon, R.J., Bond, J.S., Eds.; Oxford University Press: Oxford, NY, USA, 2001; pp. 131–147. [Google Scholar]
- Da Costa, H.P.S.; Oliveira, J.T.A.; Sousa, D.O.B.; Morais, J.K.S.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Viegas, R.A.; Vasconcelos, I.M. JcTI-I: A novel trypsin inhibitor from Jatropha curcas seed cake with potential for bacterial infection treatment. Front. Microbiol. 2014, 5, 5. [Google Scholar] [CrossRef]
- Rivera del Rio, A.; Keppler, J.K.; Boom, R.M.; Janssen, A.E.M. Protein acidification and hydrolysis by pepsin ensure efficient trypsin-catalyzed hydrolysis. Food Funct. 2021, 12, 4570–4581. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.S. Parietal Cells. In Encyclopedia of Gastroenterology; Johnson, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 140–144. ISBN 9780123868602. [Google Scholar] [CrossRef]
- Rakhshi, E.; Nau, F.; Hiolle, M.; Floury, J. Pepsin diffusion in complex food matrices. J. Food Eng. 2022, 324, 111011. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J. Potato industry by-products as a source of protein with beneficial nutritional, functional, health-promoting and antimicrobial properties. Appl. Sci. 2021, 11, 3497. [Google Scholar] [CrossRef]
- Pęksa, A.; Kita, A.; Kułakowska, K.; Aniołowska, M.; Hamouz, K.; Nemś, A. The quality of protein of coloured fleshed potatoes. Food Chem. 2013, 131, 2960–2966. [Google Scholar] [CrossRef]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Spelbrink, R.E.J.; Witteman, B.J.; Giussepin, M.L.F. Digestion kinetics of potato protein isolates in vitro and in vivo. Food Sci. Nutr. 2013, 64, 787–793. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant-and animal sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Bahniwal, R.; Holloway, T.M.; Lim, C.; McLeod, J.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Potato protein isolate stimulates muscle protein synthesis at rest and with resistance exercise in young women. Nutrients 2020, 125, 1235. [Google Scholar] [CrossRef]


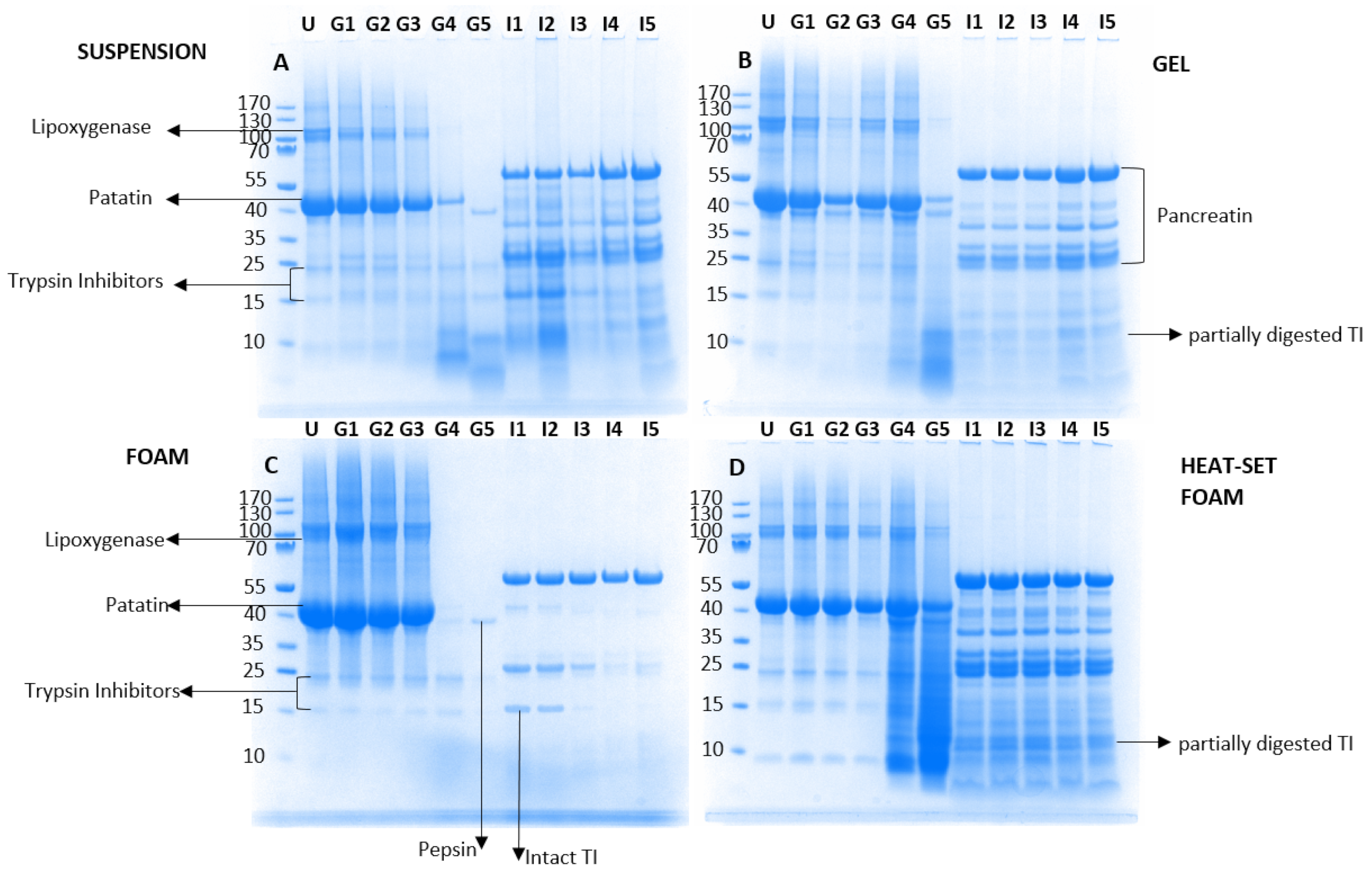
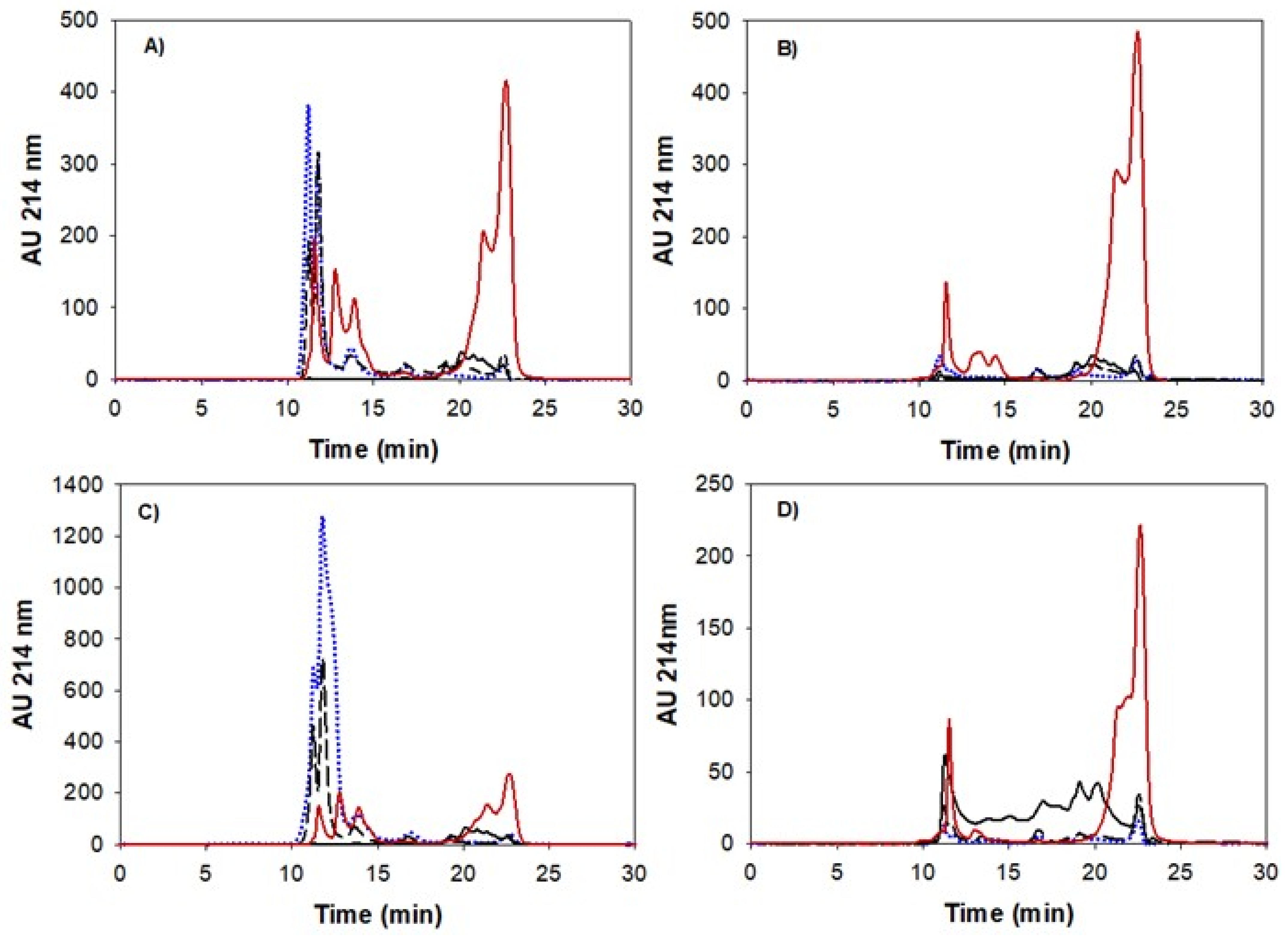
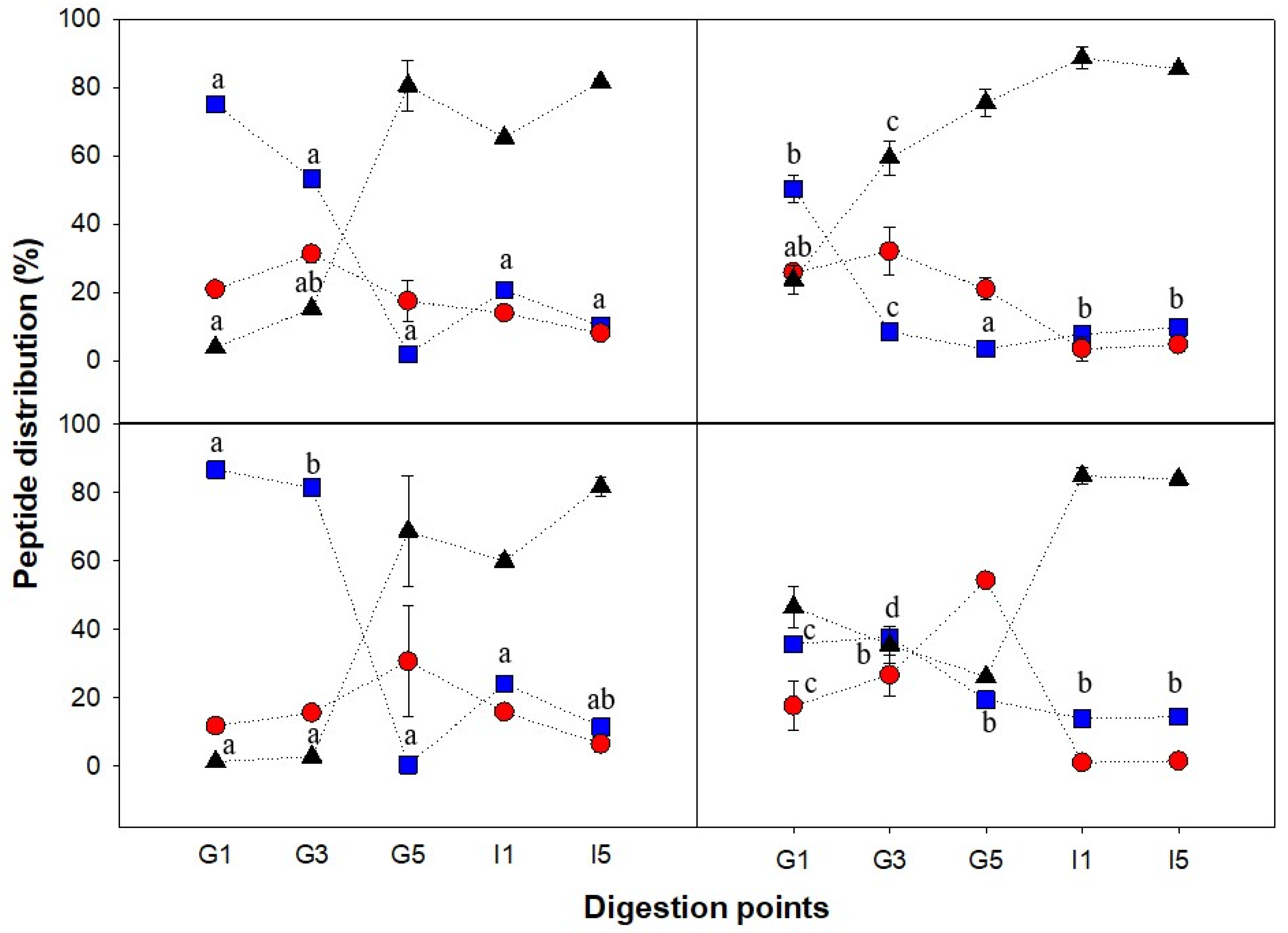
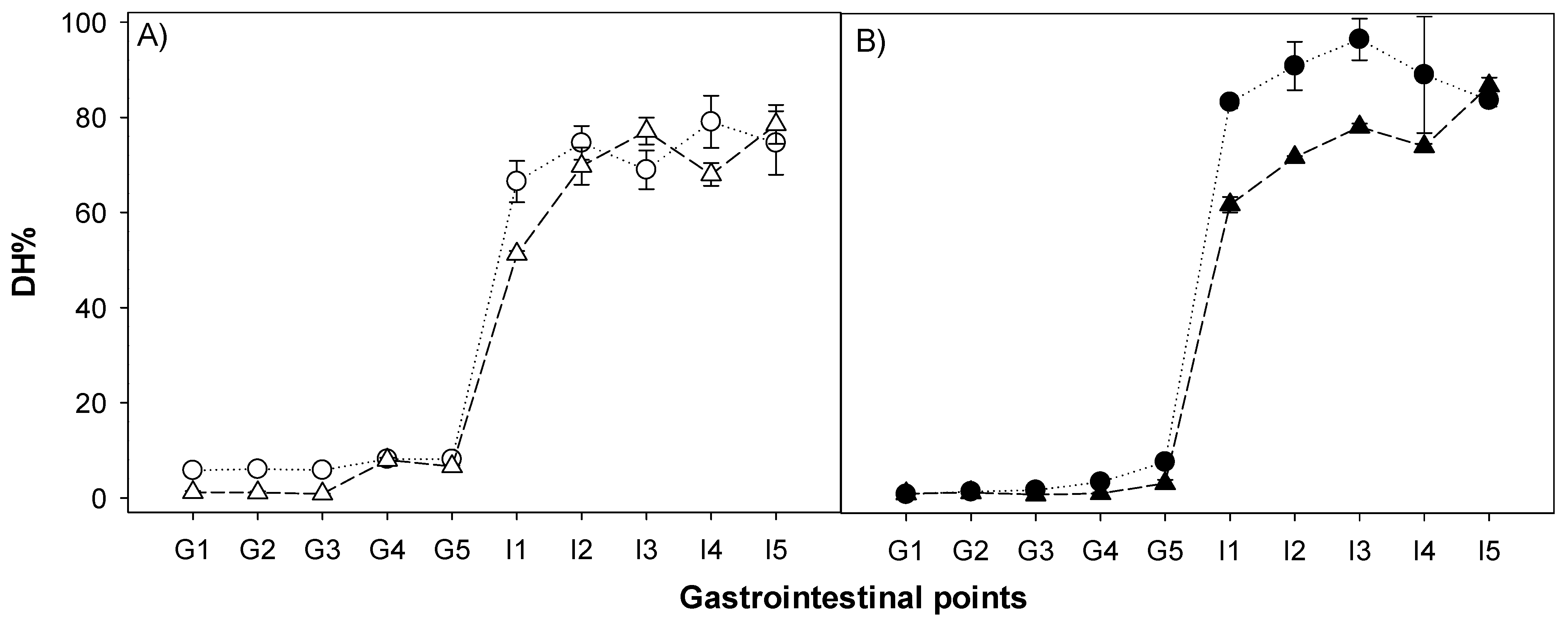
| Trypsin Inhibitory Activity | PoPI-S | PoPI-G | PoPI-F | PoPI-HF |
|---|---|---|---|---|
| TI (U/mg PoPI) | 3.1 ± 0.0 a | 2.7 ± 0.0 b | 2.9 ± 0.1 a | 1.1 ± 0.0 c |
| TIA (%) | 72.8 ± 1.1 a | 61.9 ± 1.2 b | 67.9 ± 2.9 a | 25.6 ± 0.3 c |
| mg Free Amino Acids/g Soluble Protein | PoPI-S | PoPI-F | PoPI-G | PoPI-HF |
|---|---|---|---|---|
| Essential amino acids | ||||
| Valine | 33.5 ± 0.1 a | 33.3 ± 7.3 a | 24.2 ± 1.1 a | 23.4 ± 0.5 a |
| Leucine | 78.5 ± 2.4 a | 46.8 ± 15.8 a | 55.5 ± 0.2 a | 58.5 ± 2.4 a |
| Isoleucine | 119.1 ± 1.3 a | 81.2 ± 28.0 a | 97.8 ± 3.6 a | 96.0 ± 2.5 a |
| Methionine | 9.9 ± 0.1 a | 6.0 ± 2.0 a | 6.9 ± 0.05 a | 7.4 ± 0.16 a |
| Phenylalanine | 43.3 ± 2.1 b | 25.5 ± 8.0 a | 38.9 ± 1.2 ab | 28.8 ± 2.2 ab |
| Threonine | 30.3 ± 0.5 a | 20.6 ± 7.5 a | 23.5 ± 1.13 a | 23.3 ± 0.3 a |
| Lysine | 92.5 ± 2.1 b | 56.1 ± 19.7 a | 66.8 ± 1.6 a | 67.6 ± 1.7 a |
| Histidine | 45.4 ± 1.77 b | 32.2 ± 11.7 a | 31.7 ± 2.2 a | 36.8 ± 3.2 a |
| Tryptophan | 35.7 ± 0.9 a | 23.5 ± 7.3 a | 31.6 ± 0.8 a | 27.2 ± 1.2 a |
| Non-essential amino acids | ||||
| Tyrosine | 30.4 ± 0.2 a | 19.7 ± 6.3 a | 26.3 ± 0.4 a | 26.0 ± 0.9 a |
| Asparagine | 17.2 ± 0.4 a | 41.8 ± 6.2 b | 21.4 ± 0.3 a | 27.6 ± 2.3 ab |
| Cysteine | 10.0 ± 0.1 ab | 13.5 ± 0.4 c | 10.4 ± 0.2 b | 9.1 ± 0.07 a |
| Proline | 2.48 ± 0.0 a | 1.74 ± 0.7 a | 1.6 ± 0.03 a | 1.4 ± 0.06 a |
| Glycine | 17.1 ± 4.1 a | 25.6 ± 3.2 a | 15.8 ± 0.6 a | 16.7 ± 0.6 a |
| Glutamic acid | 69.7 ± 0.2 a | 38.7 ± 16.0 a | 47.6 ± 0.2 a | 44.4 ± 2.0 a |
| Aspartic acid | 172.2 ± 12.6 a | 102.8 ± 42.2 a | 119.5 ± 1.3 a | 116.1 ± 7.2 a |
| Alanine | 58.2 ± 0.8 a | 35.0 ± 11.4 a | 43.9 ± 0.4 a | 40.8 ± 0.2 a |
| Serine | 15.4 ± 1.3 a | 10.4 ± 4.9 a | 14.18 ± 1.1 a | 11.9 ± 1.0 a |
| Glutamine | 64.1 ± 3.7 a | 46.1 ± 14.6 a | 45.7 ± 2.3 a | 42.6 ± 4.4 a |
| Arginine | 0.1 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| TFAA | 1000 ± 35 b | 695 ± 224 a | 762 ± 20 a | 743 ± 34 a |
| Essential Amino Acids | Amino Acid Content in the Reference Pattern Values (mg/g) * | Potato Protein Isolate Free Amino Acids Released Ileum (mg/g). Experimental Data | Theoretically Predicted DIAAS Based on Initial Amino Acid Composition of the Source (Before Digestion) [53] | Calculated DIAAS Based on INFOGEST In Vitro Experimental Bioaccesible Values (In Vitro DIAAS Adults) |
|---|---|---|---|---|
| Valine | 39 | 34 | 1.38 | 0.87 |
| Leucine | 59 | 78.5 | 1.43 | 1.33 |
| Isoleucine | 30 | 119.12 | 1.56 | 3.97 |
| Met + Cys | 22 | 20 | 1.15 | 0.91 |
| Phe + Tyr | 38 | 73.7 | 2.10 | 1.93 |
| Lysine | 45 | 92.5 | 1.22 | 2.05 |
| Threonine | 25 | 30.4 | 1.65 | 1.22 |
| Tryptophan | 6.6 | 35.7 | 1.28 | 5.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Munoz, L.; Tsochatzis, E.D.; Corredig, M. Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System. Nutrients 2022, 14, 2505. https://doi.org/10.3390/nu14122505
Jiménez-Munoz L, Tsochatzis ED, Corredig M. Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System. Nutrients. 2022; 14(12):2505. https://doi.org/10.3390/nu14122505
Chicago/Turabian StyleJiménez-Munoz, Luis, Emmanouil D. Tsochatzis, and Milena Corredig. 2022. "Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System" Nutrients 14, no. 12: 2505. https://doi.org/10.3390/nu14122505
APA StyleJiménez-Munoz, L., Tsochatzis, E. D., & Corredig, M. (2022). Impact of the Structural Modifications of Potato Protein in the Digestibility Process under Semi-Dynamic Simulated Human Gastrointestinal In Vitro System. Nutrients, 14(12), 2505. https://doi.org/10.3390/nu14122505








